
Pollination Efficiency of Honeybees (Apis mellifera L.) and Bumblebees (Bombus terrestris L.) in Different Cultivars of Asian Pear (Pyrus pyrifolia Nakai)
Abstract
Various cultivars of Asian pears Pyrus pyrifolia Nakai) have been cultivated in Korea. Because foraging behaviors of insect pollinators for nectar and pollen determine the fruit production, we investigated comparative pollination efficiency of the honeybee (Apis mellifera L.) and bumblebee (Bombus terrestris L.) on pear cultivars, including ‘Niitaka’, ‘Gamcheonbae’, ‘Wonhwang’, ‘Whasan’, and ‘Whangkeumbae’, that are grown in Korea. The foraging rates and time spent on the flower of honeybee and bumblebee were significantly different among cultivars. The foraging rate of the honeybee was highest in the vars. Hwasan, followed by the Whangkeumbae, Manpungbae, and Niitaka; whereas that of the bumblebee was highest in the vars. Hwasan, followed by the Manpungbae, Niitaka, and Whangkeumbae. In particular, the foraging rate preference of the honeybee was 1/3 in the Niitaka, which was lower than that of the other cultivars. Honeybee spent the longest time in flower of Hwasan, followed by the Niitaka, Whangkeumbae, and Manpungbae; whereas bumblebee spent longest time in flower of Hwasan, followed by the Niitaka, Manpungbae, and Whangkeumbae. Bumblebee showed a 2.8-fold higher rate of foraging in the pollen-producing cultivars than in the non-pollen cultivars. Fruit set by the honeybee and bumblebee was similar in most of the cultivars, except in Niitaka. Fruit set by artificial pollination was more effective than that by bee pollination in the Niitaka. However, fruit set by bee pollination was similar to that by artificial pollination in other cultivars.In fruit quality from each cultivar was not different from different pollination treatment. Therefore, it is considered that the pollination method using honeybees and bumblebees is a good option instead of the general artificial pollination in various Asian pear cultivar.
Keywords:
Honeybee, Bumblebee, Pear, Pollination, Foraging behavior, CultivarINTRODUCTION
Pears (Rosaceae, Pomoideae, Pyrus) are commercially important fruits in the world, including Korea. In Korea, it represents the fifth most-grown fruit crop, after apples, persimmons, citrus fruits, grapes (Statistics Korea, 2016). Like apples, pears are self-incompatible crop and must be pollinated by different genotypes (i.e., pollinizers) for fruit set (Cho et al., 2007; RDA, 2016), and their entomophilous flowers require insect pollinators to transfer pollen (Free, 1993). However, the majority of Korean pear cultivators grow cultivars, like ‘Niitaka’ because of consumers preference and contributing about 81.5% of the total pear cultivation area in Korea, thereby resulting in a paucity of pollinizers. On the other hand, the combination of environmental pollution and pesticide overuse has drastically reduced the density of natural insect pollinators (Potts et al., 2010). Climate change has also shortened the flowering period of pear, which has further exacerbated pollination and fruit set (Jang et al., 2002; Seo and Park, 2003), and as a result pear crops to be pollinated by artificial means instead of natural pollinators which becomes more common in Korea (Kim et al., 2003). Indeed, artificial pollination can facilitate suitable fruit set when pollinizers are limited or absent. However, the uneven germination rate of artificially pollinated pears and the prevalent of adverse weather, such as low temperature and rainfall during pear flowering limit the efficiency of artificial pollination, and increase labor requirements, thereby increasing farm management costs (Lee, 2014; RDA, 2016). Tanaka et al. (2007) reported that artificial pollination accounts for 40% of the annual working time associated with pear cultivation. Therefore, it would be highly economic to replace artificial pollination with commercial natural insect pollinators such as Apis mellifera or Bombus terrestris.
