
Body Compositional Changes of Fatty Acid and Amino Acid from the Queen of Bumblebee, Bombus terrestris during Overwintering
Abstract
The body weight and body compositions were measured on Buff-tailed Bumblebee, Bombus terrestris queen during overwintering to understand the change of the physical status and to evaluate the nutritional requirement for the oviposition by queen after overwinter. Results revealed increase in body weight and body fat (6.5 and 9.5% respectively) during the first week of overwintering initiation and subsequent decrease (11 and 79.5% respectively) in the later phase of overwintering. On the other hand, during early stage of overwinter the total body protein content increased by 12.3%, and by the end of overwintering, it was 37.6% higher than that of early stage. In the present investigation, the decreasing trend of fats and increasing trend of amino acids and thus proteins during overwintering suggest that the fats are primarily utilized for the metabolic purposes during diapause when bumble bee queens could not consume any feed, and the protein helps to sustain and lowering the freezing point in time of sub-zero temperatures of overwintering in temperate zone.
Keywords:
Metabolism, Energy consumption, Nutritional requirement, Fat, Annual nestINTRODUCTION
Native pollinators often plays essential role in pollination of wild and cultivated plants in all terrestrial ecosystems (Garibaldi et al., 2013). Bumblebees, in particular are among the most important pollinators of temperate zone plants (Inari et al., 2012). The dense hair on their bodies allows efficient pollen transfer from flower to flower. Moreover, they also exhibit a distinctive feature of sonication often helps in pollination called ‘buzz pollination’. Although bumblebees are efficient pollinators of a variety of crops including red clover, cranberries, blueberries, kiwi, almonds, apples, pears etc. but majority of the commercially reared bumblebee colonies are used in production of greenhouse tomatoes and sweet pepper (NAPPC, 2006).
In the year 1985, de Jonghe, the Belgian veterinarian and amateur bumblebee researcher first uncovered the economic benefits of using bumblebee for pollination of greenhouse tomatoes and subsequently founded the commercial rearing of Bombus terrestris company Biobest in 1987 (Velthuis and van Doorn, 2006). Couple of commercial production facilities namely Koppert Biological Systems and Bunting Brinkman Bees were initiated in the next two years. Resulting from the international trade millions of the colonies shipped throughout the world leading to deliberate releases or accidental escapes that foster B. terrestris establishments (Goulson, 2010; Inari et al., 2005; Murray et al., 2013; Velthuis and van Doorn, 2006; Lococq et al., 2016).
In general, in temperate region bumblebees are univoltine i.e. they have only one generation per year. However, observations reported that second generation could exist for B. jonellus in field (cf. Röseler, 1985; Meidell, 1968). New queen emerges at the end of the season. Young queen mates with multiple drones and stores sperms for the rest part of her life. These young queens are the only members of the colony who survive through winter. They do so by adapting a strategy called ‘diapause’ which can be defined as resting state of their life cycle. Diapause is a suspension of development that can occur at the embryonic, larval, pupal, or adult stage depending on the species. Entering into diapause is an endogenous characteristic for queen caste. Young queens participate in the process often known as ‘overwintering’. Depending on the species diapause can be facultative or obligatory. In case of bumblebee B. terrestris diapause is an obligatory part of their life cycle.
Young queens prepare themselves for entering into the diapause phase (a form of hibernation). They consume food and synthesize the reserves for diapause phase of life since the first day of eclosion. About on third day they become ready to mate. After copulation the queens become lazy, seek a suitable site for overwintering and enter diapause independent of temperature or light (Röseler, 1985). In captivity under environment controlled condition queen sit apart from the nest and do not participate any household tasks. Low juvenile hormone titre in haemolymph resulting from the inactivation of neurosecretory cells controlling corpora allata, accompanied by other physiological changes like deposition of fat and other metabolites in fat body but not in ovaries are the causes of imaginal or reproductive diapause (Röseler, 1976; 1977; Brown and Chippendale, 1978; Saunders et al., 2002). However, hardly any study is available to demonstrate the changes in body composition during the initiation and termination of overwinter in B. terrestris. An understanding such changes are fundamental in clarifying the sequences of the physiological events which would be of a great value in formulating diet for them.
