
Comparative Study of Olfactory Learning and Memory in Apis cerana and Apis mellifera Foragers
Abstract
The honeybee is an important invertebrate model organism for reward on learning and memory research. Its value as a model organism in this area is rooted in its impressive capacity for learning and memory formation. Many lines of research have been reported on learning and memory in the last few decades. However, most research on learning and memory in honeybees has been performed in the Western honeybee, Apis mellifera. Therefore, the cognitive capabilities in the Eastern honeybee, Apis cerana remain obscure, despite their biological and economical importance. In order to understand the differences of learning and memory performance in the two species of honeybees, we investigated classical olfactory conditioning according to an appetitive Pavlovian conditioning paradigm based on the olfactory conditioning of the proboscis extension response (PER). Our present study demonstrated that learning and memory performance was different between two honeybee species. During the acquisition phase, there was no statistical difference between two species. In the retention phase, A. cerana was significantly better on olfactory learning and memory than that of A. mellifera after 1 hour. On the other hand, A. mellifera showed higher learning scores than A. cerana after 24 hours. These findings extend our understandings of mechanisms underlying learning and memory capabilities, which is an important basis for the further study of behavioral responses to various ecological and biological signals in two closely related honeybee species.
Keywords:
Learning and memory, Apis mellifera, Apis cerana, Proboscis reflex extensionINTRODUCTION
The honeybee has long been served as an invertebrate model organism for learning and memory research, including social organization, division of labor, and a complex behavioral repertoire. (Menzel, 1990, 2001, 2012; Smith, 1991; Zhang et al., 2000). They have a good learning and memory ability to color (von Frisch, 1914), pattern (Horridge, 1996) and odor (Menzel et al., 1996) of targets. Moreover, the honeybee is not only able to form a concept of visual objects (Collett et al., 1983), discriminate landmarks (Giurfa et al., 2001), but it also forms long-term memory and optimizes the flight path based on specific conditions (Zhang et al., 1996).
Honeybees have a strong olfactory learning ability. They learn odor through olfactory receptor neurons localized on its antennae, and change these odor stimuli into electrical signals and finally transfer them into the mushroom body, a memory center of the insects (Laska et al., 1999). Since the proboscis extension response (PER) experiment was carried out for a learning paradigm in the honeybee by pairing an odor stimulus with a sucrose reward (Takeda et al., 1961), honeybees turned out to be a good learner in the association between an initially olfactory stimulus (the conditioned stimulus, CS), and a sucrose stimulus (the unconditioned stimulus, US). Once the association between the olfactory CS and the rewarding US has been learned, the CS alone elicits the PER. This method has been gradually improved as a classical experiment model for olfactory learning and memory (Gerber et al., 1999; Smith et al., 1994). However, our knowledge of olfactory learning and memory in honeybees predominantly stems from the studies on Apis mellifera, and as a result, there is little experimental evidence to olfactory learning and memory in other honeybee species. Some research has recently started on Asian bees as a visual and olfactory learning (Qin et al., 2012; Wang et al., 2014). A previous study investigated that Apis cerana workers can be used to study learning applying the classic PER conditioning paradigm (Wang et al., 2014). However, differences of short- and mid-term learning and memory abilities between A. cerana and A. mellifera are still unknown.
