Producing Clover and Cotton Creamed Honey under Cooling Conditions and Potential use as Feeding to Honey Bee Colonies
Abstract
In this study, the possibility to produce creamed honey from liquid clover and cotton honey using glucose powder and temperature of 5°C was investigated. Various glucose powder amounts were added to the liquid honey as 0.1, 0.3, 0.6, 1.2, 1.8 and 2.4% (w/w). Then, some parameters as well as the potential use of creamed honey as natural feeding to honey bee colonies were studied. The results showed that creamed honey can be produced after 2 weeks at 5°C using glucose powder with % of 1.2, 1.8 or 2.4. A negative correlation (r = -0.84) was found between glucose powder (%) and time needed till full crystallization. The increase in glucose (%) caused a reduction in % of fructose, sucrose and moisture. Honey bee colonies consumed creamed honey either of clover or cotton within 24 hours. Creamed cotton honey can be used to feed honey bee colonies when necessary. Especially because cotton honey has very low marketable value due to its undesirable color, scent and taste. Using creamed honey can be considered as a solution to overcome problems of using liquid honey as feeding to honey bees (e.g. the death of bee workers inside the liquid honey). The study presents a simple technology to produce creamed honey using glucose powder and a refrigerator. Also, using creamed honey as natural feeding to honey bee colonies was highlighted.
Keywords:
Honey, Crystallization, Glucose, Clover, Cotton, Honey beesINTRODUCTION
Honey is naturally produced by honey bees from natural resources or the excretions of plant sucking insects (Molan, 1996). Honey is a supersaturated solution and contains three main sugars; sucrose, glucose and fructose. The biological origin of honey can impact its physicochemical properties (e.g. sugars and water content) as found by Stelmakiené et al. (2012), even if honey samples are from the same region (James et al., 2009). Basically, there are different honey types at each country based on the available nectar sources. In Egypt, there are many pollen and nectar sources for honey bees (Abou-Shaara, 2015). However, each of citrus (during April), clover (during June), and cotton (during September) are the main honey types (Hussein, 2001). It could be said that clover honey is the most common one in Egypt. Cotton honey is not desirable by consumers due to its scent, color and taste. Citrus honey is produced relatively by few numbers of beekeepers. Egypt has the highest honey production with 5700 tons (23.97% of the total) in the Arabian countries according to FAOstat (2012).
For market purposes, honey can be sold in different forms including; liquid, chunk or creamed honey. The Egyptian market prefers mainly liquid honey followed by chunk honey. Creamed honey is not common in Egypt, mainly because most beekeepers can not produce it. The standard method described by Dyce (1975) to produce creamed honey depends on adding 5~10% (w/w) of previously granulated honey to liquid honey and store it at 14°C till full crystallization. Instead of this method Chen et al. (2009) have added 0.1% (w/w) glucose powder to liquid litchi honey and stored it at 14°C till full crystallization. The crystallization process is impacted by pasteurization (Dyce, 1975), storage temperature (Dyce, 1975 and Lupano, 1998), glucose/water ratio (Manikis and Thrasivoulou, 2001), and fructose/glucose ratio (Laos et al., 2011). Honey can be naturally crystallized on room temperature (Zamora and Chirife, 2006). Especially during cold periods but the natural crystallization of honey is not desirable and accelerates honey spoilage by yeasts. Most beekeepers can only use their own refrigerator with cooling temperature of about 5°C to produce creamed honey. Thus, developing a simple method to produce creamed honey is essential.
Food is very important for the development of honey bee colonies (Škerl and Gregorc, 2014). The main carbohydrate source for honey bees is nectar (Nicolson, 2008). A single colony requires a great amount of nectar annually with mean of 120 kg as estimated by Seeley (1995). But nectar sources are not available to honey bee colonies throughout the year. Therefore, beekeepers supply their colonies with artificial feeding using mainly sucrose solutions (sugar syrup). Sugar syrup is good as energy source for honey bees (Carrillo et al., 2015). However, brood development has been increased when the colonies have been fed on honey instead of sugar syrup (Andelković et al., 2011). Thus, searching for alternative to sugar syrup is important to enhance colony development. Using liquid honey as natural feeding to honey bee colonies has many problems such as the death of many bees inside the honey. Also, the bees could not be able to utilize the liquid honey greatly due to its high viscosity. Feeding honey bees on creamed honey could help in solving problems of using liquid honey. Thus, the study aims to develop a simple method to produce creamed honey, and to investigate the possibility to feed honey bee colonies on creamed honey.