MATERIALS AND METHODS
Bumblebee collection
Young queens of B. terrestris of three different physiological conditions viz. mated (n=19), after one week of overwinter (early stage) (n=20) and at the end of overwinter for 3 months (n=20) were obtained from Yecheon Entomology Institute, Republic of Korea. B. terrestris colonies are reared in captivity under controlled climatic condition at +28°C to +30°C and relative humidity is maintained 60%.
Body weight measurement
B. terrestris queens were narcotized by applying carbon di oxide (CO2) and body weight of individual was obtained by electronic balance. One way ANOVA and Tukey’s HSD test were performed in order to analyse difference in the body weight of queens of different physiologic conditions using SAS 9.2.
Sample preparation for chemical analyses
The queens were freeze dried for 72 hours at -50°C using the freeze dryer (Christ Alpha1-4 LD Plus, Martin Christ Gefriertrocknungsanlagen GmbH, Germany) and ground to powder form and used for further analysis. All the chemicals used in the study were of analytical grand and glass wares were meticulously clean.
Amino acid analysis
Amino acid composition was determined by S433 (Sykam GAmbH, Gemany) equipped with LCA K07/Li (PEEK-column 4.6 X 150mm) column following the standard method of AOAC (Association of Official Analytical Chemists) (1990). The powder samples were hydrolysed in 6N hydrochloric acid (HCl) at 110°C for 24 hours under nitrogen environment followed by reconstitution with dilution buffer (0.12N, pH 2.20) and injected in the analyser.
Fatty acid analysis
Fatty acid composition was analysed by Gas chromatography (GC-14B, Shimadzu, Tokyo, Japan) equipped with Flame Ionization Detector (GC-FID) and SP-2560 column following the standard method of Korean Food Standard Codex (2010). The samples were derived into fatty methyl ester (FAMEs) and injected into GC.
RESULTS AND DISCUSSION
During diapause B. terrestris queens do not consume any food. It implies that they must sequester sufficient nutritional reserve in the pre-diapause period to meet its metabolic needs during diapause and still have sufficient reserve remaining at the end of diapause to resume normal activity. Fig. 1 demonstrates the changes in body weight of mated B. terrestris queen, one week from the overwintering and at the end of overwinter process. There is a significant changed in the body weight of B. terrestris in different physiological conditions (dF=2,56; F=10.86; P<0.000). Firstly the body weight was found to be increased by 6.4% in the time of one week after the initiaiton of overwintering. After the young queens emerge, each queen mate with multiple drones and store sperms for the rest of their life. Few days after mating they go for the diapause which is a programmed strategy to survive in the winter. In the mean time they eat more and prepare themselves for the diapause by reserving the food which could be utilized during this period. Presumably this is the cause that the body weight was increased during the first few days when overwintering was induced. In time of termination we found the body weight was decreased almost 11% comparing to the mated B. terrestris which was found statistically significant (Fig.1).
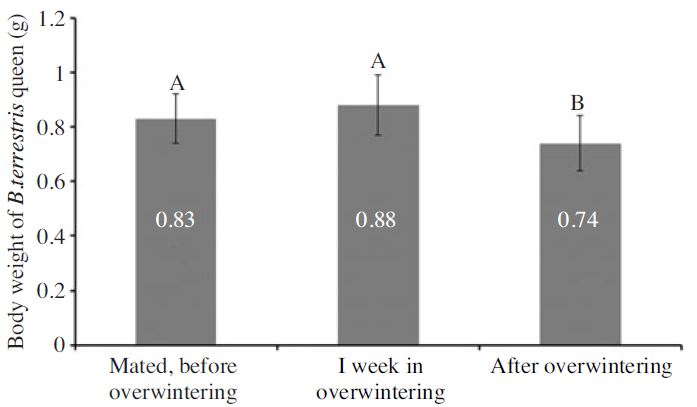
Body weight (g) of mated, one week from the initiation of overwinter and overwintered Bombus terrestris queen.
To understand the role of fat during the overwinter period we analysed the fatty acid composition of the fat fraction of the B. terrestris. Table 1 represents the fatty acid compositions of mated B. terrestris queen, after one week from the initiation of overwinter and at the termination of overwinter process. Oleic, Palmitoleic, palmitic, myristic acid were found predominated among the fatty acids. The fatty acid reflects the similar pattern observed for the body weight of B. terrestris queens. Total body fat was increased by 9.5% during the first week of the overwinter and decreases rapidly 79.5% during the latter part of overwintering. The pattern suggests that overwintering B. terrestris queens utilize fat stores as the primary metabolic substrate.

