
Head Capsule Width, Body Weight and Length Measurements for Instar Determination in Mason Bee (Osmia cornifrons) Larvae
Abstract
Osmia cornifrons is a cavity-nesting solitary species used as an apple pollinator in Korea. We collected Osmia spp larvae to examine potential correlations of larval stage. The head widths of from 1st to the 5th instar ranged from 0.4±0.1 to 1.3±0.3 mm, and growth rate of each instar was significantly highest between 1st and the 2nd instar. The fitness regression model for the head capsule width was analyzed. The head capsule width plotted against the number of instars resulted in a regression line of y=0.146x+0.551, R2=0.838. The body weights of the larvae increased with larval developmental stage, and the coefficient of variation of larval weights was high. However, the calculated regression line was y=5.122x2-12.154x+11.444, R2=0.900. The growth ratio of the larval length was clearly maximal between the 3rd and 4th instars, while that of 5th instar larvae was decreased. The calculated regression line is y=2.595x+0.472, R2=0.920. The result suggests that measurements of head capsule width, weight and length may be useful as a method to determine larval instar.
Keywords:
Mason bee, O. cornifrons, Larval development, Head width, Weight, Length, InstarINTRODUCTION
Osmia cornifrons (Radoszkowski) (Hymenoptera: Megachilidae), commonly known as the Japanese orchard bee or hornfaced bee, is an important alternative pollinator of various crops, such as apples, apricots, cherries, nectarines, peaches, pears, plums, etc. (Yamada et al., 1971; Park et al., 2018). Body size is one component of the female phenotype that has linked to variation in female reproductive success within species of mason bees. Size correlated positively with fitness through its effects on provisioning rate; provision mass, fecundity, and offspring size and sex ratio (Kim, 1997; Roulston and Cane, 2000; Bosch and Vicens, 2006; Rehan and Richards, 2010). Thus, factors that affect body size during development can affect reproductive success later in life. Heritability for body size is apparently zero or low for solitary bees (Tepedino et al., 1984; Frohlich and Tepedino, 1986; Owen and Mcorquodale et al., 1994). Instead, body sizes attained by adult females are strongly influenced by environmental conditions during larval development, including the amount of pollen and nectar they received (Roulston and Cane, 2000; Radmacher and Strohm, 2010), Size and age at maturity are important life history traits. Body length is also one of the the defining traits of a species even though maximum species range little around a species characteristic an organism’s length relates immediately to its shape and function (Schmidt-Neilson, 1984) and scaling research have end up increasingly critical in ecology and evolutionary biology (Peters, 1983; Calder, 1984; LaBarbera, 1989). Several variables have been used as an estimator of body size in bees, including body length (Gathmann and Tscharntke, 2002), radial cell length (Cane, 1987; Pouvreau, 1989; Bullock, 1999), and head width (van Nieuwstadt and Ruano Iraheta, 1996; Roulston and Cane, 2000). Larval age of larvae is distinguished by the size of the head width (Dyar and Rhinebeck 1890; Sharifi and Mills 1971). This is because the larva grows exponentially when molting without growth (Nijhout, 2013), and the keratinized part of the head during the development of the larva grows in a discontinuous or stepwise manner in order that the larva grows exponentially (Gains and Campell, 1935). In addition, Bodenheimer (1927) stated that the larva̓s body length, body width, head height, tactile length, and leg length grow in stages like the head width, and have a linear relationship with the reiki. Yoon et al. (1997) determined to larvar instar of the mulberry longicorn beetle Apriona germari using head capsule width, weight and length, it is not easy to determine its age because the form of habitat is perforated and passes within the branches of the host plant. Each larva collected different places and different temperature.
This makes a study on the extent to which size can also have an effect on some of life-records traits impossible and information on size-weight relationships of living forms is more appropriate. Two measurements, head capsule width and larval length, have been used as a correlate of body weight in O. cornifrons larval stages. Kodaira (2009) demonstrated that the two measures among different types of worker bees found head widths to be a better indicator of body size. Our report indicates in three areas of size-weight relationships in the bee O. cornifrons: 1) Correlation between larval stage and head capsule width 2) Correlation between larval stage and weight 3) Correlatation between larval stage and body length. However, the characteristics of different larval stages of O. cornifrons