High Level of Sequence-Variation in Sacbrood Virus (SBV) from Apis mellifera
Abstract
Sacbrood virus (SBV) is one of the main pathogenic RNA viruses of honeybee. SBV is found worldwide and many local strains have been reported, such as kSBV, cSBV, and wSBV. In this study, SBV-specific DNA fragments were cloned and sequenced by reverse-transcription PCR from 4 populations of A. mellifera, 4 sequences from 1 population belonged to the 2134D51 genotype (349 nucleotides, nt) and 12 sequences from 3 populations belonged to the 2100D0 genotype (400 nt) among the 16 determined sequences. A total of 87 points of mismatches were found by comparison with the most similar sequences in GenBank. Seventeen single-nucleotide polymorphisms (SNP) were detected, and 6 SNP-patterns in the 2100D0 genotype and 2 SNP-patterns in the 2134D51 genotype were identified based on SNP positions. In SNP-pattern 2, 10 SNPs were detected, but only 2 SNPs were found in SNP-pattern7. Meanwhile, one SNP-pattern was found from one RNA-sample, multi SNP-patterns were detected from other RNA-samples. Large numbers of SNP variants indicate that vast numbers of point-mutations on SBV have occurred since SBV invaded Korea and that SNP smay have been introduced individually over time. Thorough analysis of SNP variants will not only define the local infection-route, but also the relationships between SNP-pattern and SBVpathogenic abilities.
Keywords:
Sacbrood virus, Single-nucleotide polymorphism, Variant, Phylogeny, Variation, Apis melliferaINTRODUCTION
Sacbrood virus was first recorded in 1913 (White, 1917) and characterized in 1964 (Bailey et al., 1964). It belongs to the genus Iflavirus, a Picorna virus with a circular capsid that is 28nm in diameter. The SBV genome contains only one open reading frame encoding a polyprotein consisting of 2858 amino acids (Ghosh et al., 1999). SBV-infected larvae do not pupate, and their color changes from white to pale yellow followed by death (Bailey, 1975). The large number of vulnerable larvae in honeybee colonies in the spring indicate that this is a favorable period for the occurrence and spread of SBV (Bailey, 1969).
SBV has been detected in many geographically distinct regions worldwide, including Europe (Austria, Germany, and the United Kingdom; so-called “SBV”), South Asia (India and Nepal), and South Africa (Grabensteiner et al., 2001). Because of the world-wide distribution of SBV, many geological variants of SBVs have been identified, such as wSBV, cSBV and kSBV. In China, SBV (so-called “cSBV”) was first recognized in Guangdong province in 1972, which spread throughout the country (Dong et al., 1986). In Vietnam, SBV (so-called “vSBV”) occurs in both Apis cerana and Apis mellifera it was first detected in 2003 and its sequence was determined in 2004 (Le and Pham, 2004). The complete genome of SBV isolated from A. cerana was reported in 2013 (Nguyen et al., 2013).
In South Korea, the over 300,000 population of A. cerana was dramatically decreased by a variant of SBV, known as “kSBV”, in 2009. Only 20,000 colonies had survived by 2017 (Choi et al., 2010). The complete genome of kSBV was first identified from A. cerana and submitted on NCBI (accession number: HQ322114) in 2010. Genomic and phylogenetic analysis of kSBV revealed high homology among the geographical strains and a close relationship between kSBV and Chinese and other Asian SBV strains (Choe et al., 2012a). Subsequently, the analysis of kSBV genome from A. mellifera host revealed a mutation lacking a nucleotide section compared with SBV-UK. However, this genome was similar to that of the kSBV strain, which infects A. cerana(Choe et al., 2012b). A. cerana with acquired resistance to kSBV has not been detected.