Fatty acid composition (mg/100g Dry matter basis) of mated, one week from the induction of overwinter and overwintered Bombus terrestris queen
Irrespective of the physiologic situations body fat of B. terrestris queens mostly comprises of monounsaturated fatty acids (76.2~76.7%). In general, monounsaturated fatty acids (MUFA) was followed by saturated fatty acids (SFA) and less concentration was found for polyunsaturated fatty acids (PUFA) in B. terrestris which is in agreement with other scientific reports on different insect species (Bhulaidok et al., 2010; Chakravorty et al., 2011; Rumpold and Schlüter, 2013; Chakravorty et al., 2016; Ghosh et al., 2017). However, the utilization of different categories of fatty acids differed significantly. SFA and MUFA in overwintered B. terrestris queen were decreased by 83.8 and 79.3% respectively while comparing with the body mated in time of diapause initiation i.e. mated queens. However, the PUFA proportion was decreased only by 42.5%. Fatty acids serve various functions during whole life cycle of insects including biosynthesis of wax, pheromones, sex pheromones, prostaglandins and other derivative of PUFA, defensive secretions etc. to mention a few (Stanley-Samuelson et al., 1988). During nonfeeding period like diapause they play role as the primary energy sources. Although ample reports are available on fatty acid composition of many different species of insects, but study on changes in fatty acid composition attending have been addressed in few species and mostly restricted to comparative account of diapause and non-diapause states (Khani et al., 2007). Dolycoris baccarum (Hemiptera) and piezodorus lituratus (Hempitera) diapausing adult had different fatty acid composition in diapausing and prediapausing condition. Diapausing adult was reported to contain higher proportion of MUFAand lower proportion of SFA (Bashan and Cakmak, 2005). Unsaturated fatty acid/saturated fatty acids ratio (UFA/SFA) increased in total phospholipid in overwintering larvae of Eurosta solidaginis (Diptera) (Bennett et al., 1999). In the present study, UFA/SFA ration was increased from 3.6 to 4.9 in overwintering B. terrestris queen which is also in agreement few other insect species (Khani et al., 2007). Linoleic acid is a structural component of membrane to maintain peoper fluidity and permeability. Presumably, the increased proportion of unsaturated fatty acids could be attributed to the adaptation to cold in order to maintain appropriate fluidity of the food reserve to make them available as energy source (cf. Khani et al., 2007).
Many insect species like larvae of Peiris rapae (Lepidoptera), larvae of Plodia interpunctella (Lepidoptera), last instar larvae of Pectinophora gossypiella (Lepidoptera), Culex pipens (Diptera) show diapause-associated increases in reserves or body size and thus body weight (cf. Hahn and Denlinger, 2007; Kono, 1970; Tsuji, 1958; Adkisson et al., 1963; Mitchell and Briegel, 1989). Present investigation demonstrated that the B. terrestris queen belongs to this group and it also supplements previous scientific report by Fliszkiewicz and Wilkaniec (2007) in which the diapausing B. terrestris queens were characterized to contain higher dry matter in fat body and higher fat content as compared with their non-diapausing counterparts. It makes sense for diapausing individuals to increase their reserves as a strategy to deal with the energetic demands of the diapause period. Lipids are primary metabolic reserve. The trend of reducing body fat level during overwinter period is found similar to the study on parasitoid wasp Asobara tabida (Hymenoptera) in which the fat reserve and also fecundity decreased significantly with the time spent in diapause (Ellers and van Alphen, 2002). During diapause insect could not obtain water which could be generated from lipids and help insects in survival (cf. Yocum et al., 2011; Wharton, 1985; Danks, 2000). This could be one possible use of fat. In fact, the metabolic pathways leading to cell proliferation and growth are down regulated during diapause while basic cellular maintenance remain operational at reduced rate and stress resistance pathways leading to cryoprotectant and heat shock protein (HSP) synthesis etc. are likely to be up regulated (Dehlinger et al., 2005). However, on the other hand accumulation of greater reserve increases the risk of attracting natural enemies or not completing development before the onset of inclement condition (Masaki, 1977; Tauber et al., 1986). Presumably this could be a possible reason few species do not follow this trend. To cite few examples, diapausing larvae of Calliphora vicina (Diptera), diapausing pupae of Meduca sexta (Lepidoptera) do not show any significant greater store of fat or nutrients than their non diapausing counterpart (Saunders, 1997; 2000; Siegert, 1986). Diapausing pupae of Papilio polyxenes (Lepidoptera), larvae of Choristoneura fumiferana (Lepidoptera) are smaller than their non diapausing counterparts (Blau, 1981; Harvey, 1961).
Table 2 represents the amino acid content of mated B. terrestris queen, one week from the initiation of overwinter and at the termination of overwinter process. In contrast to the pattern of changes in fatty acids, a pronounced increase took place during overwintering period. During early stage of overwinter initiation i.e. one week after the initiation of overwinter the protein concentration was raised by 12.3% from the mated queen and subsequently it was increased by 37.6%.

