
Body Fatty and Amino Acid Composition of a Native Bumblebee, Bombus ignitus Relative to B. terrestris of Foreign Origin in Korea
Abstract
Bombus terrestris is imported and now commercially produced, while B. ignitus is the native bumblebee species, which is in the process of commercialization in Korea. For the successful rearing, understanding the body composition is important parameter to understand the diet requirement. We measured and compared body fat and amino acid composition of two bumblebee species under mated condition. The results revealed that there was significant difference in the body weight of two tested species. The quantity of body fat (fatty acids) and proteins (amino acids) was found higher in B. ignitus than that was reported for B. terrestris. However, individual fatty acid and amino acid distribution was similar in both species. Dominant fatty acids were oleic acid and palmitoleic acid of among monounsaturated fatty acid, palmitic of saturated fatty acid, and linoleic acid of polyunsaturated fatty acid. Among amino acids, glutamic acid was predominant followed by glycine and leucine. To foster the population of native bumblebee i.e. B. ignitus through rearing, appropriation of feed formulation could be based on this result which could lead the purpose of feed development for indigenous population.
Keywords:
Bombus ignitus, Nutrition, Commercialization, Feed, Mass productionINTRODUCTION
Bumblebees are important pollinators in the wild as well as in agricultural production system. Since Roland de Jonghe first uncovered the economic benefits of using bumblebee for pollination particularly in greenhouse cultivation, Eurasian bumblebee B. terrestris and North American bumblebee B. locurum, B. impatiens have been started to be reared extensively on an industrial scale and widely spread though commercially far beyond of their native geographical range (Velthuis and Doorn, 2006). Resulting from the international trade millions of colonies of these species were shipped throughout the world leading to deliberate release or accidental escapes that foster their establishment (Goulson, 2010; Inari et al., 2005; Murray ey al., 2013; Lococq et al., 2016). The scenario even becomes more complex when the country which is not native for B. terrestris starts to produce colonies for commercial purpose. In Korea, increasing trend is observed in producing B. terrestris colonies domestically rather than importing them (unpublished data). Although little is known about the possible effects of the exotic species on the native species, however, considerable volume of evidences suggest competition among the invasive and native species which could render negative impacts on the native species leading to threatened condition (Goulson, 2003). Thus, in order to avoid the undesirable chance of extinction and maintain ecological balance native bumblebee species can be reared and used in place of invasive species.
In Korea, there are twenty native bumblebee species. Among the most abundant seven native species, B. ignitus was found to be more efficient in pollination and become a promising candidate for rearing in captive condition in commercial scale (Yoon et al., 1999). At the start of the year queen emerges from winter rest (diapause) and begins to feed greater quantity of food i.e. pollen in order to gain strength in preparation of building nest and rearing young. Pollen with high protein stimulates the young queen’s ovaries and start producing eggs. Female worker bees emerged from the first batch of the eggs and new queens are produced at the end of the season. Before winter diapause, new queen mate with multiple drones and store sperms for the rest of the life and they are the only members of the colony to survive through winter by entering a resting state ‘diapause’ or ‘overwintering’ (Katayama, 1971, 1973; Goulson, 2010).
Although not for commercial purpose, but the domestication research of bumblebee is more than hundred years old. Two queen method, a queen together with some conspecific workers or cluster of cocoons from another colony (Sladen, 1912), queen stimulating by workers of closely related species (Velthuis and Doorn, 2006; Ono et al., 1994; Ptacek, 1985, 1991, 2001; van Heemert et al., 1990; van den Eijinde et al., 1991) are the significant studies in the way of today’s commercial production of bumblebee colonies. Also the studies of photoperiodic regimen (Tasei and Aupinel, 1994) and climatic conditions including humidity are highly remarkable (Yoon et al., 2002). Honeybee-collected pollen is usually used to rear bumblebee in captivity. Studies reveal that fresh and frozen pollen is a good source of nutrition comparing with the dried pollen (Roseler, 1977). Also pollen from multiple floral sources, pollen with high protein content shows the rearing success. The amount of calories one needs to maintain body weight is influenced by body composition. Thus, body composition is important parameter to understand the diet requirement. As there are many factors influencing body composition including gender, age, activity level, and genes. To cite some example, the age specific protein requirement of honeybee is remarkable like young adults undergoing physiological changes such as maturation of flight muscle, suboptimal diet delaying the growth etc. (Hersch et al., 1978; Hagedorn and Moeller, 1968). However, the data on the body composition of the different bumblebee species is scanty. Thus, this present paper deals with the comparison study of body composition of two bumblebee species namely B. ignitus and B. terrestris under mated condition which could lead the appropriation of feed formulation especially for B. ignitus to foster native population