Pear crops in Europe typically rely on natural insect pollination, rather than artificial pollination, owing to the pollen-rich nature of Western pear cultivars (Mayer, 1994; Van den Eijnde, 1995), and when required for efficient fruit set and breeding, growers introduce A. mellifera and B. terrestris (Farkas and Orosz-Kovz-K, 2002; Webster, 2002). In Japan, oriental pear crop is pollinated either artificially or by bees (Itai, 2007), and in China, pear crops are largely dependent on artificial pollination (Wu et al., 2011). In Korea, about 79% of pear crops are pollinated artificially (Lee et al., 2016), whereas only 4% are pollinated by commercial insect pollinators (Yoon et al., 2017).
Furthermore, artificial pollination is even more common in Asian pear because of less attractiveness of flowers to bees than other flower sources (Delaplane and Mayer, 2000). The foraging preferences of A. mellifera and B. terrestris for nectar and pollen depend on sugar and protein content, respectively (Graham, 1993; Konzmann and Lunau, 2014). Benedek and Ruff (1998) reported that the foraging behavior of bees on 13 pear cultivars was assoiated with sugar content of nectar. In addition, the cultivar preference of A. mellifera and B. terrestris depend on pollen tube growth (Jacquemart et al., 2006). Before commercially use and adopt commercially available insects to pollinate pear crops, it is necessary to conduct a comparative study of pollination effect of insect pollinators on the pear cultivars, including ‘Niitaka’, ‘Gamcheonbae’, ‘Wonhwang’, ‘Whasan’, and ‘Whangkeumbae’, those are grown in Korea. Accordingly, the present study was conducted in order to compare the foraging behavior and pollination efficiency of A. mellifera and B. terrestris on several pear cultivars that are cultivated in Korea.
MATERIALS AND METHODS
Insect and pear materials
The study was conducted from April 12 to 14, 2016, at the National Institute of Horticultural Science in Naju (Jeonnam Province, Korea) and the cultivars included in the study were ‘Niitaka’, ‘Manpungbae’, ‘Whasan’ and ‘Whangkeumbae’. Bombus terrestris was used in 10 generations, following the artificial rearing conditions (26°C, RH 80% of the Insect Industry Division, Agricultural Biology Department, National Institute of Agricultural Science and Technology, and A. mellifera (Italian yellow) were purchased from a beekeeper. The pear trees included were 12-year-old, and the full-bloom day of the target cultivars were April 9 (‘Niitaka’), 11 (‘Manpungbae’), 12 (‘Whasan’), and 16 (‘Whangkeumbae’). The meteorological information during the full-bloom day in the Naju area was measured using the Rural Development Agency's Agricultural Weather Information Service (http://weather.rda.go.kr; Table 1).
Study sites
The study site included net screen house and open field. In the net screen house, we selected two sites of 540m2 each included 8 trees of each cultivar ‘Niitaka’, ‘Manpungbae’, and ‘Whasan’. The entire experiment was enclosed with 2mm×2mm mesh. Both A. mellifera (2 flames, over 2,000 bees/colony) and B. terrestris (100 bees/colony) were placed at each study site. At each site, artificial pollination and control were added, and the bee colonies were kept 4 days before the full-bloom day. Pear pollen was collected from ‘Cuwhang’ cultivar for artificial pollination, and the collected pollen was mixed with pollen extender (Seoksongja; Korea Agriculture Materials, Gwangju, Korea) at a 1 : 3 ratio and artificial pollination was conducted on three cultivars (‘Niitaka’, ‘Manpungbae’, and ‘Whasan’) of each pear tree in each experiment site before bee colonies were installed. Immediately after and the artificial pollination, trees were covered with 1mm×1mm mesh to prevent further pollination by insects (A. mellifera and B. terrestris). The non-treatment area included one tree of each cultivar.
In the open field, we selected two study sites of 4,000m2 each included 24 trees of each cultivars ‘Niitaka’, ‘Manpungbae’, and ‘Whangkeumbae’. Both A. mellifera (5 flames, over 10,000 worker bees/colony) and B. terrestris (5th generation, 200 worker bees/colony×5) were introduced to each study site. In order to reduce the interference of insects (A. mellifera and B. terrestris), the sites were established at a distance of > 1km. Otherwise, the methods were identical to those used in the net screen house.