commercialized in Korea remain unclear. In addition, the precise number of instars that O. cornifrons larvae go through at the optimal temperature of 25°C is almost not precisely known. Using body length, head capsule width and larval growth measurement to study Osmia spp length-weight relationship using simple linear measures, such as the head capsule width and body length. In order to verify our conclusions on growth and size regulation we developed a new strategy of the larval stage model that incorporates the new findings described in this paper and that accurately reproduces growth trajectories under a variety of temperatures and nutritional conditions. We characterized the developmental mechanisms shaping body size in the mason bee pollinator, O. cornifrons. This study manipulates larval development in mason bee to understand how developmental mechanism shape adult body size, we based our approach on the insect body size, weight, and length of head capsule width for each instar.
MATERIALS AND METHODS
1. Experimental insect
Mason bee eggs and larvae, used as experimental insects, were collected by trap nesting from an insect garden in the middle eastern part of the Korean Peninsula in Yeongwol (37°12ʹ55ʺN, 128°21ʹ46ʺE), Jeongseon (37°16ʹ04ʺN, 128°44ʹ31ʺE) and Jecheon (37°04ʹ03ʺN, 128°09ʹ51ʺE) of the Republic of Korea, in the early to late 2017. Periodically, we collected a trap-nest straw we plugged with a mud and then brought into the rearing room at 25℃, 65% relative humidity (RH), and continuous darkness (Lee et al., 2016). Harvested bamboo nest straws were sliced longitudinally along one side to allow access to the nest contents for parasite removal; then, we collected egg and larvae from the nest straws. The collected eggs and larva were moved into transparent 24-well cell culture plates marked to indicate. Egg and larvae were reared in the rearing room to investigate the species growth rate, length and head width and when the larvae become cocoons; we sorted the species of O. cornifrons from the cocoons of Osmia spp. using the manuscript by Maeta (1978) and book by Yoon et al. (2015).
2. Measurement of head width capsule, length, and weight of O. cornifrons larvae
To determine the growth rate for O. cornifrons, larvae we investigated the period and sizes of head width, body length, and weight of each instar. The sizes of head widths, body lengths, and weights were measured using a HVC-2000A (Cisvision, Korea) digital inspection microscope. The growth ratio for larval development was determined on the basis of head width, body weight and body length of 1st to 5th after molting. Widths of the molted head and body lengths were measured using the HySCALER software for image analysis (HySCALER software; version 1.4; Cisvision, Korea). Larval weight was recorded using an electronic balance (Scaltec, Germany). Additionally, the larval period of each instar was monitored.
3. Statistical analysis
The statistical analyses were conducted using the one-way ANOVA test (followed by the post-hoc Tukey HSD test) and regression analysis. In particular, the Welch’s ANOVA was performed if the data were not of equal variance. The one-way ANOVA test was used to investigate differences in the average head width, body length, body weight and duration in each larval instar. The relationships between larval size (head capsule width, weight, and body length) and larval stage were confirmed using a regression analysis, and each regression equation was derived if a significant regression model was confirmed. In addition, fitness was confirmed by substituting measurement data of head width capsule, length, and weight into respectively derived regression equations. All data were statistically processed after normality was tested using the Shaprio-Wilks test. All statistical analyses were performed using the SPSS PASW 22.0 package for Windows (IBM, Chicago, IL, USA).
RESULTS
1. Determination of growth rate by head capsule widths instar-wise in larvae
More than 1000 larvae were collected from 16 April 2017 to August 2017. Among these, 90% larvae were of mason bee O. cornifrons passes through larval instars to become a pupa. The head capsule widths of O. cornifrons ranged from 0.7 to 0.1 mm. The head capsule widths of O. cornifrons larvae reared individually through all five instars under laboratory conditions were in the range of 0.7±0.1 mm, 0.9±0.1 mm, 1.0± 0.1 mm, 0.1±0.1 mm, and 1.3±0.1 mm, 1st to 5th instar respectively (Table 1). There were significant differences among the mean head capsule widths of different instars (one-way ANOVA test F3,120=9.208, p=0.0001). Growth peaked in the 2nd instar. The growth ratio of each instar in head capsule width was significantly highest between 1st and 2nd instars. The coefficient variation (CV) in the head width was highest (16.4%) duration the 1st instar, and gradually decreased until the 4th instar. When the larval head capsule width was plotted against the number of instars, the calculated simple linear regression model was y=0.146x+0.551 (R2=0.838, ANOVA test: F1,164=849.587, p=0.0001, DW=1.625), (Fig. 2). The fitness to the linear regression model for the larval head width shows a relatively poor fitness (97%), but using the 1st instar compared with better fitness than when based on the other instars (Table 2). The fitness for the larval head width from the total instars was 98.5%. However, the fitness difference between the observed and theoretical values was clear.