MATERIALS AND METHODS
Bumblebee collection
Young mated queens of B. terrestris (n=19) and B. ignitus (n=20) of about 10 days old were obtained from Yecheon Entomology Institute, Republic of Korea. The colonies were reared in captivity under controlled climatic condition at 28°C to 30°C and relative humidity is maintained 60%, but using the same diet for the generations for both species. Sucrose solution and multifloral pollen mix were provided as their diet to maintain the population. Originally, Japanaese population the B. ignitus population was reared by a Belgium based commercial production unit Biobest and imported in Korea in the month of August, 2016. Then in Korea the population was maintained in Yecheon Bumblebee Research Center.
Body weight and body composition analysis
Body weight: Individuals of both species were narcotized by applying carbon dioxide (CO2) and body weight of individual insect was obtained by electronic balance. T-test and Tukey’s HSD test were carried out to analyse difference in the body weight of two species.
Sample preparation: The samples of B. ignitus (n=20) were freeze dried for 72 hours at -50°C using the freeze dryer (Christ Alpha 1-4 LD plus, Germany), ground to powder and used for further analyses. All chemicals used in the study were of analytical grade and glass wares were meticulously clean. Further analyses of fatty acid and amino acid were conducted with B. ignitus only for this study and data of B. terrestris were taken from Ghosh et al., 2017 for the comparative purpose.
Fatty acid analysis: Fatty acid composition was analysed by Gas Chromatography (GC-14B, Shima-dzu, Tokyo, Japan) equipped with Flame Ionization Detector (GC-FLD) and SP-2560 column following the standard method of Korean Food Standard Codex (2010). The samples were derivatized into fatty acid methyl ester and injected into GC.
Amino acid analysis: Amino acid composition was determined by S433 (Sykam GamBH, Germany) equipped with LCA K07/Li (PEEK-column 4.6 150mm) column following the standard method of AOAC (1990). The powdered sample were hydrolysed in 6 N hydrochloric acid (HCl) at 110°C for 24 hours under nitrogen environment followed by reconstitution with dilution buffer (0.12 N, pH 2.20) injected in the analyser.
RESULTS AND DISCUSSION
Body weight of B. ignitus was significantly higher than the body weight of B. terrestris (t=2.502, df=33, p=0.0087) (Fig. 1). Bodyweight is an important determinant of survival especially prior to overwintering (Holm, 1972). In a study on B. terrestris, it was found that bumblebee with a wet weight below 0.6 g prior to diapause (overwintering) did not survive and for those queens exceeding this threshold a higher pre-diapause weight did not increase their post-diapause performance (Beekman et al., 1998). Before winter diapause new queen mate with multiple drones and store sperms for the rest of the life and they are the only members of the colony to survive through winter by entering a resting state ‘diapause’ (overwintering). Bodyweight is of prime importance for diapause survival. Few days after mating they go for the diapause and in the meantime they eat more and prepare themselves for the diapause and presumably this is the cause in the start of the overwintering bodyweight has been increase (Ghosh et al., 2017). Weight loss was observed during the overwintering as they utilize the fat during overwintering and when queen emerge from the overwintering in spring their bodyweight becomes less (Holm, 1972; Ghosh et al., 2017). Also in case of wasps like Vespa affinis and Vespula vulgaris (Hymenoptera: Vespidae) which has life cycle similar to bumblebee similar observation were made (Martin, 1993; Harris and Beggs, 1995).
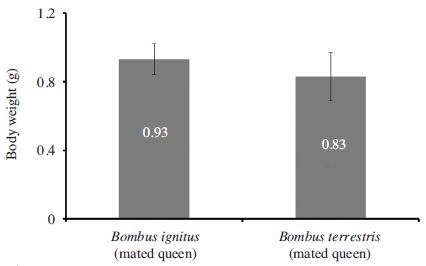
Body weight (g) of mated Bombus ignitus and B. terrestris queen. Data of mated Bombus terrestris queen was obtained from Ghosh et al. 2017.
The total fatty acids and thus lipid content of mated B. ignitus queen was found higher than that was reported for mated B. terrestris queen. However, the distribution of individual fatty acid was found similar (Table 1). In both cases, oleic acid was predominating followed by another monounsaturated palmitoleic acid. Votavova et al. (2015) also reported oleic acid as the most abundant fatty acid, however, followed by palmitic and myristic acid. Palmitic acid was found predominated among saturated fatty acids in the present study. Young queens after mating consume greater quantity of food which can be considered as preparatory stage for undergoing overwintering. In case of B. terrestris queen, monounsaturated fatty acid fraction was increased in the starting of the overwintering (Ghosh et al., 2017) and similar observation was found in another study where monounsaturated fatty acid level increased before hibernation at the expense of saturated analogues that are preferably consumed during winter (Votavová et al., 2015).