Based on the analysis with full-length genomes and deduced amino acid sequences of 32 SBVs, 5 genotypes, 2134D51 (kSBV, vSBV), 2119D39 (cSBV), 2119D30 (iSBV), 2100D0 (wSBV), and 2134D3 (eSBV), were proposed (Lee et al., 2017). However, the relations hips among these genotypes and detailed characteristics, particularly the infecting-pattern or infecting-ability of each genotype, remain unclear. Furthermore, the large deletion in genomes of kSBV and vSBV (2134D51; Lee et al., 2017) indicate a close relationship between these geographical SBVs. Because vSBV was detected earlier than kSBV, kSBV may be a type of vSBV that was accidentally introduced from Vietnam through an unknown route.
This study was conducted to develop a useful tool for evaluating pathogenic SBV. Sequences from larvae of independently SBV-infected populations of A. mellifera were separately determined and then compared, particularly in the region of the 2100 deletion in the SBV genome. Additionally, genotypes and resulting phenotypes and single nucleotide polymorphisms (SNP) were evaluated to identify the phylogenetic relationships among the SNP patterns.
MATERIALS AND METHODS
SBV-infected honeybee samples
Total RNA was extracted from the larvae of SBV-infected honeybee in Seongju, South Korea in 2017. From 4 different populations of Apis mellifera, the 4 RNA samples were named as 1, 2, 3, and 4 and isolated separately.
To detect SBV in these RNA-samples, SBV-specific RT-PCR was performed with the 4 RNA samples using SBV-specific primer pair, SBVD-F1 (5 -GCAGAGTCTAAAATGAGAGTG-3 ) and SBVD-R1, (5 -ATCTCCTGATTTATATTGCATC-3 ), respectively. Briefly, reverse transcription was conducted for 10 min with the oligo dT primer, followed by PCR amplification. In 40 cycles, PCR was performed as follows: 30 s denaturation at 95°C; 30 s annealing at 51°C; 30 s polymerization at 72°C in each cycle, preceded by 5 min pre-denaturation at 95°C and followed by 7 min post-polymerization at 72°C. From the 4 RNA-samples, 582 or 531-base pair (bp) SBV-specific PCR products were amplified, respectively. These PCR-products were modified and used for molecular cloning.
Molecular cloning of 4 different SBV-specific PCR products
From the RNA samples, 582 or 531 bp PCR products were amplified, respectively. On all PCR-products, two restriction sites, KpnI and XbaI, were located at 400 bp (genotype 2100D0) or 349 bp (genotype 2134D51).
Each SBV-specific DNA-fragment and pBX-vector was cut using restriction enzyme KpnI (New England BioLabs, Ipswich, MA, USA) and XbaI (Fermentas, Waltham, MA, USA). The 400- or 349-bp fragments were ligated with cutting pBX and were transformed into competent Escherichia coli. Four recombinant plasmids from E. coli clones using each ligation-mixture originated from each RNA sample 1, 2, 3, and 4 were selected.
Determination of DNA sequence for 4 independent clones from each RNA sample
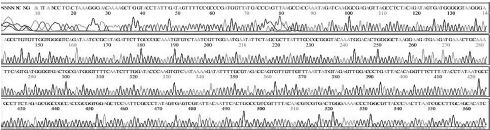
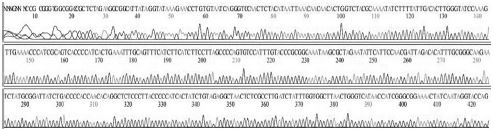
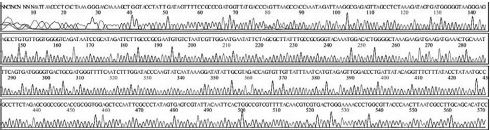
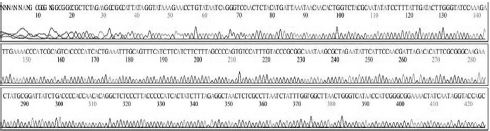
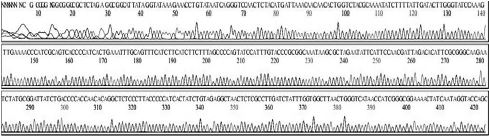
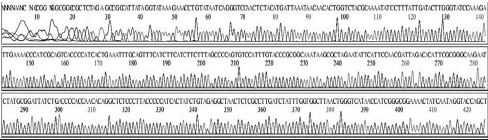
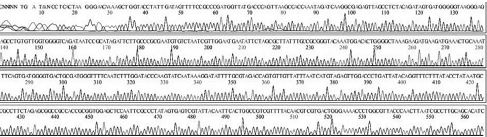
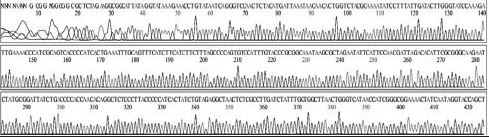
Four DNA sequences from each RNA sample were correctly determined by sequencing in both directions. A total of 32 DNA sequences of 16 recombinant DNAs were determined by either T7 or M13-20R sequencing primers (SolGent, Daejeon, Korea). Sequences in both directions were compared and corrected manually using the chromatograms (Supplement Table s1-s4). Sixteen DNAsequences were acquired from 4 different origins in the same geological location of Seongju, South Korea.