Correlation between larval stage and head capsule during lrava period in the laboratory condition. The reared condition was maintained 25℃, 60% (RH). Average head width (±SD) and applied the exponential regression model (R2=0.838, p=0.0001, y=0.146x+0.551).
2. Determination of growth rate by body weight instar-wise in larvae
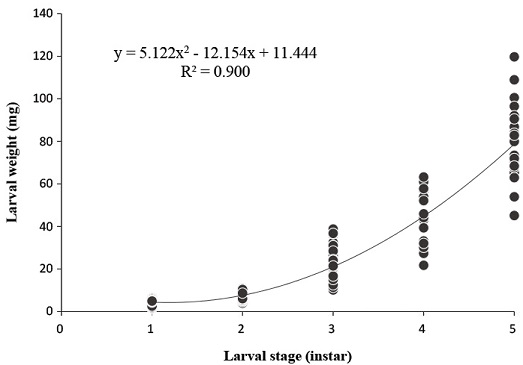
The larval body weights of O. cornifrons were in the range of 3.9±1.1 mm to 78.3±16.1 mm (Fig. 1 and Table 3). There were significant differences among the mean larval body weight of the different instars. The growth ratio for larval weight was based on 1st and 5th instars, and no significant difference in growth ratio over each instar (one-way ANOVA test F3,167=5.474, p=0.001) was apparent. The growth ratio of larval weight was highest between the 3rd and 5th instars. The growth ratio was higher in the 3rd and 4th instar, whereas that of the 5th instar exhibited a decrease. The average larval weight CV was 24.7% higher than comparative percentages related to head capsule width and length. The calculated exponential curve model was y=5.122x2-12.154x+11.444 and R2 was 0.900 (ANOVA test: F2,290=1299.383, p=0.0001) (Fig. 3). The fitness to the exponential curve model for the larval weight from total instars was 98.7% (Table 4), showing a relatively poorer relationship than for the larval head width.
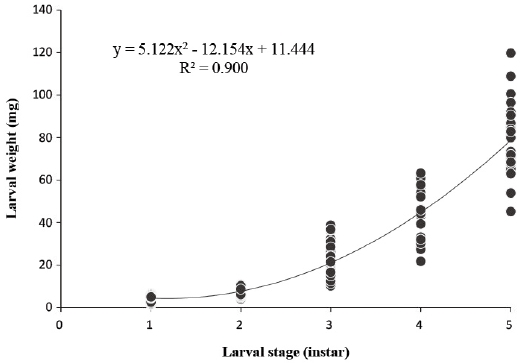
Correlation between larval stage and weight during lrava period in the laboratory condition. The reared condition was maintained 25℃, 60% (RH). Average weight (±SD) and estimated exponential curve model (R2=0.900, p=0.0001, y=5.122x2-12.154x+11.444) in regression analysis.
3. Determination of growth rates by body length instar-wise in larvae
The larval lengths ranged from 3.7±0.6 mm to 13.6±1.3 mm. The body length all five instars of O. cornifrons larvae were measured (Fig. 1), and significant differences in connection with the growth ratio for each instar (one-way ANOVA test F3.121=9.614, p=0.0001) (Table 5). The growth ratio of larval length was highest between the 3rd and the 4th instars, whereas that after the 4th instar showed a decrease. The coefficient variation in the larval length was highest with 15.7% in the 1st instar. The correlation between larval stage and body length obeyed the simple linear regression model (y=2.595x+0.472, R2=0.920, ANOVA test: F1,164=1887.908, p=0.0001, DW=1.235) (Fig. 4). The fitness to lst linear regression model covering larval lengths of all instars was 91.2% (Table 6).
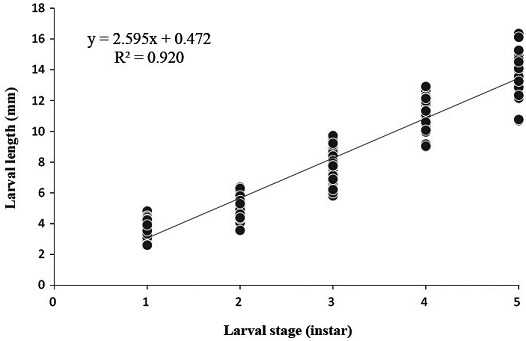
Correlation between larval stage and body length during lrava period in the laboratory condition. The reared condition was maintained 25℃, 60% (RH). Average body length (±SD) was applied the simple liner regression model (R2=0.920, p=0.0001, y=2.595x+0.472).
DISCUSSION
We confirmed the characteristics of potential correlations of instar and larval stage of O. cornifrons larvae. However, 1st and 5th, showed smaller significant differences head capsule width and 2nd instar showed an increased growth ratio. Thus, it agreement with studies involving lepidopteran species (Calvo and Molina, 2008), this study recorded a variable number of molts for S. panda larvae. A variable number of instars is well recorded in the literature for many insect species. This is largely attributable to the nutritive value of the food of the larvae, which is affected by environmental factors (Gaines and Campbell, 1935; Fogal and Kwain, 1972; Schmidt et al., 1977). Temperature was constant in this study; thus, the main source of variation for both head capsule width and instar number is likely to be food type (namely, plant species) and quality. Regression models have been employed by many researchers for determining the number of instars in several species worldwide (Hansen et al., 1981; Cave and Smith, 1983; Logan et al., 1998; Chen and Seybold, 2013; Cazado et al., 2014; Castaneda-Vildozola et al., 2016; Chen et al., 2017). Panzavolta (2007) used the Gaines and Campbell method (the linear regression). In the present study, growth ratios were calculated both for the observed and theoretical data on instar-wise head capsule widths of O. cornifrons. Furthermore, the linear relationship between the natural logarithm of mean head capsule widths and their corresponding instars indicated perfect geometric progression in the growth of the O. cornifrons larvae. This supports, which reiterates the geometric growth of head capsule widths through the successive instars. The highly significant linear regression equation (P≤0.0001; R2≥0.838) for the head capsule widths of successive instars indicates a representation of all instars.