Diapause is a genetically programmed response occurring at a specific stage which is species specific. During diapause (overwintering) the metabolic pathways leading to cell proliferation and growth are down regulated while basic cellular maintenance remain operational at reduced rate and stress resistance pathways leading to cryoprotectant and heat shock protein synthesis etc. are likely to be up regulated (Denlinger et al., 2005). Moreover during this overwintering insect could not obtain water which could be generated from lipids and helps them to survive (Yocum et al., 2011; Wharton, 1985; Danks, 2000).High depletion of lipid was found in queen B. terrestris during overwintering which clearly explain that lipid reserve meet energy demands during overwintering period (Ellers and van Alphen, 2002; Votavova et al., 2015; Ghosh et al., 2017). Also the amount of lipid to be used during overwintering depends on overwintering temperature (Vesterlund et al., 2014).
Among polyunsaturated fatty acids (PUFAs) only linoleic acid was present in the lipid fraction of both species. Linoleic acid is involved in the biosynthesis of arachidonic acid (C20:4) which is the precursor of prostaglandins and other eicosanoids playing diverse functions including hormonal activities necessary for the development of insect (Stanley et al., 2002; Stanley and Kim, 2014). Also linoleic and other PUFAs have impact on the fecundity by ensuring fluidity of spermatozoa required for zygote formation in human (Wathes et al., 2007) although data is limiting in case of insect including bumblebee. Earlier studies showed that few insect species belonging to orders Orthoptera, Homoptera, Isoptera, and Neuroptera are capable of synthesizing linoleic acid (Stanley-Samuelson et al., 1988). However, recent study demonstrated Nasonia vitripennis (Hymenoptera: Pteromalidae) possesses △12 desaturase which makes the wasp capable of linoleic acid synthesis from oleic acid (Blaul et al., 2014). Hardly any report is available on bumblebee in this context. Report on worker honeybee Apis mellifera (Hymenoptera: Apidae) showed adult to contain linoleic acid (7.8%) in lipid fraction although it was not available in brood (larvae and pupae) (Ghosh et al., 2016). Presumably diet contamination could explain the obtained linoleic acid in the lipid fraction of bumblebees.
Similarly the total amino acids was found higher in B. ignitus queen in comparison to mated queen of B. terrestris and the distribution of individual amino acid was found similar in both species (Table 2). Glutamic acid was found predominated following alanine. Glutamate plays role as an excitatory neurotransmitter at the insect neuromuscular junction (Briley et al., 1981). It has also importance as icenucleating agent (INA) which is required for adopting cold hardiness strategies. INA from the freeze-tolerant hornet Vespula maculate (Hymenoptera: Vespidae) constituted 74 kDa hydrophilic protein found in haemolymph, in which almost 20% of the amino acids was found either glutamate or glutamine (Bale 2002; Duman and Patterson, 1978). During the preparative phase insect takes decision of entering into diapause accompanying with alterations which results sequestration of additional energy reserve, waterproofing agents etc. To cite few examples, prediapausing larvae of Diatraea grandiosella (Lepidoptera: Crambidae) were found to store an electrophoretically discrete protein fraction which is makes up 20% of the soluble proteins of the fat body of the newly diapaused individuals (Brown and Chippendale, 1978). In Colorado potato beetle Leptinotarsa decemlineata (Coleoptera: Chrysomelidae), diapausing adult female accumulate markedly greater quantity of hexamerin protein.First they accumulate a hexamerin protein in haemolymph and later in fat body often known as ‘diapause protein’ (Brown and Chppendale, 1978; de Kort and Koopmanschap, 1994; de Kort, 1990).
Body weight is of prime importance for the survival and it is influenced by body composition. The amount of calories, in turn diet is one of the most important factor to maintain the body weight besides the other factors like gender, stage of life cycle, activity level etc. The present study indicated the body weight and quantitative body fat (fatty acids) and protein (amino acids) differs between the two bumblebee species. To foster the population of native bumblebee i.e. B. ignitus through rearing, appropriation of feed formulation is essential and the study provides a basic understanding of the body composition which could lead the purpose of feed development for indigenous population.
Acknowledgments
The authors are thankful to Gyeongbuk Bumblebee project (2017) to CJ and research fund to support SG. Some part of nutritional analyses was done with the instruments of Insect industry R&D Center (GB Pollinator) in ANU.
References
- AOAC, (1990), Official methods of analysis, 15th ed., Association of Official Analytical Chemists, Washington DC, USA.
-
Bale, J. S., (2002), Insects and low temperatures: from molecular biology to distributions and abundance, Phil. Trans. R. Soc. Lond. B, 357, p849-862.
[https://doi.org/10.1098/rstb.2002.1074]