MATERIALS AND METHODS
Crystallization process
Egyptian clover and cotton honeys were obtained from a commercial apiary located at Damanhour district, El- Behera governorate, Egypt. The honey was firstly heated using water bath at about 70°C for 20 min to get rid of any natural crystals and to kill sugar tolerant yeast. It was then cooled directly using tap water. After that, honey was divided into six groups (450g per group). Then, glucose monohydrous powder (C6H12O6.H2O, El Nasr Pharma ceutical Chemicals Co., Abu Zaabal, Egypt) was used as seed. In a study by Chen et al. (2009) glucose powder was used successfully at 0.1% (w/w) and storage temperature of 14°C to obtain creamed honey of Taiwanese litchi honey. In this study, storage temperature of 5°C was used, thus higher amounts of glucose was tested. Glucose powder with % of 0.1, 0.3, 0.6, 1.2, 1.8 and 2.4 (w/w) was added to the 6 groups, respectively for each honey type. Glucose powder was mixed well with liquid honey using mixer. The seeded honey was left for up to 24 hours at room temperature, and then all air bubbles were removed from honey surface. The honey was placed into plastic jars (for each honey type, 3 jars per group was used, and in each jar 150g honey was added). The jars were stored at 5°C in refrigerator till full crystallization. A thermometer was used to check refrigerator temperature daily during the experiment.
Studied parameters
The time needed till full crystallization was recorded for each treatment. After full crystallization, samples of creamed honey of each group were subjected to chemical analysis. Each of pH values, moisture, glucose, fructose and sucrose contents were determined of each honey sample according to AOAC (1990 and 2000). To determ ine the moisture content, 2.0g of each sample was dried to constant weight in hot air oven at 70°C. Subsequently, the moisture was calculated on dry basis. A digital pH meter (Model HI 9321; HANNA instrument, Porto, Portugal) was used to measure the pH of honey samples. To measure sugars, firstly 20 grams of each sample were softened by mixing with an equivalent amount of warmed distilled water at 40±1°C. The whole mixture was kept for 5 min at room temperature till analysis. Secondly, sucrose content was determined using the Layne-Enyon method. Finally, glucose and fructose were determined using the resorcinol reagent method.
Feeding honey bee colonies on creamed honey
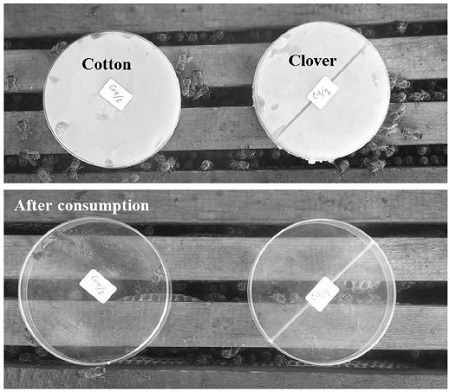
The potential use of creamed clover and cotton honey to feed honey bee colonies were tested. Fifteen honey bee colonies at equal strength (with 5 frames covered with bees, of them three brood frames and two frames of stored food) were used in the experiment. One hundred grams of creamed clover, and cotton honey were presented to each colony in plastic Petri dishes (three replicates/each treatment per honey type were used). Preference of honey bees to consume creamed clover or cotton honey was investigated as well as the consumption time. The experiment was done during winter at the experimental apiary, Faculty of Agriculture at El-Bostan Region, El-Behera Governorate.
Statistical analysis
The experiment was designed as completely randomized design (CRD). Analysis of Variance (ANOVA) was performed and means of measured parameters were compared using Duncan multiple range test at 5% level of significance. Also, pair wise Pearson correlation coefficients (r) at 5% level of significance between crystallization time and/or added glucose powder (%) and measured parameters were calculated. SAS 9.1.3 program (SAS Institute, 2004) was used to perform the analysis. The data of the feeding experiment were not statistically analyzed because no variations suitable to statistical analysis were found between treatments as shown in the result section.
RESULTS
Characteristics of creamed clover and cotton honey
Liquid clover and cotton honey with 0.1% (w/w) glucose powder showed no ability to fully crystallize after 7 months on 5°C. Thus, this treatment was not considered while the results of the other treatments are presented in Table 1.