Effect of cultivar on bee foraging behavior
In order to compare the foraging behavior of A. mellifera and B. terrestris on different pear cultivars, the numbers of foraging worker bees of A. mellifera and B. terrestris were counted for 5 minutes hourly from 10 am to 7 pm during April 12 to 14, when the pear cultivars were in full bloom. The numbers of foraging bees were counted in all 8 trees of each cultivar in the net screen house and in 24 trees for each cultivar in the open field, and for each time interval, foraging rates were calculated as the percentages of total worker bees active on each cultivar. Using the same hourly time intervals, we also compared the time spent staying in the flowers as the time spent collecting pollen or nectar from one flower by one worker and the time taken to move from one flower to another.
Effect of pollen availability on bees foraging behavior
In order to determine whether pollen production affects bee activity, we compared the foraging behavior of bees on pollen-producing cultivars such as ‘Manpungbae’ and ‘Whasan’, (i.e., ‘Fertile’ group), and ‘Niitaka’ and ‘Whangkeumbae’ (i.e., ‘Sterile’ group). More specifically, we compared the ratio of A. mellifera and B. terrestris foraging behavior, time spent on flowers, and visiting time from flower to another flower in each group.
Effect of pollination method on fruit set
The rate of fruit set was surveyed on May 3 and 4, when flower fertilization could be determined definitively. The rate of fruit set was measured in three trees from the beepollinated, artificially pollinated, and non-pollinated groups, respectively. The rate of fruit set was calculated as the percentage of flowers that were pollinated on 20 randomly selected branches per tree. Meanwhile, the rate of fruit set in flower clusters was calculated as the percentage of flower clusters in each tree that contained at least one pollinated flower, following Lee et al. (2010).
Effect of pollination method on fruit quality
Fruit quality was determined by randomly selecting 30 fruits from each cultivar and experiment by measuring the weight, firmness, length, diameter, seed number (‘Whangkeumbae’ excluded), soluble solids, and titratable acidity of five of the fruits, as described previously (Lee et al. 2015). The weight of fruits was weighed using an electronic balance (AND, CB-3000, Seoul, Korea) and length and diameter were measured using Vernier calipers (Mistutoyo, 500-181, Kawasaki, Japan). Fruit shape index and malformed index were expressed as percentage of fruits with an length/diameter (L/D) value over 0.87 or higher and with a difference of 5mm or more between right and left sides, respectively. Meanwhile, firmness was evaluated using the 8-mm measuring rod of a TMS-Pro (Food Technology, Sterling, VA, USA), and the vertical maximum pressure was measured on the fruit's equatorial plane (5 mm sample move, 100 mm min-1). Next, juice was extracted from the flesh of the point of the median equatorial plane of fruit using cheesecloth, and the concentration of soluble solids in the juice samples was measured using a digital sugar meter (PR-32α, Atago, Tokyo, Japan).Juice was extracted from five fruits of each treatment group, 5ml of each juice sample was diluted with 35ml distilled water, each sample’s pH was neutralized to 8.3 using 0.1 N NaOH, and titratable acidity was determined using malic acid.
Statistical analysis
The t-test was used to compare the foraging behavior, time spent on flowers, and time spent moving from flower to another flower of A. mellifera and B. terrestris, and the effects of pear cultivars were analysed using one-way ANOVA. The foraging behavior of A. mellifera and B. terrestris on the ‘Fertile’ and ‘Sterile’ cultivar groups was also analyzed using the t-test, and effects of pollination method on fruit quality were analysed using one-way ANOVA. Furthermore, both fruit shape and the percentage of malformed fruit were compared using the chi-square test. Significant differences identified by ANOVA and the Tukey’s test was used for post-hoc analysis. All statistical analyses were performed using the SPSS PASW 22.0 package for Windows (IBM, Chicago, IL, USA).