Each DNA-sequence was designated as the “pSBV” plus “genotype, such as D0 or D51”, plus “number of RNA-sample, such as 1, 2, 3, and 4”, and plus “number of recombinant DNA”. For example, “pSBVD0-1-2” means that the DNA sequence of SBV-specific recombinant plasmid 2 belonged to the D0-genotype originated from RNA-sample 1.
SBV-specific DNA sequences analysis
DNA sequences were analyzed by Program Clustal X (2.0) for alignment. Phylogenetic trees were created using Program Treeview X (0.5.0). The sequences in this study were compared to the sequences in GenBank using the Nucleotide Basic Local Alignment Search Tool (BLAST).
RESULTS AND DISCUSSION
Genome of SBVs belong to 2 different genotypes
RNA genomes of SBVs from SBV-infected larvae of A. mellifera were converted to DNA fragments by molecular cloning. PCR products produced with the specific primer SBVD-F1/R1were either 582- or 531-bp SBV-specific fragments, and the final 400- or 349-bp SBV-specific sequences were fixed by molecular cloning using KpnI and XbaI (Fig. 1).
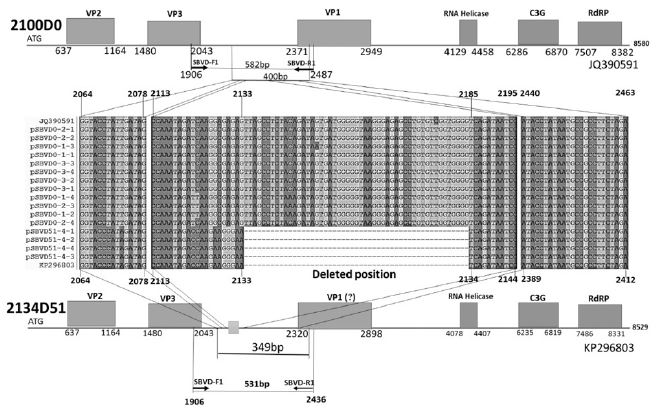
SBV genotyping group of 16 sequences from 4 RNA-samples. From 4 SBV-infected larvae of Apis mellifera in Seongju of Korea, 16 SBV-specific DNA-sequences were determined. Twelve sequences (400 nt) from 3 larvae belong to genotype 2100D0, 4 sequences (349 nt) from 1 larva belong to genotype 2134D51. Among the reported sequences of SBV in GenBank, the closest relationship was found for strain AmSBV-Kor21 (JQ390591) for 2100D0, and strain AcSBV-Kor4 (KP296803) for 2134D51, respectively. Numbers of nucleotides were from the start point of CDSs in JQ390591 or KP296803 in GenBank, respectively.
From 4 independent RNA-samples, 16 sequences were confirmed without mismatch (Supplement Table s1-s4). Among the 16 sequences, 12 sequences from RNA-samples 1, 2, and 3 belonged to genotype 2100D0, while 4 sequences from only RNA sample 4 belonged to genotype 2134D51 according to BLAST analysis.