Larval development proceeds through five instars and we collected larva from different places and different temperature (Torchio, 1989) and takes approximately a month under controlled conditions (Bosch and Kemp, 2000). Maeta (1978) reported that larval development involved 5th instars. Temperature thresholds for egg and larval development have been estimated at 10~14℃ and 7~14℃, respectively (Maeta, 1978, 2006). In O. lignaria and O. cornifrons, egg and larval developmental rates increase with increasing temperatures from 18℃ to 26℃, and then stabilize at 29~30℃ (Bosch and Kemp, 2000; Maeta et al., 2006). On consuming the pollen-nectar provision and completing defaecation, the fifth instar spins a thick multilayered cocoon with secretions originating from its salivary glands (Torchil, 1989). The larval developmental at 26℃ took 26 days in O. lignaria (Bosch and Kemp, 2000; Sgolastra, 2007).
Hubner (1820) has considered a pest of several ornamental and fruit plants in the Mediterranean area (Hemiptera Coccoidea) (Balachowsky, 1966; Zhang, 1994; Molina and Calvo, 2005), however, information about this insect is scarce. There are no published data on either instar numbers, or head capsule width as a function of the instar in our results. Brief anatomical descriptions of larval stages are available for this species (Amphibian) (Huertas, 1980; Bogner, 1999; Gomez and Aizpurua, 2002; Calvo and Molina, 2008), but no morphometric studies have been published (Molina, 2004). An increment of instar numbers mainly due to adverse developmental conditions can be found in other (Lasiocampidae) lepidopteran species, such as Forest tent caterpillars (Malacosoma disstria), Huber (Esperk et al., 2007). Developmental time (measured as number of instars) seems to increase head capsule width overlaps and misclassification probabilities, and must be related with the feeding strategies adopted by this species (Malacosoma neustrium) along its life cycle.
The mason bee (O. cornifrons) appeared to be highly related in the width of larval head capsule, weight and length. A thorough understanding of its biology and unambiguous identification of larval instars, in particular, are pre-requisites for managing this mason bee successfully by any appropriate means. Based on the studies carried out during the sixties measurement of larval stage, it was concluded Maeta (1978), that O. cornifrons pollinator undergoes four or five molts. Among several morphological variables tested, head capsule width, body weight, and length were observed to be the most appropriate features in determining the actual number of instars in O. cornifrons by regression analysis (Yoon et al., 2015). We have now determined the number of instars in O. cornifrons after quite a long period. This is the first report of instar determination based on the regression analysis of head capsule width, larval weight and length. The regression analysis of fixing the instars has also been validated by counting the number of molts upon individually rearing all instars until pupation under laboratory condition. The mean growth ratios for both instar-derived and theoretical data from the regression analysis were the same. The mean growth ratios of 1.1~1.3 indicate that the number of instars in O. cornifrons is five. The linear regression with R2 values of more than 0.838 between the larval stage (instar) numbers and their corresponding mean head capsule widths reaffirms that no instar has been missed or added in the larva. It is thus, evident from the study that the instar determination by regression analysis is appropriate and reliable. Ours is the first report that the regression analysis to determine the number of instars in O. cornifrons. Furthermore, after scanning through the available literatures thoroughly, to the best of our knowledge, this is probably the detailed study, which proves that the mason bee of O. cornifrons through five larval instars to reach its pupal stages.
Acknowledgments
This work was supported by a grant from the National Institute of Agricultural Sciences, Rural Development Administration, Republic of Korea (Project No.: PJ0135592020).
References
- Balachowsky, A. S. 1966. Entomology Applied to Agriculture. Tome II. Lépidoptères. Paris, Masson et Cie, 1: 99-145.
-
Bosch, J. and N. Vicens. 2006. Relationship between body size, provisioning rate, longevity and reproductive success in females of the solitary bee Osmia cornuta. Behav. Ecolo. & Socio. 60: 26-33.
[https://doi.org/10.1007/s00265-005-0134-4]