RESULTS
Effect of cultivar on bee foraging behavior
On the ‘Niitaka’ trees in the net screen house, the average foraging rate of A. mellifera was 18.6±2.8%, which was five times higher than that of B. terrestris (3.7 ±0.5%) (t-test: t(4)=9.062, p=0.001; Table 3). However, there was no significant difference in foraging rates of A. mellifera and B. terrestris on either the ‘Manpungbae’ and ‘Whasan’ trees in the net screen house or on any of the cultivars in the open field (Table 2).

The foraging rate (mean±SD%) of A. mellifera and B. terrestris on the flowers of different pear cultivars in the net screen house

The time spent (mean±SD) on the flowers of different pear cultivars by A. mellifera and B. terrestris
Furthermore, in the net screen house, A. mellifera exhibited the highest foraging rate (47.0±8.4%) on the ‘Whasan’ trees, followed by the ‘Manpungbae’ and ‘Niitaka’ trees (ANOVA: F(2,6)=10.944, p=0.010; Table 3), respectively. The foraging rate of the A. mellifera on the ‘Whasan’ trees was 2.5 times greater than that on the ‘Niitaka’ trees. B. terrestris exhibited high foraging rates on both the ‘Whasan’ and ‘Manpungbae’ trees (49.7 and 46.6%, respectively), 12~13 times higher than the foraging rate observed for ‘Niitaka’ trees (ANOVA F(2,6)=5.820, p=0.039). However, there were no significant differences between the foraging rates of either bee species among cultivars in the open field (p>0.05; Table 2).
A. mellifera spent the longest time as 4.7±1.0 seconds on ‘Whasan’ flowers, followed by ‘Niitaka’ (4.2±0.8 s), ‘Whangkeumbae’ (3.7±3.0 s) and ‘Manpungbae’ (2.9±0.8 s) (welch’s ANOVA F(3,14.947)=7.099, p=0.003). B. terrestris spent the longest time 4.3±2.5 s and 4.2±1.7 s on ‘Whasan’ and ‘Niitaka’ flowers, respectively, followed by ‘Manpungbae’ (3.0±1.1 s) and ‘Whangkeumbae’ (1.2 ±0.7 s) (F(3,23)=4.836, p=0.009). Furthermore, A. mellifera spent 3.7±3.0 s on ‘Whangkeumbae’ flowers, which was 2.5 s longer than the time spent of B. terrestris (t-test: t(13)=2.132, p=0.05). However, there were no significant differences in the time spent between A. mellifera and B. terrestris on flowers of any of the other cultivars (Table 3).
In regard to the time spent from flower to another flower, time spent by A. mellifera was not statistically different among cultivars (p>0.05). B. terrestris spent slightly less time (2.2 s) in ‘Manpungbae’ than in the other cultivars without statistical differences. In addition, there were also no significant differences between the time spent from flower to flower by A. mellifera and B. terrestris for any of the cultivars (p>0.05; Table 4).
Effect of pollen availability on bees foraging behavior
The foraging rate of A. mellifera on ‘Fertile’ flowers 39.5±11.9%, which was 1.4 times greater than that observed for the species on ‘Sterile’ flowers; however, the difference was insignificant (p>0.05) (Fig. 1A). In contrast, significant difference was observed in the foraging rate of B. terrestris on the ‘Fertile’ cultivars was 47.8±17.2%, which was 2.9 times greater than that observed on ‘Sterile’ cultivars (t-test: t(13)=3.149, p=0.008; Fig. 1B). A. mellifera also spent a similar amount of time foraging on ‘Fertile’ and ‘Sterile’ flowers (3.9±2.1 s and 3.7±1.3 s, respectively) (p>0.05; Fig. 2-A), whereas B. terrestris spent more time on ‘Fertile’ flowers (3.7±2.0 s ) than on ‘Sterile’ flowers (2.8±2.0 s), although no significant difference was observed (p>0.05; Fig. 2B). Furthermore, A. mellifera spent about 1 s longer foraging on ‘Sterile’ flowers than B. terrestris. A. mellifera spent 2.6±0.6 s, 0.4 s longer from flower to flower when foraging on ‘Fertile’ flowers than on ‘Sterile’ flowers, and B. terrestris spent slightly longer from flower to flower when foraging on ‘Fertile’ flowers (2.6±0.9 s) than when foraging on ‘Sterile’ flowers (2.9±1.9 s); however, none of these differences were significant(p>0.05; Fig. 3). Thus, pollen availability affected the preference of A. mellifera or B. terrestris for flowers, but did not have a great influence on either time spent on flower.