Although there were few mismatches between each of the 12 sequences (2100D0) and 2064-2463nt region in the coding DNA sequence (CDS) of JQ390591 in GenBank, this reported sequence showed the highest similarity to each of the 12 sequences (Supplement Fig. 1). However, in 4 sequences from RNA sample 4 (genotype 2134D51), 51-bp deletions were detected at position 2134-2184 in the CDS of JQ390591 (corresponding to position 2133-2134 in the CDS of KP 296803). These 4 sequences showed the highest similarity to KP 296803, including a few mismatches.
According to the higher infection rate of SBV in A. cerana than in A. mellifera and higher mortality rate of A. cerana larvae than A. mellifera larvae when infected with the same type of SBV (Gong et al., 2016), A. mellifera may be resistant to kSBV (2134D51), while A. cerana has no resistance against kSBV. This may explain why populations of A. cerana decreased more dramatically than populations of A. mellifera in recent years in Korea.
Recently, some strains of kSBV (2134D51) were isolated from A. mellifera, but their rate of occurrence was relatively low compared to that in A. cerana. In this study, kSBV (2134D51) was found as the only pathogenic strain of SBV in A. mellifera, in a ratio of one in four independent larvae. This finding and ratio may be related to virushost interactions, although the number of cases was too low to draw conclusions.
SNP identification in analyzed sequences
Using the Clustal X (2.0) program, 400-nt 12 sequences from 3 different RNA samples belonging to genotype 2100D0 were aligned and compared with the same region of JQ390591 in GenBank. A total of 78 mismatches were detected from 4800 nt of 12 sequences (98.38% similarity). Because some mismatches were found at the same position on 400 nt, 15 points of significant mutations or SNPs were detected in the region from 2064 to 2463nt in the CDS of JQ390591 (Fig. 2).
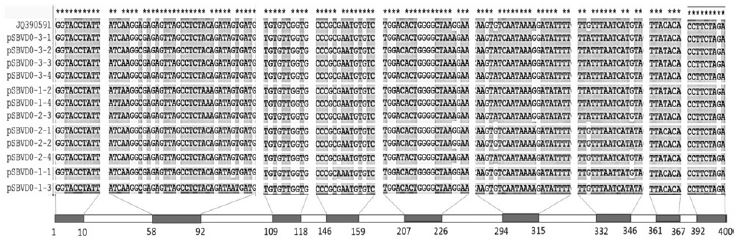
Alignment of 12 sequences from RNA-samples 1, 2, and 3 belonging to 2100D0. Only variable regions compared with JQ390591are illustrated. The homologous nucleotides are denoted by “*”. A total of 78 mismatches were detected in 4800 nt of all analyzed sequences.
Among the 15 SNPs, 2 common SNPs named as SNP65 (A=>C, transversion) and SNP114 (C=>T, transition) were found in all 12 sequences, and 3 different SNPs named as SNP223, SNP298, and SNP364 were found in only 8 sequences; however, some SNPs were found to have only one sequence, such as SNP87, SNP151, SNP211, and SNP341 (Fig. 3).
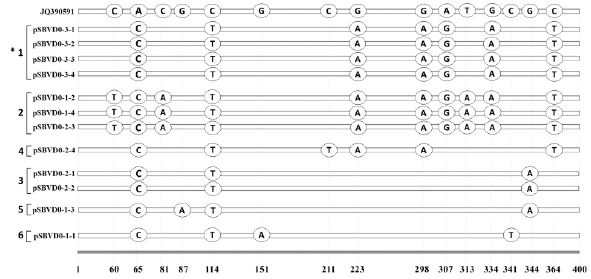
SNP variants based on 400-bp sequences belonging to genotype 2100D0. The variation positions in 12 sequences from 3 different RNAsamples were detected by comparing to the most similar sequences JQ390591 in GenBank. “*” indicates number of SNP-patterns. A total of 6 SNP-patterns were classified. Four, 3, and 2 individual sequences belonged to SNP-pattern1, 2, and 3, respectively. Three remain sequences were SNP-pattern4, 5, and 6. “pSBVD0-3-1” indicates the first sequence cloned from RNA-sample 3 and belonging to genotype 2100D0.