Two honeybee species, A. mellifera and A. cerana have evolved in different ecological environments, so they have established distinct behavioral characterization in their society (Ruttner, 1988). In the majority of cases, no hybrids were observed even when colonies of both types were kept at the same locality, implying that the two bee species had allopatric speciation in evolution (Ruttner and Maul, 1983). A. mellifera has been commonly distributed in the world and introduced for high productivity of honey and royal jelly (Jianke et al., 2010). In contrast, A. cerana is a species that is bred locally in China and Korea and southeastern Asian counties, has stronger merits in resisting diseases, wasps, bee mites, and extreme climates, although it provided lower quantity of honey and royal jelly (Peng et al., 1987; Su et al., 2005). Recent studies have shown that geographical isolation and evolutionary divergence are occurred by representing between the two species in biological differences such as their ecosystems, chemosensation, and morphological structures (Buchler et al., 1992; Jung et al., 2014). The size and surface area of the antennae in A. cerana and A. mellifera workers appear to be identical, but the distributions of sensory hairs on the antennae are significantly different. Four classes of olfactory sensilla (placodea, trichodeum types A and B, basiconica) are significantly more abundant on the antennae of A. cerana worker than on that of A. mellifera worker. However, A. mellifera workers have a greater abundance of other sensilla, such as sensilla campaniformia, s. coeloconica, s. ampullaca, and s. chaetica, than A. cerana workers (Jung et al., 2014). Despite significant differences in olfactory-driven behaviors, preference to nectar sources, and foraging behaviors between the A. mellifera and A. cerana honeybees, little is known about the differences on olfactory learning ability in regards to their specific species.
In an attempt to contribute some baseline information about the A. cerana learning and memory behavior, and comparative olfactory learning performance in A. cerana and A. mellifera, this study was carried out by using a PER paradigm to investigate the olfactory learning and memory of these two honeybee species in Korea.
MATERIALS AND METHODS
Honeybees
Two honeybee species, A. mellifera and A. cerana, were maintained on apiaries of Incheon National University campus in Incheon, Korea. Before we initiated our experiments, we equalized the colonies such that each contained four frames covered with adult workers and at least two frames of brood and two frames of and pollen. For learning and memory, pollen-foraging honeybees of both species were directly captured near an entrance of the hives. For each experiment, we used 20 foraging honeybees from each species per learning trial. Also, we repeated this experiment four times with an additional colony. This experiment has been carried out between June and July 2017.
Learning paradigm: Proboscis extension reflex(PER)
Collected honeybees were harnessed and starved for overnight and trained in a standard paradigm of classical conditioning as described previously (Fig. 1, Menzel et al., 1973). Prior to learning behavioral experiments, the antenna of each bee was applied for 1M sucrose solution without feeding. If bees did not respond with a PER to first sucrose solution and could not extend their proboscis freely, they were discarded from the experiments. Linalool (CS) at 10-2 concentration (v/v) in mineral oil (Sigma-Aldrich, St. Louis, USA) as an olfactory cue was delivered to the honeybees by using a plastic pipette tip. Air velocity to the honeybees was about 1.5m/s consisting of an airstream carrier. The antennae were touched with a toothpick soaked in 1M sucrose solution (US), upon which the bees extended their proboscis to obtain the reward. When one bee was placed in front of the odor stimulator, the CS was presented to the bee for 5 sec. Three seconds after onset of the CS, the US was applied for the 2 sec to the antennae of the bee. Presented odors after stimulation into the arena were quickly removed with an exhauster connected with a vacuum. The bee was placed in the conditioning sets for 10 sec. Seven trials with 7 min interval were employed as training trials. During conditioning experiments, responses of the bee to the CS following trials were recorded (PER or no PER). Memory retention experiment was performed in bees 1 h and 24 h after the acquisition phase. The number of tested bees for this experiment of two species is 80 and 80 respectively.
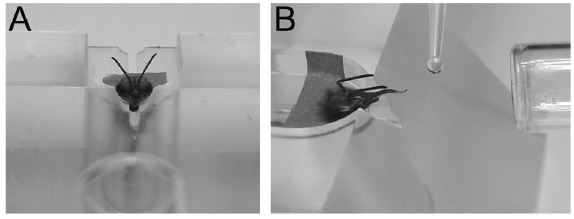