-
Bosch, J. and W. P. Kemp. 2000. Development and emergence of the orchard pollinator Osmia lignaria (Hymenoptera: Megachilidae). Environ. Entomol. 29: 8-13.
[https://doi.org/10.1603/0046-225X-29.1.8]

-
Brooke, P. and R. Bullock. 1999. Validation of a 6-item cognitive impairment test with a view to primary care usage. Int. J. Geriatric Psych. 14: 936-940.
[https://doi.org/10.1002/(SICI)1099-1166(199911)14:11<936::AID-GPS39>3.0.CO;2-1]

- Calder, W. A. 1996. Size, function, and life history. Courier Corporation.
-
Calvo, D. and J. M. Molina. 2008. Head capsule width and instar determination for larvae of Streblote panda (Lepidoptera: Lasiocampidae). Ann. Entomol. Soc. America 101: 881-886.
[https://doi.org/10.1093/aesa/101.5.881]

- Cane, J. H. 1987. Estimation of bee size using intertegular span (Apoidea). J. Kansas Entomol. 1: 145-147.
-
Castañeda-Vildózola, Á., H. González-Hernández, A. Equihua-Martínez, J. Valdez-Carrasco, J. E. Peña, L. E. Cazado and O. Franco-Mora. 2016. Head capsule width is useful for determining larval instar in Heilipus lauri (Coleoptera: Curculionidae). Florida Entomol. 99: 822-825.
[https://doi.org/10.1653/024.099.0448]