Based on the positions of SNPs in each sequence, 12 sequences belonging to the 2100D0 genotype were classified into 6 different SNP-patterns. For example, four sequences, named as pSBVD0-3-1, -2, -3, and -4, belonged to SNP-pattern1, which has 7 characteristic SNPs, including 2 common SNPs (SNP65 and SNP114) and SNP223, SNP298, SNP307, SNP334, and SNP364.
According to this classification, three sequences, pSBVD0-1-2 and -4 and D0-2-3, belonged to SNP-pattern2. Two sequences, pSBVD0-2-1 and D0-2-2, belonged to SNP-pattern3. Each sequence of pSBVD0-2-4, D0-1-3, and D0-1-1 belonged to SNP-pattern4, 5, and 6, respectively.
Interestingly, SNP-pattern1 was found only in the 4 sequences from RNA sample 3. Thus, the infected SBV strain may be the only SBV-variants, such as SNP-pattern1. However, in the 4 sequences from RNA sample 1, three different SNP patterns were found: SNP-pattern2, 5, and 6. Thus, three different types of SBV-variants might co-invade one population of A. mellifera. In the 4 sequences from RNA sample 2, three different SNP patterns were also found: SNP-pattern2, 3, and 4.
The SNP-pattern2 was detected for three sequences from 2 different RNA samples. In the SNP-pattern2, 10 characteristic SNPs were detected, including 3 unique SNPs named as SNP60, 81, and 313, which were not found in other SNP-patterns, and 7 additional SNPs that were the same as those found in SNP-pattern1.
RNA-sample 1, 2, and 3 were isolated independently from 3 different populations of A. mellifera in the same location, Seongju, Korea in 2017. In this study, common SNPs, such as SNP65 and SNP114 were found in all 12 sequences from 3 RNA-samples. Because strain AmSBV-Kor21 (JQ390591) was collected in 2011, the more recently acquired SBV in the Seongju area may have inherited these common SNPs after 2011.
However, SNP223, SNP298, and SNP364 were found in only 8 sequences (66.7%) in 3 different RNA samples, and SNP307 and SNP334 were found in only 7 sequences (58.3%). Thus, the addition of acquired SNPs is currently occurring, and the detected SNPs in this study were acquired from 2011 to 2017.
Meanwhile, four 349-bp DNA-sequences belonged to Genotype 2134D51 and were aligned and compared with the same region of KP296803 using Clustal X (2.0). Nine mismatches were detected in 1396 nt of 4 sequences (99.36% similarity). Because some mismatches were found in the 2 positions in the 349nt, only 3 points of significant mutations or SNP were detected in the region from 2064 to 2412nt in CDS of KP296803 (Fig. 4).
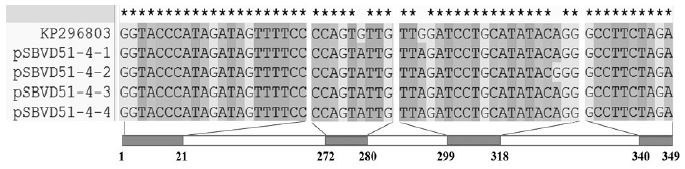
Alignment of 4 sequences from RNA-samples 4 belonging to 2134D51. Only variation region compared with KP296803 is illustrated. Homologous nucleotides are denoted by “*”. Nine mismatches were detected in 349nt of all analyzed sequences (from 2064 to 2412 in CDS of KP296803). Three SNPs were detected.
Among 3 SNPs, 2 common SNPs, named as SNP277 (G=>A, transition) and SNP301 (G=>A, transition) were found in all 4 sequences, while SNP316 (A=>G, transition) was found only one sequence, pSBVD51-4-2 (Fig. 5).