Experimental setup for learning performance of the honey bee. (A) Tethered bee (untreated control) used for the proboscis extension reflex (PER) paradigm. (B) Conditioning of the PER on restrained bees. The PER is a reflex shown by bees when their antennae are contacted with sucrose solution. During conditioning, an odor (CS) is presented in temporal association with sucrose solution to the antennae and to the proboscis (US), so that the odor progressively gains control over the PER.
Data analysis
All statistics were performed using SPSS version 20.0 (IBM, NY, USA) statistical software package version 20.0. Learning scores were recorded in dichotomous form because our response variable was yes/no (PER or no PER) during olfactory conditioning. Cochran’s Q test was used within group for comparing the performance index of acquisition trials. In addition, we used Mann-Whitney U test to compare acquisition success and memory retention between groups.
RESULTS AND DISCUSSION
Learning and memory
Using linalool as the CS during an associative learning paradigm revealed typical acquisition curves. Responses of two honeybee species to the CS increased during the following trials (Cochran Q test, Q=112.3(A. cerana), 121.9(A. mellifera), df=6, P<0.01, Fig. 2), suggesting that they learned to associate the CS with sucrose, which was played as a US. In A. mellifera, most of the learning happened by trial 3 and very little learning happened in trial 3-6. This is visible in the Fig. 2 and was verified by the non-significant difference in correct responses of trial 3 versus trial 7. However, the learning performance pattern of A. cerana increased steadily until trial 6. At trial 3, 75% of A. mellifera and 50% of A. cerana have learned the odor association as indicated by a proboscis extension, suggesting that A. cerana is a slow learner. Although the number of bees responding to the CS during trial 5 was different attendency, the percentage of correct responses between two species was similar at trial 7 (Mann-Whitney U test, Z=1.51, P=0.15, Fig. 2).
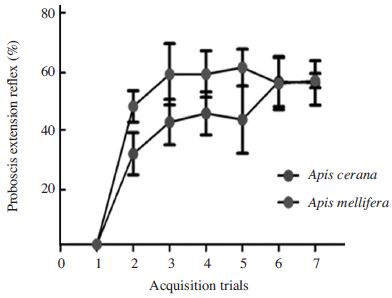
Comparison of olfactory learning abilities between A. mellifera and A. cerana. Both species of these honeybees perform similar to a learning assay (Mann-Whitney U test, P=0.15). However, A. mellifera (red, N=80) learned faster than A. cerana (blue, N=80) until third trial. Data represents percentage of bees of each species demonstrating a learned associative response to linalool for trials 1-7. Error bars depict mean±SE.
In addition to different memory acquisition between two species, capability of retention showed significantly different after 24 h. In contrast, memory retention 1 h after training was slightly different in two species, demonstrating 73 % PER in A. cerana and 62 % of PER in A. mellifera (Mann-Whitney U-test, Z=1.99, P<0.05). On the other hand, A. mellifera have better memory to remember the odor association after 24 h learning trials compared to A. cerana (32% of A. cerana versus 48% of A. mellifera, Mann-Whitney U-test, Z=2.11, P<0.05). This PER results indicated the retention ability in A. mellifera is significantly larger than A. cerana after 24 h training with linalool odors. Notably, A. cerana showed quick decrease of PER responses in 24 h after training (73% PER) compared to 1h (32% PER, Mann-Whitney U-test, Z=2.67, P<0.01), while PER performance in A. mellifera demonstrated 62% PER at 1h after training and 48% PER at 24h after training (Mann-Whitney U-test, Z=2.04, P<0.05). PER responses at 24h time point after training showed significantly different in two species (Fig. 3).
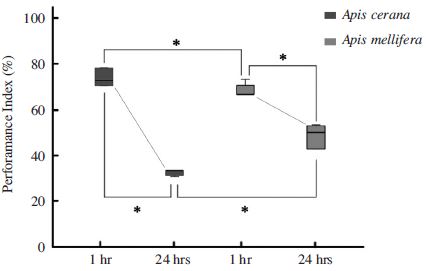
Retention tests (memory) of learned behaviors of two honeybee speices. Mean percent±SEM PER in olfactory memory retention 1 h and 24 h after training to associate odor between A. mellifera and A. cerana. A. mellifera (red box) is better at remembering the odor association 24 h after learning compared to A. cerana (Blue box). In contrast, A. cerana perform better in memory retention 1 h after training compared to A. mellifera (Mann-Whitney U test, P<0.05). Significance was determined by Mann-Whitney U tests (*=P<0.05). Error bars depict mean±SE.