-
Cave, G. L. and C. M. Smith. 1983. Number of instars of the rice water weevil, Lissorhoptrus oryzophilus (Coleoptera: Curculionidae). Ann. Entomol. Soc. America 76: 293-294.
[https://doi.org/10.1093/aesa/76.2.293]

-
Cazado, L. E., G. A. Van Nieuwenhove, C. W. O̓brien, G. A. Gastaminza and M. G. Murúa. 2014. Determination of number of instars of Rhyssomatus subtilis (Coleoptera: Curculionidae) based on head capsule widths. Florida Entomol. 97: 639-643.
[https://doi.org/10.1653/024.097.0241]

-
Chen, Y. and S. J. Seybold. 2013. Application of a frequency distribution method for determining instars of the beet armyworm (Lepidoptera: Noctuidae) from widths of cast head capsules. J. Econo. Entomol. 106: 800-806.
[https://doi.org/10.1603/EC12367]

- Davidowitz, G., L. J. D̓Amico and H. F. Nijhout. 2004. The effects of environmental variation on a mechanism that controls insect body size. Evol. Ecol. Res. 6: 49-62.
-
Davidowitz, G. 2008. Population and environmental effects on the size-fecundity relationship in a common grasshopper across an aridity gradient. J. Orthoptera. Res. 17: 265-271.
[https://doi.org/10.1665/1082-6467-17.2.265]

-
Dyar, H. G. 1890. The number of molts of lepidopterous larvae. Psyche. 5: 420-422.
[https://doi.org/10.1155/1890/23871]

-
Esperk, T., T. Tammaru and S. Nylin. 2007. Intraspecific variability in number of larval instars in insects. J. Econo. Entomol. 100: 627-645.
[https://doi.org/10.1093/jee/100.3.627]

-
Flatt, T. and A. Heyland. Mechanisms of Life History Evolution: The Genetics and Physiology of Life History Traits and Trade-Offs. Oxford: Oxford University Press; 2011.
[https://doi.org/10.1093/acprof:oso/9780199568765.001.0001]

- Fogal, W. H. and M. J. Kwain. 1972. Host plant nutritive value and variable number of instars in a sawfly, Diprion similis. Israel J. Entomol. 7: 63-72.
-
Frohlich, D. R. and V. J. Tepedino. 1986. Sex ratio, parental investment, and interparent variability in nesting success in a solitary bee. Evolution 40: 142-151.
[https://doi.org/10.1111/j.1558-5646.1986.tb05725.x]

-
Gaines, J. C. and F. L. Campbell. 1935. Dyar̓s rule as related to the number of instars of the corn ear worm, Heliothis obsoleta (Fab.), collected in the field. Ann. Entomol. Soc. America 28: 445-461.
[https://doi.org/10.1093/aesa/28.4.445]