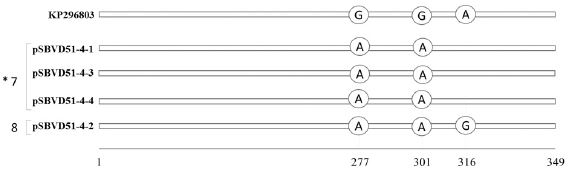
SNP variants based on 349 nt sequences belonging to genotype 2134D51. The variation positions on 4 sequences from a single RNA sample 4 were identified by comparison with the most similar sequences on NCBI (KP296803). “*” indicate number of SNP-patterns. Two SNP-patterns were determined among 4 sequences. Three sequences, pSBVD51-4-1, pSBVD51-4-3, pSBVD51-4-4, belonged to SNP-pattern7 by detecting of D51-common SNPs, such as SNP227 and SNP301. A single sequence, pSBVD51-4-1, belonged to SNPpattern8 based on the detection of additional SNP316.
Based on the positions of the SNPs in each sequence, 4 sequences belonging to the 2134D51 genotype were classified into 2 different SNP patterns. Three sequences including only SNP277 and SNP301 showed SNP-pattern7. One sequence, pSBVD51-4-2, which included SNP277 and SNP301 and additional SNP316, belonged to SNP-pattern 8.
Interestingly, SNP-pattern7 and 8 were detected in the 4 sequences from the single RNA sample 4. Thus, two types of kSBV-variants co-infected a single population of A. mellifera. Although only 3 SNPs and 2 SNP patterns were found in Genotype 2134D51, a limited number of 2134D51 sample was available.
Phylogeny on SNP patterns in genotype 2100D0
The alignment of 12 sequences belonging to genotype 2100D0 was phylogenetically analyzed using Treeview X software (0.5.0). The deduced phylogenic tree isshown in Fig. 6.
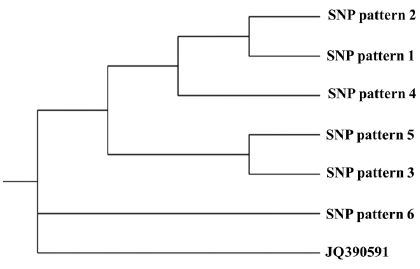
Phylogenetic tree of 6 SNP patterns in genotype 2100D0. Sequences from CDS position 2064 to 2463 (400bp) of 6 SNP patterns and AmSBV-Kor21 (JQ390591) were aligned using Clustal X (2.0). The aligned result was utilized for phylogenetic tree creation. The graph showed that SNP pattern6 was most closely related to the ancestral strain of the 6 SNPpatterns as well as strain AmSBV-Kor21.
Based on the SNP and SNP patterns determined in this study, time-dependent SNP development in this region of SBV was determined. After 2011, the collection year of SBV strain (JQ390591), pre-SNP-pattern 3 may have been present, although it was not detected in this study. This prepattern may have only two SNPs, such as SNP65 and SNP114, excluding SNP344 in SNP-pattern3. From this pre-SNP-pattern3, SNP-pattern3 and 6 may have independently evolved by acquiring SNP344, or by acquiring SNP151 and SNP341, respectively. Thereafter, SNP-pattern5 may have originated from SNP-pattern3 by adding SNP87.
From pre-SNP-pattern3, pre-SNP-pattern4 may be evolved, although it was not detected in this study. This pre-SNP-pattern4 may contain three additional SNPs, such as SNP223, SNP298, and SNP364, including SNP65 and SNP114 and excluding SNP211. Independently, SNPpattern4 or SNP-pattern1 may be divided from pre-SNP-pattern4 by acquiring SNP211 or by acquiring SNP307 and SNP334, respectively. Finally, SNP-pattern2 may have originated from SNP-pattern1 by adding SNP60, SNP81, and SNP313.
There may be other explanations for the development of SNPs in recent years, based on limited evidence. Expanding the number of SBV samples and detailed analyses are needed to understand the origin and migration of SBV in detail.
In this study, we found that large numbers of SNP variants currently exist and co-invade honeybees in Korea, and that SNPs are developing in time-dependent and hostdependent manners. It would be a possible process to convert strong- or weak-pathogenic SBVs. Determining these characteristics in an easier manner is important for analyzing SNP variants for detecting individual pathogenic characteristics of individual SBVs.