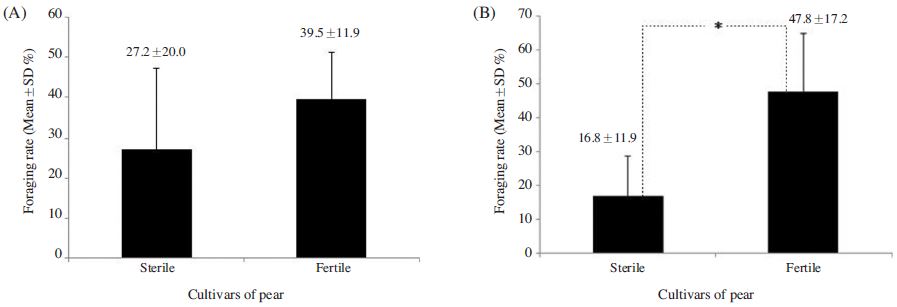
Comparison of the foraging rate of Apis mellifera (A) and Bombus terrestris (B) between the sterile and fertile pollens of the pear cultivars. * indicates significant difference at p<0.05 (t-test) between treatment plots.
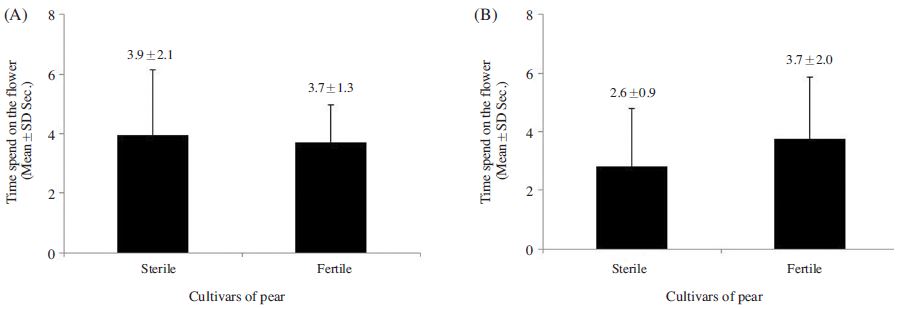
Comparison of the time spent by Apis mellifera (A) and Bombus terrestris (B) between the sterile and fertile pollens of the flower of the pear cultivars. There was no significant difference between the sterile and fertile pollens of the pear cultivars (t-test p>0.05).
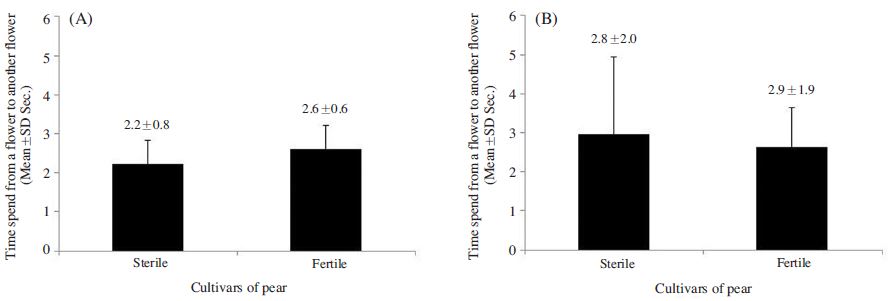
Comparison of the time spent from a flower to another flower by Apis mellifera (A) and Bombus terrestris (B) between the sterile and fertile pollens of pear cultivars. A. mellifera and B. terrestris did not differ significantly between the sterile and fertile pollens of the pear cultivars (t-test p>0.05).