Amino acid composition (g/100g Dry matter basis) of mated, one week from the induction of overwinter and overwintered Bombus terrestris queen
Similar kind of observation was reported for Pectinophora gossypiella (Lpidoptera). Concentration of total protein was considerably increased by 62.3% in haemolymph during diapause though a drop occurred in fat body protein concentration in late phase of diapause (Rostom et al., 1992). Post-feeding larvae of Diatraea grandiosella (Lepidoptera) and diapausing adult female of Leptinotarsa decemlineata (Coleoptera) accumulate markedly greater quantity of hexamerin protein (Brown and Chippendale, 1978; de Kort and Koopmanschap, 1994). Rise in protein might add to the concentration of solutes, which in turn, lower the freezing point of the haemolymph, that enables the species to withstand unfavourable low temperature during diapause. Prediapausing larvae of Diatraea grandiosella were found to store protein fraction which makes up 20% of the soluble proteins of the fat body of the newly diapaused individuals (Brown and Chippendale, 1978). Moreover, in the diapause associated protein of D. grandiosella aspartate, leucine, lysine and glutamate was found predominate (Brown and Chippendale, 1978). In the present study also, B. terrestris queen contained these amino acids in higher proportion. In addition higher proportion of alanine and glycine were found (Table 2). In Colorado potato beetle L. decemlineata protein accumulates first in haemolymph and later in fat body often known as ‘diapause protein’ which acts as a storage protein. The other blood proteins also increase under short day, resulting almost 3 times higher concentration of total proteins in haemolymph and remains high during diapause (cf. de Kort, 1990; de Loof and de Wilde, 1970).
In the present investigation the decreasing trend of fats and increasing trend of amino acids and thus proteins during overwintering suggest that the fat primarily are utilized for the metabolic purposes as they could not feed at that time and on the other hand the protein helps to sustain in time of sub-zero temperature by lowering the freezing point. Thus, high fat diet could be supplied to these overwintered queens in captivity.
Acknowledgments
The authors are thankful to Gyeongbuk FTA grant (2016) to CJ and research fund to support SG. Some nutritional analyses were done with instruments of Insect industry R&D Center (GB Pollinator) in ANU.
LITERATURE CITED
-
Adkisson, T. A., R. A. Bell, and S. G. Wellso, (1963), Environmental factors controlling the induction of diapause in the pink bollworm, Pectinophora gossypiella (Saunders), J. Insect Physiol, 9, p299-310.
[https://doi.org/10.1016/0022-1910(63)90107-0]