In addition, we conducted SNP analysis to evaluate SBV to determine the detailed identification, origin, or migration of invasion, and pathogenic characteristics of SBV variants. Unfortunately, SBV variants collected for this study lack a proper storage method. This is the first study using SNP analysis for SBV; this method may be useful for overcoming kSBV-outbreaks by using NGS-sequencing technologies and analyzing mega-data by expanding sample numbers and sequencing.
LITERATURE CITED
-
Bailey, L., (1969), The multiplication and spread of Sacbrood virus of bees, Ann. Appl. Biol, 63, p483-491.
[https://doi.org/10.1111/j.1744-7348.1969.tb02844.x]

-
Bailey, L., (1975), Recent research on honey bee viruses, Bee World, 56, p55-64.
[https://doi.org/10.1080/0005772x.1975.11097544]

-
Bailey, L., A. J. Gibbs, and R. D. Woods, (1964), Sacbrood virus of the larval honey bee (Apis mellifera Linnaeus), Virology, 23, p425-429.
[https://doi.org/10.1016/0042-6822(64)90266-1]

- Choe, S. E., Th. Th. D. Nguyen, B. H. Hyun, J. H. Noh, H. S. Lee, Ch. H. Lee, and S. W. Kang, (2012a), Genetic and phylogenetic analysis of South Korean Sacbrood virus isolates from infected honey bees (Apis cerana), Veterinary Microbiology, 157, p32-40.
- Choe, S. E., L. T. K. Nguyen, J. H. Noh, Ch. H. Kweon, K. E. Reddy, H. B. Koh, K. Y. Chang, and S. W. Kang, (2012b), Analysis of the complete genome sequence of two Korean sacbrood viruses in the Honey bee, Apis mellifera, Virology, 432, p155-161.
- Choi, Y. S., M. Y. Lee, I. P. Hong, N. S. Kim, H. K. Kim, K. G. Lee, and M. L. Lee, (2010), Occurrence of Sacbrood virus in Korean Apiaries from Apis cerana (Hymenoptera: Apidae), Journal of Apiculture, 25(3), p187-191.
- Dong, B. Y., Y. Z. Fang, Z. Q. Guo, X. J. Li, Y. Zhang, and K. H. Bi, (1986), Study on Chinese sacbrood bee virus, Apic. China, 2, p6-8.
- Ghosh, R. C., B. V. Ball, M. M. Willcocks, and M. J. Carter, (1999), The nucleotide sequence of sacbrood virus of the honeybee: an insect picornavirus, J. Gen. Virol, 80, p1541-1549.
- Gong, H. R., X. X. Chen, Y. P. Chen, F. L. Hu, J. L. Zhang, Z. G. Lin, J. W. Yu, and H. Q. Zheng, (2016), Evidence of Apis cerana Sacbrood virus infection in Apis mellifera, Appl Environ Microbiol, 82, p2256-2262.
-
Grabensteiner, E., W. Ritter, M. J. Carter, S. Davison, H. Pechhacker, J. Kolodziejek, O. Boecking, I. Derakhshifar, R. Moosbeckhofer, E. Licek, and N. Nowotny, (2001), Sacbrood virus of the honeybee (Apis mellifera): rapid identification and phylogenetic analysis using reverse transcription-PCR, Clin Diagn Lab Immunol, 8(1), p93-104.
[https://doi.org/10.1128/cdli.8.1.93-104.2001]

- Le, T. H., and V. L. Pham, (2004), Preliminary studies on the origin and the phylogenetic relatedness of the Sacbrood virus isolated in Vietnam, J. Biol, 26, p37-42, (In Vietnamese).
-
Lee, C. W., M. S. Yoo, S. J. Lim, J. M. Kim, Y. S. Cho, and B. S. Yoon, (2017), A proposal on the new genotyping of Sacbrood viruses for the definition of Korean Sacbrood virus (kSBV), J. Apiculture, 32(2), p89-97.
[https://doi.org/10.17519/apiculture.2017.06.32.2.89]

-
Nguyen, N. T. B., and T. H. Le, (2013), Complete genome sequence of Sacbrood virus strain SBm2, isolated from the honeybee Apis cerana in Vietnam, Genome Announc, 1(1), e00076-12.