-
Gathmann, A. and T. Tscharntke. 2002. Foraging ranges of solitary bees. J. Ani. Ecol. 71: 757-764.
[https://doi.org/10.1046/j.1365-2656.2002.00641.x]

-
Harries, F. H. and C. F. Henderson. 1938. Growth of insects with reference to progression factors for successive growth stages. Ann. Entomol. Soc. America 31: 557-572.
[https://doi.org/10.1093/aesa/31.4.557]

-
Honek, A. 1993. Intraspecific variation in body size and fecundity in insects: a general relationship. Oikos 66: 483-492
[https://doi.org/10.2307/3544943]

- Huertas Dionisio, M. 1980. Pachygastria trifolii (Denis & Schiff., 1775) en la zona costera de Huelva y Cádiz, con la descripción de una subsp. nueva (Lep. Lasiocampidae) (I parte) (4ª contribución al estudio de los Lasiocampidae). SHILAP Revta. Lipid. 8: 223-227.
- Kim, W. T., S. W. Bae, H. C. Park, K. H. Park, S. B. Lee, Y. C. Choi and Y. H. Koh. 2010. The larval age and mouth morphology of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). Int. J. Ind. Entomol. 21: 185-187.
-
Kingsolver, J. G. and D. W. Pfennig. 2004. Individual-level selection as a cause of Cope̓s rule of phyletic size increase. Evol. 58: 1608-1612. PMID: 15341162
[https://doi.org/10.1111/j.0014-3820.2004.tb01740.x]

-
LaBarbera, M. 1989. Analyzing body size as a factor in ecology and evolution. Ann. Rev. Eecol. & System. 20: 97-117.
[https://doi.org/10.1146/annurev.es.20.110189.000525]

-
Lee, K. Y., H. J. Yoon, K. S. Lee and B. R. Jin. 2016. Development and mating behavior of Osmia cornifrons (Hymenoptera: Megachilidae) in the constant temperature. J. Asia-Pacific Entomol. 19: 281-287.
[https://doi.org/10.1016/j.aspen.2016.03.003]

- Maeta, Y. 1978. Comparative studies on the biology of the bees of the genus Osmia of Japan, with special reference to their managements for pollinations of crops (Hymenoptera: Megachilidae). Bul.Tohoku National Agricul. Experi. Stat. 57: 1-221.
-
Molina, J. M. and D. Calvo. 2005. Developmental rates of the Lappet Moth “Streblote panda” Hübner (1820) (Lepidoptera: Lasiocampidae) at constant temperatures. Spanish J. Agricul. Res. 3: 319-325.
[https://doi.org/10.5424/sjar/2005033-155]

-
Nijhout, H. F., G. Davidowitz and D. A. Roff. 2006. A quantitative analysis of the mechanism that controls body size in Manduca sexta. J. Biol. 5: 1-16.
[https://doi.org/10.1186/jbiol43]

-
Owen, R. E. and D. B. McCorquodale. 1994. Quantitative Variation and Heritability of Postdiapause Development Time and Body Size in the Alfalfa Leafcutting Bee (Hymenoptera: Megachilidae). Ann. Entomol. Soc. America 87: 922-927.
[https://doi.org/10.1093/aesa/87.6.922]

-
Panzavolta, T. 2014. Instar determination for Pissodes castaneus (Coleoptera: Curculionidae) using head capsule widths and lengths. Environ. Entomol. 36: 1054-1058.
[https://doi.org/10.1603/0046-225X(2007)36[1054:IDFPCC]2.0.CO;2]

-
Park, M. G., N. K. Joshi, E. J. Rajotte, D. J. Biddinger, E. J. Blitzer, J. E. Losey and B. N. Danforth. 2018. Apple grower pollination practices and perceptions of alternative pollinators in New York and Pennsylvania. Renew. Agric. Food Syst. 35: 1-14.
[https://doi.org/10.1017/S1742170518000145]