-
Beekman, M., P. van Stratum, and R. Lingeman, (1998), Diapause survival and post-diapause performance in bumblebee queens (Bombus terrestris), Entomol. Exp. Appl, 89, p207-214.
[https://doi.org/10.1046/j.1570-7458.1998.00401.x]

-
Blaul, B., R. Steinbauer, P. Merkl, R. Merkl, and H. Tschochner, (2014), Oleic acid is a precursor of linoleic acid and the male sex pheromone in Nasoniavitripennis, Insect Biochem. Mol. Biol, 51, p33-40.
[https://doi.org/10.1016/j.ibmb.2014.05.007]

-
Briley, P. A., M.T. Filbin, G. G. Lunt, and P. D. Turner, (1981), Glutamate receptor binding in insects and mammals, Mol. Cell Biochem, 39, p347-356.
[https://doi.org/10.1007/bf00232584]

-
Brown, J. J., and G. M. Chippendale, (1978), Juvenile hormone and a protein associated with the larval diapause of the southwestern corn borer, Diatraeagrandiosella, Insect Biochem, 8, p359-367.
[https://doi.org/10.1016/0020-1790(78)90022-7]

-
Danks, H. V., (2000), Dehydration in dormant insects, J. Insect Physiol, 46, p837-852.
[https://doi.org/10.1016/s0022-1910(99)00204-8]

- deKort, C.A.D., and A.B. Koopmanschap, (1994), Nucleotide and deduced amino acid sequence of a cDNA clone encoding Diapause Protein1, an arylphorin-type storage hexamer of the Colorado potato beetle, J. Insect Physiol, 40, p527-535.
- deKort, C.A.D., (1990), Thirty-five years of diapause research with the Colorado potato beetle, Entomol. Exp. Appl, 56, p1-13.
-
Denlinger, D. L., G. D. Yocum, and J. L. Rinehart, (2005), Hormonal control of diapause, p615-650, In Comprehensive Molecular Insect Science, eds. by L.I. Gilbert, K. Iatrou, and S.S. Gill, 3rd vol.@, Elsevier Press, Amsterdam.
[https://doi.org/10.1016/b0-44-451924-6/00043-0]

-
Duman, J. G., and J. L. Patterson, (1978), The role of ice nucleators in the frost tolerance of overwintering queens of the bald faced hornet, Comp. Biochem. Physiol. A, 59, p69-72.
[https://doi.org/10.1016/0300-9629(78)90308-0]