Means ± S.E. of crystallization time (CT), fructose (%), glucose (%), fructose/glucose (F/G) ratio, sucrose (%), moisture (%), and pH values of creamed honey resulted from adding glucose powder with % of 0.3,0.6,1.2,1.8 and 2.4 (w/w) to liquid clover and cotton honey
Liquid honey treatments with different glucose powder (%) lasted from 15 to 59 days and from 27 to 66 days till full crystallization for clover and cotton honey, respectively. All replicates of each glucose powder treatment within each honey type were fully crystalized at the same time. Cotton honey crystallized faster than clover honey at all the treatments. Significant differences (P<0.05) were found between treatments of clover and cotton honey. The fast crystallization process was happened when 1.2, 1.8, or 2.4% powder glucose was added to liquid clover or cotton honey.
When more glucose powder was added to liquid honey samples, glucose (%) was increased. But % of fructose and sucrose were decreased. Creamed cotton honey had less reducing sugars than creamed clover honey. Significant differences (P<0.05) were found between reducing sugars of creamed clover and cotton honey at each glucose powder treatment. Fructose (%) at all treatments was higher than % of glucose and sucrose. Glucose powder treatments of 1.2, 1.8 and 2.4% accelerated crystallization process for clover and cotton honey. But all parameters were decreased when more glucose powder was added except pH values. Honey with less fructose/glucose (F/G) ratio showed fast crystallization than honey with more F/G ratio. Significant differences (P<0.05) were found among treatments of each honey type. Insignificant (P>0.05) variations were detected at each glucose powder treatment between creamed clover and cotton honey.
The less moisture (%) was found in creamed clover and cotton honey with 2.4% glucose powder treatment. No significant differences (P>0.05) were found between moisture (%) of clover and cotton honey at each glucose powder treatment. When more glucose powder was added pH values were increased from 3.84 to 4.3 and from 3.41 to 4.35 for clover and cotton honey, respectively. Creamed clover honey had higher pH values than cotton honey. Significant differences (P<0.05) were found between pH values of creamed clover and cotton honey at each glucose powder treatment.
A negative correlation (r = -0.84) was found between glucose powder (%) and time needed till full crystallization. Increasing the amount of glucose powder added to liquid honey increased glucose (%) and pH value (positive correlations), but reduced % of fructose, sucrose and moisture (negative correlations). Negative correlations between crystallization time and glucose (%) were found. Crystallization time correlated positively with the % of fructose, sucrose and moisture. Fructose/glucose (F/G) ratio correlated positively with crystallization time (r = 0.81) and negatively with % of glucose powder (r =-0.97). Increasing glucose powder (%) reduced F/G while low F/G led to short crystallization time. All the correlations were significant (P<0.05) as shown in Table 2.
Feeding honey bee colonies on creamed honey
The field experiment showed that creamed honey either of cotton or clover highly attracted honey bee workers. The presented amounts for each colony (15 colonies in the total) were consumed completely within short period of time (Fig. 1). All creamed clover and cotton honey obtained using different glucose (%) were consumed completely by bees within 24 hours. In other words, the creamed clover and cotton honey were consumed by the 15 colonies during 1±0.00 day. There was no specific preference of honey bee workers to clover or cotton honey. It could be said that creamed honey produced using glucose powder and a refrigerator can be used successfully to feed honey bee colonies.
DISCUSSION
Characteristics of creamed clover and cotton honey