- Peters, R. H. and R. H. Peters. 1986. The ecological implications of body size (Vol. 2). Cambridge university press.
-
Plowright, R. C. and S. C. Jay. 1968. Caste differentiation in bumblebees (Bombus Latr.: Hym.) I. The determination of female size. Insect. Soc. 15: 171-192.
[https://doi.org/10.1007/BF02223465]

- Pouvreau, B. 2004. Un politique en architecture: Eugène Claudius-Petit, 1907-1989. Moniteur. Press, Schmidt-Neilson, K. (1984). Scaling: Why is Animal Size So Important? Cambridge Univ. Press, Cambridge, Mass.
-
Radmacher, S. and E. Strohm. 2010. Factors affecting offspring body size in the solitary bee Osmia bicornis (Hymenoptera, Megachilidae). Apidol. 41: 169-177.
[https://doi.org/10.1051/apido/2009064]

-
Rehan, S. M. and M. H. Richards. 2010. The influence of maternal quality on brood sex allocation in the small carpenter bee, Ceratina calcarata. Ethology 116: 876-887.
[https://doi.org/10.1111/j.1439-0310.2010.01804.x]

- Roff, D. A. 1992. The Evolution of Life Histories. New York: Chapman and Hall.
-
Roulston, T. A. H., J. H. Cane and S. L. Buchmann. 2000. What governs protein content of pollen: pollinator preferences, pollen-pistil interactions, or phylogeny? Ecological Monographs 70: 617-643.
[https://doi.org/10.1890/0012-9615(2000)070[0617:WGPCOP]2.0.CO;2]

-
Schmid-Hempel, P. 1990. Reproductive competition and the evolution of work load in social insects. Ame. Natur. 135: 501-526.
[https://doi.org/10.1086/285059]

-
Sharifi, S. and R. B. Mills 1971. Radiographic studies of Sitophilus zeamais Mots. in wheat kernels. J. Sto. Prod. Res. 7: 195-206.
[https://doi.org/10.1016/0022-474X(71)90007-5]

-
Tepedino, V. J., R. Thompson and P. F. Torchio. 1984. Rapid Communication Heritability for Size in The Megachilid Bee Osmia Lignaria Propinqua Cresson. Apidologie 15: 83-88.
[https://doi.org/10.1051/apido:19840108]

-
Torchio, P. F. 1989. In-nest biologies and development of immature stages of three Osmia species (Hymenoptera: Megachilidae). Ann. Entomol. So. America 82: 599-615.
[https://doi.org/10.1093/aesa/82.5.599]

-
Turner, T. L., A. D. Stewart, A. T. Fields, W. R. Rice and A.M. Tarone. 2011. Population-based resequencing of experimentally evolved populations reveals the genetic basis of body size variation in Drosophila melanogaster. PLoS Genet. 7: e1001336.
[https://doi.org/10.1371/journal.pgen.1001336]

-
Van Nieuwstadt, M. G. L. and C. R. Iraheta. 1996. Relation between size and foraging range in stingless bees (Apidae, Meliponinae). Apidologie 27: 219-228.
[https://doi.org/10.1051/apido:19960404]

- Yamada, Y., N. Oyama, N. Sekita, S. Shirasaki and C. Tsugawa. 1971. The ecology of the megachilid bee Osmia cornifrons and its utilization for apple pollination. Bull. Aomori. Apple Exp. Stn. 26: 39-77.
- Yoon, H. J. and Y. I. Mah. 1997. The Estimate of Larval Growth of Mulberry Longicorn Beetle, Apriona germari Hope on the basis of the Larval Head Capsule Width, Larval Weight and Length. Korean J. Seric. Sci. 39: 174-179.
- Yoon, H. J., K. Y. Lee, H. C. Park, Y. B. Lee, S. Y. Kim, N. Kim and P. D. Kang. 2015. Insect pollinator, mason bees. RDA, printed in Korea. p. 92.