-
Ellers, J., and J. M. van Alphen, (2002), A trade-off between diapause duration and fitness in female parasitoids, Ecol. Entomol, 27, p279-284.
[https://doi.org/10.1046/j.1365-2311.2002.00421.x]

-
Ghosh, S., K. Choi, S. Kim, and C. Jung, (2017), Body compositional changes of fatty acid and amino acid from the queen of Bumblebee, Bombus terrestris during overwintering, J. Apic, 32, p11-18.
[https://doi.org/10.17519/apiculture.2017.04.32.1.11]

-
Ghosh, S., C. Jung, and V.B. MeyerRochow, (2016), Nutritional value and chemical composition of larvae, pupae and adults of worker honey bee, Apis mellifera ligustica as a sustainable food source, J. Asia Pac. Entomol, 19, p487-495.
[https://doi.org/10.1016/j.aspen.2016.03.008]

- Goulson, D., (2003), Effects of introduced bees on native ecosystems.Annu, Rev. Ecol. Evol. Syst, 34, p1-26.
- Goulson, D., (2010), Bumblebees: behaviour, ecology and conservation, 2nd Edition, Oxford University Press, New York.
-
Hagedorn, H. H., and F. E. Moeller, (1968), Effect of the age of pollen used in pollen supplements on their nutritive value for the honeybee. I. Effect on thoracic weight, development of hypopharyngeal glands and brood rearing, J. Apic. Res, 7, p89-95.
[https://doi.org/10.1080/00218839.1968.11100195]

- Harris, R. J., and J. R. Beggs, (1995), Variation in the quality of Vespa vulgaris (L.) queens (Hymenoptera: Vespidae) and its significance in wasp population dynamics, New Zeal. J. Zool, 22, p131-142.
- Hersch, M. I., R. M. Crewe, H. R. Hepburn, P. R. Thompson, and N. Savage, (1978), Sequential development of glycolytic competence in muscles of worker honeybee, Comp. Biochem. Physiol. B, 61, p427-431.
-
Holm, S. N., (1972), Weight and life length of hibernating bumblebee queens (Hymenoptera, Bombidae) under controlled condition, Ent. Scand, 3, p313-320.
[https://doi.org/10.1163/187631272x00184]

-
Inari, N., T. Nagamitsu, T. Kenta, K. Goka, and T. Hiura, (2005), Spatial and temporal pattern of introduced Bombus terrestris abundance in Hokkaido, Japan and its potential impact on native bumblebees, Popul. Ecol, 47, p77-82.
[https://doi.org/10.1007/s10144-004-0205-9]

- Katayama, E., (1971), Observations on the brood development in Bombus ignites (Hymenoptera, Apidae) I. Egg laying habits of queens and workers, Kontyu, 39, p189-203.
- Katayama, E., (1973), Observations on the brood development in Bombus ignitus (Hymenoptera, Apidae) II. Brood development and feeding habits, Kontyu, 41, p203-216.
- Korean Food Standard Codex, (2010), Ministry of Food and Drug Safety, Republic of Korea.
- Lococq, T., C. Audrey, D. Michez, N. Brasero, and J-V. Rasplus, (2016), The alien’s identity: consequences of taxonomic status for the international bumblebee trade regulations, Biol. Conserv, 195, p169-176.
-
Martin, S. J., (1993), Weight changes in adult hornets, Vespa affinis (Hymenoptera: Vespidae), Ins. Soc, 40, p363-368.
[https://doi.org/10.1007/bf01253899]

-
Murray, T. E., M. F. Coffey, E. Kohoe, and F. G. Horgan, (2013), Pathogen prevalence in commercially reared bumblebees and evidence of spillover in conspecific populations, Biol. Conserv, 159, p269-276.
[https://doi.org/10.1016/j.biocon.2012.10.021]