- AOAC, (1990), Official methods of analysis, 15th ed, Association of Official Analytical Chemists, Washington DC, USA.
- Bashan, M., and O. Cakmak, (2005), Changes in composition of phospholipid and triacylglycerol fatty acids prepared from prediapausing and diapausing individuals of Dolycoris baccarum and Piezodorus lituratus (Hemiptera: Pentatomidae), Ann. Entomol. Soc. Am, 98, p575-579.
-
Bennett, V.A., N.L. Pruitt, and R.E. Lee, (1997), Seasonal changes in fatty acid composition associated with coldhardening in third instar larvae of Eurosta solidaginis, J. Comp. Physiol, 167, p249-255.
[https://doi.org/10.1007/s003600050071]

- Bhualaidok, S., O. Sihamala, L. Shen, and D. Li, (2010), Nutritional and fatty acid profiles of sun-dried edible black ants (Polyrhachis vicina Roger), Maejo Int. J. Sci. Technol, 4, p101-112.
-
Blau, W. S., (1981), Life history variation in the black swallowtail butterfly, Oecologia, 48, p116-122.
[https://doi.org/10.1007/bf00346997]

-
Brown, J. J., and G. M. Chippendale, (1978), Juvenile hormone and a protein associated with the larval diapause of the southwestern corn borer, Diatraea grandiosella, Insect Biochem, 8, p359-367.
[https://doi.org/10.1016/0020-1790(78)90022-7]

-
Chakravorty, J., S. Ghosh, and V.B. Meyer-Rochow, (2011), Chemical composition of Aspongopus nepalensis Westwood 1837 (Hemiptera; Pentatomidae), a common food insect of tribal people in Arunachal Pradesh (India), Int. J. Vitam. Nutr. Res, 81, p49-56.
[https://doi.org/10.1024/0300-9831/a000050]

- Chakravorty, J., S. Ghosh, K. Megu, C. Jung, and V.B. Meyer- Rochow, (2016), Nutritional and anti-nutritional composition of Oecophylla smaragdina (Hymeoptera: Formicidae) and Odontotermes sp. (Isoptera: Termitidae): two preferred edible insects of Arunachal Pradesh, India, India. J. Asia Pac. Entomol, 19, p711-720.
-
Danks, H. V., (2000), Dehydration in dormant insects, J. Insect Physiol, 46, p837-852.
[https://doi.org/10.1016/s0022-1910(99)00204-8]

- de Kort, C. A. D., (1990), Thirty-five years of diapause research with the Colorado potato beetle, Entomol. Exp. Appl, 56, p1-13.
-
de Kort, C. A. D., and A.B. Koopmanschap, (1994), Nucleotide and deduced amino acid sequence of a cDNA clone encoding Diapause Protein-1, an arylphorin-type storage hexamer of the Colorado potato beetle, J. Insect Physiol, 40, p527-535.
[https://doi.org/10.1016/0022-1910(94)90126-0]

-
de Loof, A., and J. de Wilde, (1970), Hormonal control of synthesis of vitellogenic female protein in the Coloradobeetle, Leptonotarsa decemlineata, J. Insect Physiol, 16, p1455-1466.
[https://doi.org/10.1016/0022-1910(70)90144-7]

- Denlinger, D.L., G.D. Yocum, and J.L. Rinehart, (2005), Hormonal control of diapause, p615-650, In Comprehensive Molecular Insect Science, eds. by L.I. Gilbert, K. Iatrou, and S.S. Gill, 3rd vol., Elsevier Press, Amsterdam.
-
Ellers, J., and J.M. van Alphen, (2002), A trade-off between diapause duration and fitness in female parasitoids, Ecol. Entomol, 27, p279-284.
[https://doi.org/10.1046/j.1365-2311.2002.00421.x]