Foragers of the honeybee have to fly several kilometersup to more than 10 kilometers away to collect pollen and nectar (Menzel et al., 1996). Thus, it is essential for honeybees to learn and remember not only the color, shape of flowers, and floral scents but also the location of food sources (Collins et al., 1983; Seeley 1985; Pahl et al., 2011). A variety of studies have examined the behavioral and physiological characterization of learning and memory in honeybees and have tried to compare the capacity for learning and memory formation between honeybee species (Giurfa and Sandoz, 2012). Previous works have been conducted in comparing visual learning of various contexts with other bee races (Menzel et al., 1973; Qin et al., 2012). They have reported the differences not only between species of the genus Apis, but also within the species: in a test between A. m. ligustica, A. mellifera lamarckii, A. mellifera carnica, and A. cerana. A. cerana outperformed the others, learning the quickest and reaching the highest level in horizontal and vertical color cross paradigms (Menzel et al., 1973). Another research showed that A. cerana has greater learning and memory capacity on both color and grating patterns than that of A. mellifera (Qin et al., 2012). However, little was known about the differences of olfactory learning and memory abilities between two species, A. mellifera and A. cerana. Comparing the ability of olfactory learning and memory of A. mellifera and A. cerana, the results of the present study showed that there were significant differences between A. cerana and A. mellifera in olfactory learning and memory performance. During acquisition phase, two species showed similar learning pattern and percentages of acquisition success. However, in memory retention phase, A. mellifera may have a stronger memory capability than A. cerana after 24 h (Fig. 3). A recent study has reported in comparison of learning ability and memory recall between two species (Wang et al., 2014). A. mellifera possess greater learning ability than that of A. cerana. However, the performances of memory retention were similar these two sibling species (Wang et al., 2014). These contradictory results compared to our results may be ascribed to many experimental parameters. The olfactory conditioning and memory retention rely on age of returning foragers, the state of satiation in individual bees and different kind of odorants (CS). In addition, we cannot exclude the possibility that honeybees used to experiments may have different genetic backgrounds to honeybee population of China.
If it is assumed that differences in learning abilities occur in response to the natural conditions under which animals live (Dukas 1998; Raine et al., 2008), some different ecological strategies that A. cerana adopt might make it less important for them to be good olfactory long term learners. A. mellifera foragers tend to fly long distances (median distance = 6.1km) to find flowers (Beekman et al., 2000). The flight distance of A. mellifera is farther than that of A. cerana (Shah et al., 1980). On their return, they must remember foraging site locations and resource properties, as well as the route back to the nest. This requires substantial associative learning and memory. There have been few molecular comparative studies on learning and memory between two species. In comparison of antennal proteome profiles (Woltedji et al., 2012), the exclusive expression of carbonic anhydrase in the antennae of the A. mellifera worker bee is probably associated with more cognitively demanding tasks performed by foragers because it plays important roles in synaptic plasticity and cognition as in spatial learning memory (Whitfield et al., 2003; Sun et al., 2002). More experiments are required to reveal the understanding of the differences in the learning abilities of the two species.
Acknowledgments
This work was mainly carried out with the support of Cooperative Research Program for Agriculture Science and Technology Development (Project Number: PJ012285) and this work also partly supported from Cooperative Research Program for Agriculture Science and Technology Development (Project Number: PJ012526) to KK and HWK. RI was supported from the postdoctoral fellowships from Incheon National University.
LITERATURE CITED
-
Beekman, M., and F. L. W. Ratnieks, (2000), Long-range foraging by the honeybee, Apis mellifera L, Funct. Ecol, 14, p490-496.
[https://doi.org/10.1046/j.1365-2435.2000.00443.x]