In the present study, liquid clover and cotton honey with 0.1% (w/w) glucose powder and storage temperature of 5°C showed no ability to fully crystallize after 7 months. This result is on the contrary with the work of Chen et al. (2009). They were able to obtain Taiwanese creamed litchi honey by adding 0.1% (w/w) glucose powder and using storage temperature of 14°C. This result confirms the importance of storage temperature during the crystallization process. Cotton honey reached to full crystallization faster than clover honey under all glucose powder treatments. It could be explained by the natural variations between the two types of honey. Differences between Egyptian clover and cotton honey in regard to sugars, acidity, phenolic acid and flavonoids were found (Hamdy et al., 2009). Increasing the glucose powder amount had accelerated crystallization process of clover and cotton honey at the same temperature. This result is in line with the findings of Manikis and Thrasivoulou (2001). They found that honey with high glucose content had high crystallization ability. In general, glucose has a great role in causing crystallization even under room temperature (Zamora and Chirife, 2006). The results showed that the fast crystallization correlated with the less F/G ratio. This result is in accordance with the work of Laos et al. (2011), they found that honey with more glucose content and less F/G had high crystallization rate.
Honey with more glucose/water (G/w) tends to have high crystallization rate (Manikis and Thrasivoulou, 2001). G/W ratios of studied honey samples using different levels of glucose powder 0.3, 0.6, 1.2, 1.8, and 2.4% were 1.83, 1.88, 1.98, 2.1 and 2.22 for clover honey and 1.76, 1.81, 1.82, 1.94 and 2.03 for cotton honey, respectively. This result supports the idea that more G/W causes rapid crystallization. It could be said that the level of glucose and storage temperature are the key factors in regard to crystallization followed by F/G and G/W. The obtained % of glucose, fructose and moisture of creamed clover and cotton honey were relatively near to those of Taiwanese creamed litchi honey (34, 39.3, and 17%, respectively) as found by Chen et al. (2009). But in the present study, moisture was decreased when more glucose was added. In general, honey tends to have low pH value. Accordingly, the lowest pH value was 3.76 in the forest and rape honey in a study by Stelmakienè et al. (2012). All samples of cotton creamed honey had less pH values than clover honey. This could help in explaining the undesirable taste of cotton honey.
Feeding honey bee colonies on creamed honey
This is likely the first study to investigate the potential use of creamed honey as feeding to honey bees. Creamed honey showed high attractiveness to honey bees. The bees consumed the creamed honey within 24 hours. These results suggest the high possibility of using creamed honey to feed honey bee colonies. It is known that nectar is the natural carbohydrate source to honey bees and the extra amount of nectar is stored as honey. Thus, it could be expected that creamed honey has more benefits to honey bee colonies over sugar syrup. It was found that feeding honey bees on honey can induce health-related physiological variations over feeding honey bees on sugar syrup or high fructose corn syrup (Wheeler and Robinson, 2014). This method of bees feeding (i.e. using creamed honey) is suitable to be used particularly with unsold honey or honey with low marketable value (cotton honey).
CONCLUSION
The study presents a simple technology to produce creamed honey using glucose powder and a refrigerator. Crystallization time was greatly impacted by glucose powder amount. The creamed honey of liquid clover or cotton honey can be obtained using glucose powder with % of 1.2, 1.8, or 2.4 (w/w) and storage temperature of 5°C for about two weeks. Honey with low marketable value (namely, cotton) or unsold honey can be used by beekeepers to feed their colonies (as creamed honey). Especially creamed honey can be used to overcome problems of using liquid honey to feed honey bees. Further studies on the impacts of using creamed honey as feeding on the development and productivity of honey bee colonies are required.
References
-
Abou-Shaara, H.F., (2015), Potential honey bee plants of Egypt, Agron. Res. Moldavia, 48(2), p99-108.
[https://doi.org/10.1515/cerce-2015-0034]