- Fliszkiewicz, M., and Z. Wilkaniec, (2007), Fatty acids and amino acids in the fat body of bumblebee Bombus terrestris (L.) in diapausing and non-diapausing queens, J. Apic. Sci, 51, p55-63.
-
Garibaldi, L. A., I. Steffan-Dewenter, R. Winfree, M. A. Aizen, R. Bommarco, S. A. Cunningham, C. Kremen, L. G. Carvalheiro, L. D. Harder, O. Afik, I. Bartomeus, F. Benjamin, V. Boreux, D. Cariveau, N. P. Chacoff, J. H. Dudenhoffer, B. M. Fretas, J. Ghazoul, S. Greenleaf, J. Hipolito, A. Holzschuh, B. Howlett, R. Issacs, S. K. Javorek, C. M. Kennedy, K. M. Krewenka, S. Krishnana, Y. Mandelik, M. M. Mayfield, I. Motzke, T. Munyuli, B. A. Nault, M. Otieno, J. Petersen, G. Pisanty, S. G. Potts, R. Rader, T. H. Ricketts, M. Rundlof, C. L. Seymour, C. Schuepp, H. Szentgyorgyi, H. Taki, T. Tscharntke, C. H. Vergara, B. F. Viana, T.C. Wanger, C. Westphal, N. Williams, and A. M. Klein, (2013), Wild pollinators enhance fruit set of crops regardless of honey bee abundance, Science, 339, p1608-1611.
[https://doi.org/10.1126/science.1230200]

- Ghosh, S., S. M. Lee, C. Jung, and V. B. Meyer-Rochow, (2017), Nutritional composition of five commercial edible insects in South Korea, J. Asia Pac. Entomol.
- Goulson, D., (2010), Bumblebees: behaviour, ecology and conservation, Oxford University Press, New York.
-
Hahn, D. A., and D. L. Denlinger, (2007), Meeting the energetics demands of insect diapause: nutrient storage and utilization, J. Insect Physiol, 53, p760-773.
[https://doi.org/10.1016/j.jinsphys.2007.03.018]

-
Harvey, G. T., (1961), Second diapause in spruce budworm from eastern Canada, Canadian Entomologist, 93, p594-602.
[https://doi.org/10.4039/ent93594-7]

-
Inari, N., T. Hiura, M. J. Toda, and G. Kudo, (2012), Pollination linkage between canopy flowering, bumblebee abundance and seed production of understorey plants in a cool temperate forest, J. Ecol, 100, p1534-1543.
[https://doi.org/10.1111/j.1365-2745.2012.02021.x]

-
Inari, N., T. Nagamitsu, T. Kenta, K. Goka, and T. Hiura, (2005), Spatial and temporal pattern of introduced Bombus terrestris abundance in Hokkaido, Japan and its potential impact on native bumblebees, Popul. Ecol, 47, p77-82.
[https://doi.org/10.1007/s10144-004-0205-9]

-
Khani, A., S. Moharramipour, M. Barzegar, and H. Naderi-Manesh, (2007), Compariosn of fatty acid composition in total lipid of diapause and non-diapause larvae of Cydia pomonella (Lepidoptera: Tortricidae), Insect Sci, 14, p125-131.
[https://doi.org/10.1111/j.1744-7917.2007.00134.x]

- Kono, Y., (1970), Photoperiodic induction of diapause in Pieris rapae crucivora Boisduval (Lepidoptera: Pieridae), Appl. Entomol. Zool, 5, p213-224.
- Korean Food Standard Codex, (2010), Ministry of Food and Drug Safety, Republic of Korea.
- Lococq, T., C. Audrey, D. Michez, N. Brasero, and J-V. Rasplus, (2016), The alien’s identity: consequences of taxonomic status for the international bumblebee trade regulations, Biol. Conserv, 195, p169-176.
- Masaki, S., (1977), Life cycle programming, p31-60, in Adaptation and speciation in the fall webworm, ed. by T. Hidaka, Kodansha, Tokyo.
- Meidell, O., (1968), Bombus jonellus (Kirby) (Hym., Apidae) has two generations in a season, Norsk. Ent. Tidsskr, 14, p31-32.
- Mitchell, C. J., and H. Briegel, (1989), Inability of diapausing Culex pipiens (Diptera: Culicidae) to use blood for producing lipid reserve for overwintering survival, J. Med. Entomol, 26, p318-326.
-
Murray, T. E., M. F. Coffey, E. Kohoe, and F. G. Horgan, (2013), Pathogen prevalence in commercially reared bumblebees and evidence of spillover in conspecific populations, Biol. Conserv, 159, p269-276.
[https://doi.org/10.1016/j.biocon.2012.10.021]