-
Bitterman, M. E., R. Menzel, A. Fietz, and S. Schafer, (1983), Classical conditioning of proboscis extension in honeybees (Apis mellifera), J. Comp. Psychol, 97, p107-119.
[https://doi.org/10.1037//0735-7036.97.2.107]

- Buchler, R., W. Drescher, and I. Tornier, (1992), Grooming behaviour of Apis cerana, Apis mellifera and Apis dorsata and its effect on the parasitic mites Varroa jacobsoni and Tropilaelaps clareae, Exp. Appl. Acarol, 16, p313-319.
-
Collett, T. S., and B. A. Cartwright, (1983), Eidetic images in insects: their role in navigation, Trends. Neurosci, 6, p101-105.
[https://doi.org/10.1016/0166-2236(83)90048-6]

-
Collins, A. M., and M. S. Blum, (1983), Alarm responses caused by newly identified compounds derived from the honeybee sting, J. Chem. Ecol, 9, p57-65.
[https://doi.org/10.1007/bf00987770]

-
Dukas, R., (1998), Cognitive Ecology: The evolutionary ecology of information processing and decision making, 1st ed., p430, University of Chicago Press.
[https://doi.org/10.1016/s1364-6613(98)01205-4]

- Gerber, B., and J. Ullrieh, (1999), No evidence for olfactory blocking in honeybee classical conditioning, J. Exp. Biol, 202, p1839-1854.
-
Giurfa, M., S. Zhang, A. Jenett, R. Menzel, and M. V. Srinivasan, (2001), The concepts of ‘sameness’ and ‘difference’ in an insect, Nature, 410, p930-932.
[https://doi.org/10.1038/35073582]

-
Giurfa, M., and J. C. Sandoz, (2012), Invertebrate learning and memory: Fifty years of olfactory conditioning of the proboscis extension response in honeybees, Learn. Mem, 19, p54-66.
[https://doi.org/10.1101/lm.024711.111]

-
Horridge, G. A., (1996), The honeybee (Apis mellifera) detects bilateral symmetry and discriminates its axis, J. Insect Physiol, 42, p755-764.
[https://doi.org/10.1016/0022-1910(96)00026-1]

-
Jianke, L., F. Mao, D. Begna, F. Yu, and Z. Aijuan, (2010), Proteome comparison of hypopharyngeal gland development between Italian and royal jelly producing worker honeybees (Apis mellifera L.), J. Proteome Res, 9, p6578-6594.
[https://doi.org/10.1021/pr100768t]

-
Jung, J. W., K. W. Park, H. W. Oh, and H. W. Kwon, (2014), Structural and functional differences in the antennal olfactory system of worker honeybees of Apis mellifera and Apis cerana, J. Asia-Pac. Entomol, 17, p639-646.
[https://doi.org/10.1016/j.aspen.2014.01.012]

-
Laska, M., C. Galizia, M. Giurfa, and R. Menzel, (1999), Olfactory diserimination ability and odor structureactivity relationships in honeybees, Chem. Senses, 24, p429-438.
[https://doi.org/10.1093/chemse/24.4.429]

- Menzel, R., (1990), Learning, memory, and “cognition” in honeybees, p237-292, In Neurobiology of comparative cognition R. P. Kesner eds. by, and D. S. Olton, 1st ed., p488Lawrence Erlbaum Associated, Hillsdale.
-
Menzel, R., (2001), Searching for the memory trace in a minibrain, the honeybee, Learn. Mem, 8, p53-62.
[https://doi.org/10.1101/lm.38801]

- Menzel, R., (2012), The honeybee as a model for understanding the basis of cognition, Nat. Rev. Neurosci, 11, p758-68.
-
Menzel, R., H. Freudel, and U. R?hl, (1973), Rassenspezifische Unterschiede im Lernverhalten der Honigbiene (Apis mellifera L.), Apidologie, 4, p1-24.
[https://doi.org/10.1051/apido:19730101]

-
Menzel, R., M. Hammer, U. Müller, and H. Rosenboom, (1996), Behavioral, neural and cellular components underlying olfactory learning in the honeybee, J. Physiol Paris, 90, p395-398.
[https://doi.org/10.1016/s0928-4257(97)87928-4]

-
Pahl, M., H. Zhu, J. Tautz, and S. Zhang, (2011), Large Scale Homing in Honeybees, Plos One, 6, e19669.
[https://doi.org/10.1371/journal.pone.0019669]