[https://doi.org/10.1128/genomea.00076-12]

- White, G. F., (1917), Sacbrood, U. S. Dep. Agric. Bull, 431, p1-55.
SUPPLEMENTARY DATA
Appendix

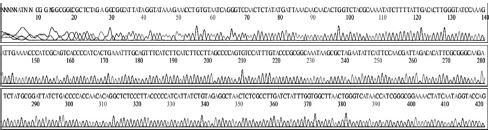
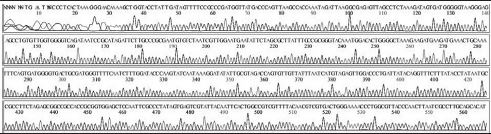
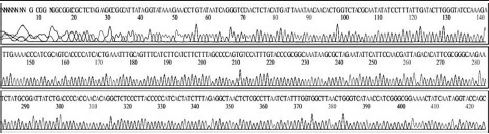
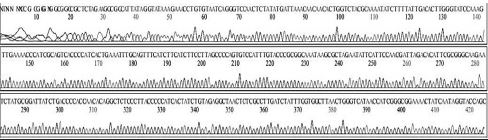
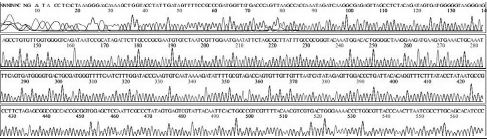
Variation positions of 6 SNP-patterns belonging to Genotype 2100D0 in comparison with genome of strain AmSBV-Kor21 on NCBI. The DNA fragment 400bp from position of CDS 2063 to 2462 (2242 to 2641 in complete genome) were searched on NCBI for the similarity sequences. The highest similar sequences belong to strain AmSBV-Kor21 (JQ390591) was observed. The non-homologous nucleotides were marked in picture 1 to 6. The dissimilar nucleotides of SNP-pattern 1, 2, 3, 4, 5, 6 in comparison with sequences belong to strain AmSBV-Kor21 are 10, 7, 6, 4, 3, 4, respectively.
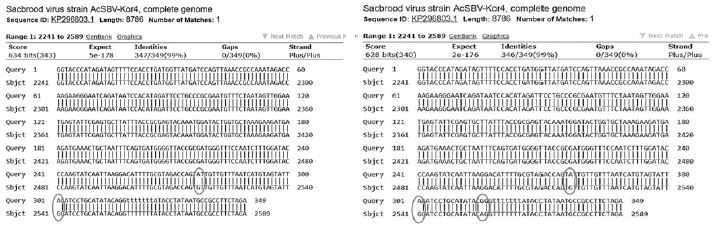
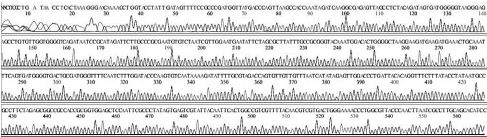
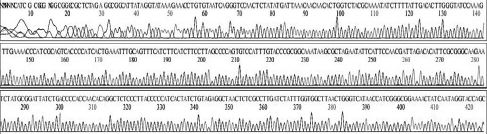
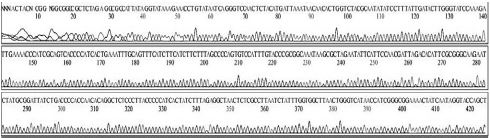
Variational positions of 2 SNP-patterns belonging to Genotype 2134D51 in comparison with genome of strain AcSBV-Kor4 on NCBI. The DNA fragment 349bp of SNP-pattern 7 and 8 belonging to Genotype 2134D51 were searched on NCBI for the similarity sequences. The highest similar sequences belong to strain AcSBV-Kor4 (KP296803) was observed. The nonhomologous nucleotides were marked in pictures. The dissimilar nucleotides of SNP-pattern 7, 8 in comparison with sequences belong to strain AcSBV-Kor4 (KP296803) are 2 and 3.