- Ono, M., M. Mitsuhata, and M. Sasaki, (1994), Use of introduced Bombus terrestris worker helpers for rapid development of Japanese native B. hypocrite colonies (Hymenoptera, Apidae), Appl. Entomol. Zool, 29, p413-419.
- Ptacek, V., (1985), Testing three methods of bumble bee rearing, Sb. Ved. Prac. VSUP Troubsko, 9, p59-67, (in Czech with English summary).
- Ptacek, V., (1991), Trials to rear bumble bees, ActaHortic, 288, p144-148.
- Ptacek, V., (2001), Some biological aspects of bumble bee (Bombus, Hymenoptera) management, Acta Hortic, 561, p279-286.
- Roseler, P-F., (1977), Rearing bumblebee colonies, Proc. 8th Int. Congr. IUSSI, Wageningen, 312.
- Sladen, F.W.L., (1912), The humble-bee.304p, MacMillan, London.
-
Stanley, D. W., A. R. N. Aliza, H. Tunaz, S.M. Putnam, Y. Park, and J. C. Bedick, (2002), Eicosanoids in insect biology, Neotrop. Entomol, 31, p341-350.
[https://doi.org/10.1590/s1519-566x2002000300001]

-
Stanley, D., and Y. Kim, (2014), Eicosanoid signalling in insects: from discovery to plant protection, Crit. Rev. Plant Sci, 33, p20-63.
[https://doi.org/10.1080/07352689.2014.847631]

-
Stanley-Samuelson, D. W., R. A. Jurenka, C. Cripps, G.J. Blomquist, and M. de Renobales, (1988), Fatty acids in insects: composition, metabolism and biological significance, Arch. Insect Biochem. Physiol, 9, p1-33.
[https://doi.org/10.1002/arch.940090102]

- Tasei, J-N, and P. Aupinel, (1994), Effect of photoperiodinc regimens on the oviposition of artificially overwintering Bombus terrestris L. queens and the reproduction of sexuals, J. Apic. Res, 33, p27-33.
- vanHeemert, C., A. de Ruijter, E. J. van den, and S. J. van der, (1990), Year-round production of bumble bee colonies for crop pollination, Bee World, 71, p54-56.
- van den Eijinde, J., R. A. de, and S.J. van der, (1991), Method for rearing Bombus terrestris continuously and the production of bumblebee colonies for pollination purposes, ActaHortic, 288, p154-158.
-
Velthuis, H. H. W., and A. V. Doorn, (2006), A century of advances in bumblebee domestication and the economic and environmental aspects of its commercialization for pollination, Apidologie, 37, p421-451.
[https://doi.org/10.1051/apido:2006019]

-
Vesterlund, S. R., T. M. Lilley, T. van Ooik, and J. Sorvari, (2014), The effect of overwintering temperature on the body energy reserves and phenoloxidase activity of bumblebee Bombus lucorum queens, Insestes Soc, 61, p265-272.
[https://doi.org/10.1007/s00040-014-0351-9]

- Votavová, A., A. Tomcala, E. Kofronova, M. Kudzejova, J. Sobotnik, P. Jiros, O. Komzakova, and I. Valterova, (2015), Seasonal dynamics in the chemistry and structure of the fat bodies of bumblebee queens, PLoSONE, 10, e0142261.
-
Wathes, D. C., D. R. E. Abayasekara, and R. J. Aitken, (2007), Polyunsaturated fatty acids in male and female reproduction, Biol. Reprod, 77, p190-201.
[https://doi.org/10.1095/biolreprod.107.060558]

-
Wharton, G. W., (1985), Water balance in insects, p565-601, in Comprehensive Molecular Insect Science vol. 4, eds., L.I. Gilbert By, K. Iatrou, and S.S. Gill, Elsevier, Amsterdam.
[https://doi.org/10.1016/b978-0-08-030805-0.50020-1]

- Yocum, G. D., J. S. Buckner, and C. L. Fatland, (2011), A comparison of internal and external lipids of nondiapausing and diapausing initiation phase adult Colorado Potato beetles, Leptinotarsadecemlineata, Comp. Biochem. Physiol. Part B, 159, p163-170.
-
Yoon, H-J., S. E. Kim, and Y. S. Kim, (2002), Temperature and humidity favourable for colony development of the indoor-reared bumblebee, Bombus ignitus, Appl. Entomol. Zool, 37, p419-423.
[https://doi.org/10.1303/aez.2002.419]

- Yoon, H-J., Y-I. Mah, M-Y. Lee, I-G. Park, and M. Bilinski, (1999), Ecological characteristics of Bombus ignitus Smith in Korea, Korean J. Appl. Entomol, 38, p101-107.