Effect of pollination method on fruit set
In the net screen house, artificial pollination yielded higher fruit set in the ‘Niitaka’ cultivar (65.4±6.1%) than pollination by either A. mellifera or B. terrestris (ANOVA F(3,8)=95.501, p=0.0001; Table 5). In ‘Manpungbae’, pollination by A. mellifera yielded the greatest fruit set (41.3±6.6%), followed by B. terrestris, artificial pollination, and non-pollination, respectively, and the differences were significant (ANOVA F(2,8)=52.609, p=0.0001). In ‘Whasan’, artificial pollination yielded a fruit set that was two times greater than achieved through pollination by either bee species (F(2,8)=26.654, p=0.0001). In terms of flower cluster fruit set in the net screen house (Table 6), artificial, A. mellifera, and B. terrestris pollination yielded similar levels of fruit set: 88~100% in ‘Niitaka’ (F(2,6)=1.000, p=0.422), 93~100% in ‘Manpungbae’ (F(2,6)=0.912, p=0.451), and 81~97% in ‘Whasan’ (F(2,6)=1.566, p=0.284). However, the notreatment group set no fruit at all.

The percentage (mean±SD) of the fruit set in different pear cultivars pollinated by artificial, A. mellifera and B. terrestris in the net screen house

The percentage (mean±SD) of the fruit set per flower clusters in different pear cultivars pollinated by artificially, A. mellifera B. terrestris in the net screen house
In the open field, artificial pollination yielded a fruit set (54.3±3.2%) in the ‘Niitaka’ cultivar that was greater than that achieved through pollination by either bee species and that was about four times higher than the fruit set achieved by bee pollination (ANOVA F(3,8)=89.652, p=0.0001). In ‘Manpungbae’, artificial pollination yielded a fruit set (35.8 ±4.8%) that was 1.9~2.8 times greater than that achieved through bee pollination(ANOVA F(2,8)=37.042, p=0.0001), and in ‘Whangkeumbae’, artificial pollination yielded a fruit set (50.3±5.3%) that was 2.1~2.9 times greater than that achieved through bee pollination (ANOVA F(2, 8)=111.471, p=0.0001; Table 7).

The fruit set (mean±SD) for pollination by artificially, A. mellifera and B. terrestris, in different pear cultivars in the open field
In terms of flower cluster fruit set in the open field, artificial pollination of the ‘Niitaka’ cultivar yielded the greater fruit set (100%) than bee pollination (F(3,8)=89.652, p=0.0001), which only yielded fruit sets of 62.3~66.6%. Artificial pollination also yielded the greatest flower cluster fruit set in the ‘Manpungbae’ cultivar (77.4±5.1%), followed by the fruit set of the B. terrestris, A. mellifera, and no-pollination treatments; however, there was no significant difference between pollination methods (F(2,6)=2.457, p=0.166). Finally, in ‘Whangkeumbae’, the flower cluster fruit sets achieved through artificial and A. mellifera pollination were statistically similar (90%) and significantly greater than the flower cluster fruit set achieved through B. terrestris pollination (F(2,8)=111.471, p=0.0001; Table 8).