- NAPPC (North American Pollinator Protection Campaign), (2006), Importation of non-native bumble bees into North America: Potential consequences of using Bombus terrestris and other non-native bumble bees for greenhouse crop pollination in Canada, Mexico and United States, [www.pollinator.org/Resources/BEEIM PORTATION_AUG2006.pdf.].
- Röseler, P. F., (1976), Juvenile hormone and queen rearing in bumblebees, p55-61, in Phase and caste determination in insects, ed. by M. Lüscher, Pergamon Press, Oxford.
- Röseler, P. F., (1977), Endocrine control of polymorphism in bumblebees, Proc. VIII Int. Congr. I.U.S.S.I. Wageningen, p22-23.
- Röseler, P. F., (1985), A technique for year-round rearing of Bombus terrestris (Apidae, Bombini) colonies in captivity, Apidologie, 16, p165-170.
- Rostom, Z. M. F., M. S. Salama, A. M. Salem, and A. M. El-Shafei, (1992), Changes in the patern of protein in the haemolymph and fat body of the pink bollworm Pectinophora gossypiella Saunders during the active and diapause phases of the larva, J.K.A.U. Sci, 4, p127-139.
- Rumpold, B.A., and O.K. Schlüter, (2013), Nutritional composition and safety aspects of edible insects, Mol. Nutr. Food Res, 00, p1-22.
-
Saunders, D. S., (1997), Under-sized larvae from short-day adults of the blow fly, Calliphora vicina, side-step the diapause programme, Physiological Entomol, 22, p249-255.
[https://doi.org/10.1111/j.1365-3032.1997.tb01165.x]

-
Saunders, D. S., (2000), Larval diapause duration and fat metabolism in three geographical strains of the blow fly, Calliphora vicina, J. Insect Physiol, 46, p509-517.
[https://doi.org/10.1016/s0022-1910(99)00137-7]

- Saunders, D. S., C. G. H. Steel, X. Vafopoulou, and R. D. Lewis, (2002), Insect Clock, 3rd ed, p560, Elsevier Science B.V., Amsterdam.
-
Siegert, K. J., (1986), The effects of chilling and integumentary injury on carbohydrate and lipid metabolism in diapause and non-diapause pupae of Manduca sexta, Comp. Biochem. Physiol, 85A, p257-262.
[https://doi.org/10.1016/0300-9629(86)90248-3]

-
Stanley-Samuelson, D.W., R.A. Jurenka, C. Cripps, G.J. Blomquist, and M. de Renobales, (1988), Fatty acids in insects: composition, metabolism and biological significance, Arch. Insect Biochem. Physiol, 9, p1-33.
[https://doi.org/10.1002/arch.940090102]

- Tauber, M. J., C. A. Tauber, and S. Masaki, (1986), Seasonal adaptations of insects, Oxford Press, New York.
- Tsuji, H., (1958), Studies on the diapause of the Indian meal moth, Plodia interpunctella Hübner. I. The influence of temperature on the diapause and the type of diapause, Japanese J. Appl. Entomol. Zool, 2, p17-23.
-
Velthuis, H. H. W., and A. V. Doorn, (2006), A century of advances in bumblebee domestication and the economic and environmental aspects of its commercialization for pollination, Apidologie, 37, p421-451.
[https://doi.org/10.1051/apido:2006019]

- Wharton, G. W., (1985), Water balance in insects, p565-601, in Comprehensive Molecular Insect Science, vol. 4, eds. By L.I. Gilbert, K. Iatrou, and S.S. Gill, Elsevier, Amsterdam.
- Yocum, G. D., J. S. Buckner, and C. L. Fatland, (2011), A comparison of internal and external lipids of nondiapausing and diapausing initiation phase adult Colorado Potato beetles, Leptinotarsa decemlineata, Comp. Biochem. Physiol. Part B, 159, p163-170.