-
Andelković, B., G. Jevtić, M. Mladenović, M. Petrović, and T. Vasić, (2011), Influence of spring feed on the strength of honey bee colonies during spring development, Biotech. Anim. Husb, 27(4), p1757-1760.
[https://doi.org/10.2298/BAH1104757A]

- AOAC, (1990), Food composition, additives and natural contaminants, In: Official methods of analysis Helrich, K. (ed), Association of official analytical chemists international 2, 15th Edition, Arlington, VA, USA.
- AOAC, (2000), Sugars and sugar products, In: Official methods of analysis W. Horwitz (ed.), Association of official analytical chemists international, 2(44), 16th Edition, Washington, DC, p22-33.
-
Carrillo, M.P., S.M. Kadri, N. Veiga, and R.D.O. Orsi, (2015), Energetic feedings influence beeswax production by Apis mellifera L. honeybees, Acta Sci, 37(1), p73-76.
[https://doi.org/10.4025/actascianimsci.v37i1.24191]

-
Chen, Y.W., C.-H. Lin, F.-Y. Wu, and H.-H. Chen, (2009), Rheological properties of crystallized honey prepared by a new type of nuclei, J. Food Proc. Eng, 32, p512-527.
[https://doi.org/10.1111/j.1745-4530.2007.00227.x]

- Dyce, E.J., (1975), Producing finely granulated or creamed honey, In Honey: A Comprehensive Survey E. Crane ed, p293-306, William Heinemann Ltd, London, England.
- FAOstat, (2012), (accessed on 1.6. 2014).
- Hamdy, A.A.., H.M. Ismail, A.A. AL-Ahwal, and N.F. Gomaa, (2009), Determination of flavonoid and phenolic acid contents of clover, cotton and citrus floral honeys, J. Egy. Pub. Heal. Assoc, 48(3and4), p245-259.
- Hussein, M.H., (2001), Beekeeping in Africa: I-north, east, northeast and west African countries, Proc. 37th Int. Apic. Congr, Durban, South Africa.
- James, O.O., M.A. Mesubi, L.A. Usman, S.O. Yeye, K.O. Ajanaku, K.O. Ogunniran, O.O. Ajani, and T.O. Siyanbola, (2009), Physical characterisation of some honey samples from North-Central Nigeria, Int. J. Phys. Sci, 4(9), p464-470.
- Laos, K., E. Kirs, R. Pall, and K. Martverk, (2011), The crystallization behaviour of Estonian honeys, Agr. Res, 9, p427-432.
-
Lupano, C.E., (1998), DSC study of honey granulation stored at various temperatures, Food Res. Int, 30, p683-688.
[https://doi.org/10.1016/S0963-9969(98)00030-1]

- Manikis, I., and A. Thrasivoulou, (2001), The relation of physicochemical characteristics of honey and the crystallization sensitive parameters, Apiacta, 36, p106-112.
- Molan, P.C., (1996), Authenticity of honey, In Food authentication Springer US, p259-303.
-
Nicolson, S.W., (2008), Water homeostasis in bees, with the emphasis on sociality, J. Exp. Biol, 212, p429-434.
[https://doi.org/10.1242/jeb.022343]

- SAS Institute, (2004), The SAS System Version 9.1.3, SAS Institute, Cary. NC.
- Seeley, T.D., (1995), The wisdom of the hive: the social physiology of honey bee colonies, Cambridge, MA, Harvard University Press, In Nicolson, S.W., (2008), Water homeostasis in bees, with the emphasis on sociality, J. Exp. Biol, 212, p429-434.
-
Škerl, M.I.S., and A. Gregorc, (2014), A preliminary laboratory study on the longevity of A. m. carnica honey bees after feeding with candies containing HMF, J. Apic. Res, 53(4), p422-423.
[https://doi.org/10.3896/IBRA.1.53.4.07]

- Stelmakiené, A., K. Ramanauskiené, V. Briedis, and D. Leskauskaité, (2012), Examination of rheological and physicochemical characteristics in Lithuanian honey, Afr. J. Biotechnol, 11(60), p12406-12414.
-
Wheeler, M.M., and G.E. Robinson, (2014), Diet-dependent gene expression in honey bees: honey vs. sucrose or high fructose corn syrup, Scientific Reports, 4, p5726.
[https://doi.org/10.1038/srep05726]

-
Zamora, M.C., and J. Chirife, (2006), Determination of water activity change due to crystallization in honeys from Argentina, Posprint. Publ. Defin. Food Cont, 17, p59-64.
[https://doi.org/10.1016/j.foodcont.2004.09.003]