-
Peng, Y. S., Y. Fang, S. Xu, and L. Ge, (1987), The resistance mechanism of the Asian honey bee, Apis cerana Fabr., to an ectoparasitic mite, Varroa jacobsoni Oudemans, J. Invertebr. Pathol, 49, p54-60.
[https://doi.org/10.1016/0022-2011(87)90125-x]

-
Qin, Q-H., X-J. He, L-Q. Tian, S-W. Zhang, and Z-J. Zeng, (2012), Comparison of learning and memory of Apis cerana and Apis mellifera, J. Comp. Physiol. A, 198, p777-786.
[https://doi.org/10.1007/s00359-012-0747-9]

-
Raine, N. E., and L. Chittka, (2008), The correlation of learning speed and natural foraging success in bumble-bees, Proc. R. Soc. B. Biol. Sci, 275, p803-808.
[https://doi.org/10.1098/rspb.2007.1652]

-
Ruttner, F., (1988), Biogeography and taxonomy of honeybees, 1st ed., p284, Springer.
[https://doi.org/10.1007/978-3-642-72649-1]

-
Ruttner, F., and V. Maul, (1983), Experimental analysis of reperoductive interspecies isolation of Apis mellifera L. and Apis cerana Fabr, Apidologie, 14, p309-327.
[https://doi.org/10.1051/apido:19830405]

- Seeley, T. D., (1985), Honeybee ecology: a study of adaptation in social life, 1st ed., p201, Princeton University Press.
- Shah, F. A., and T. A. Shah, (1980), Flight range of Apis cerana from Kashmir, Indian Bee J, 42, p48.
- Smith, B. H., (1991), The olfactory memory of honeybee, Apis mellifera: I, Odorant modulation of short and inermediated-term memory after single trial conditioning. J. Exp. Biol, 161, p367-382.
- Smith, B. H., and S. Cobey, (1994), The olfactory memory of the honeybee, Apis mellifera, II. Blocking between odorants in binary mixtures. J. Exp. Biol, 195, p91-108.
-
Su, S., S. Albert, S. Chen, and B. Zhong, (2005), Molecular cloning and analysis of four cDNAs from the heads of Apis cerana cerana nurse honeybees coding for major royal jelly proteins, Apidologie, 36, p389-401.
[https://doi.org/10.1051/apido:2005026]

-
Sun, M. K., D. L. Alkon, (2002), Carbonic anhydrase gating of attention: memory therapy and enhancement, Trends Pharmacol. Sci, 23, p83-89.
[https://doi.org/10.1016/s0165-6147(02)01899-0]

-
Takeda, K., (1961), Classical conditioned response in the honeybee, J Insect Physiol, 6, p168-179.
[https://doi.org/10.1016/0022-1910(61)90060-9]

- Von Frisch, K., (1914), Der Farbensinn und Formensinn der Biene, Fischer, Jena.
-
Wang, Z., K. Tan, (2014), Comparative analysis of olfactory learning of Apis cerana and Apis mellifera, Apidologie, 45, p45-52.
[https://doi.org/10.1007/s13592-013-0228-3]

-
Whitfield, C. W., A. M. Cziko, and G. E. Robinson, (2003), Gene expression profiles in the brain predict behavior in individual honeybees, Science, 302, p296-299.
[https://doi.org/10.1126/science.1086807]

-
Woltedji, D., F. Song, L. Zhang, A. Gala, B. Han, M. Feng, Y. Fang, and J. Li, (2012), Western honeybee drones and workers (Apis mellifera ligustica) have different olfactory mechanisms than eastern honeybees (Apis cerana cerana), J. Proteome Res, 11, p4526-4540.
[https://doi.org/10.1021/pr300298w]

-
Zhang, S., A. Mizutani, and M. Srinivasan, (2000), Maze navigation by honeybees: learning path regularity, Learn. Mem, 7, p364-374.
[https://doi.org/10.1101/lm.32900]

- Zhang, S. W., K. Bartsch, and M. V. Srinivasan, (1996), Maze learning by honeybee, Neurobiol. Learn. Mem, 66, p267-282.