The percentage (mean±6SD) of the fruit set per flower clusters of in different pear cultivars pollinated by artificially, A. mellifera and B. terrestris, and in the open field
Furthermore, A. mellifera pollination yielded statistically similar levels of fruit set and flower cluster fruit set in all cultivars in the net screen house but, in the open field, yielded 1.6 times higher fruit set (F(2,6)=26.985, p=0.001) and 2 times higher flower cluster fruit set (F(2,6)=10.507, p=0.011) in the ‘Whangkeumbae’ cultivar than other cultivars, which is consistent with our observation of foraging rate. In contrast, the lower observed foraging rates of B. terrestris on ‘Niitaka’ and ‘Whangkeumbae’, when compared to other cultivars, suggest that B. terrestris pollination would yield lower fruit in non-pollen cultivar; however, similar levels of fruit set were observed for all the cultivars.
Effect of pollination method on fruit quality
In the ‘Niitaka’ cultivar, artificial pollination yielded heavier (Approximately 30g) fruit that was heavier than the fruit obtained through bee pollination, although not significantly different (p>0.05; Table 9), and pollination method seemed to have little effect on fruit firmness, soluble solids, or titratable acidity. Artificial and B. terrestris pollination yielded fruits with 1.4~2.1 more seeds than the fruits pollinated by A. mellifera (F(2,117)=7.145, p=0.001), and the L/D ratio of the fruits from artificial pollination (0.97) was greater than the more round fruits that were obtained from A. mellifera and B. terrestris pollination (ratio=0.91). Furthermore, B. terrestris pollination yielded fruit with a greater shape index (76.7%) than the fruit from other types of pollination, although not significantly different (p>0.05), and the percentage of malformed fruit was lowest (0.0%) in the fruits obtained through A. mellifera pollination (Table 10).
In the ‘Manpungbae’ cultivar, pollination method had no significant effect on weight and firmness (Table 11). Artificial and B. terrestris pollination yielded significantly greater soluble solid levels (F(2,117)=6.741, p=0.005), whereas A. mellifera pollination yielded greater titratable acidity (F(2,117)=6.346, p=0.002).Artificial pollination yielded fruits with 4.9 seeds, which was 1~2 more than the number of seeds in the fruits from A. mellifera and B. terrestris pollination (F(2,117)=11.957, p=0.0001). Furthermore, the L/D ratios and shape indices of the fruits from A. mellifera and B. terrestris pollination were slightly greater than those of the fruits from artificial pollination, although not significantly, and percentage of malformed fruit was 13~23% greater in the fruit from A. mellifera and B. terrestris pollination (p>0.05; Table 12).
In the ‘Whasan’ cultivar, fruit weight was lowest in the artificial pollination group and 140 g greater in the A. mellifera group, with the weight of the B. terrestris pollination group in between the others (F(2,87)=46.722, p=0.0001). On the other hand, there was no difference in frimness, sugar content, and acidity (Table 13). In terms of fruit shape, artificial pollination in seed number was 7.1 grams, which was one more than B. terrestris, but there was significant difference (F(2,87)=3.165, p=0.047), and pollination method had no significant effect on L/D ratio or shape index, whereas the percentage of malformed fruit was 7~10% lower in the fruit from A. mellifera pollination than in that from the other pollination methods, although not significantly (p>0.05; Table 14).
In the ‘Whangkeumbae’ cultivar, artificial pollination yielded fruit that weighed 510.7g, which was 65 and 97g greater than A. mellifera and B. terrestris, respectively (F(2,117)=63.261, p=0.0001). A. mellifera in firmness and B. terrestris in soluble solids showed higher results than other pollination methods (firmness: F(2,117)=17.760, p=0.0001; soluble solids: F(2,117)=3.989, p=0.021; Table 15). In the shape properties of fruits, all the pollination methods showed statistically the similar level of all of fruit shape parameters (Table 16).
DISCUSSION
To use of insect pollinators in pear production in Korea, we investigated the foraging behavior and pollination efficiency of A. mellifera and B. terrestris on different cultivars of Asian pear. Both foraging rate and time spent on flowers by A. mellifera and B. terrestris were influenced by cultivar. In particular, the foraging rate in ‘Niitaka’ was very low at 1/3 level compared with foraging rate in other cultivars. These results indicate that ‘Niitaka’ is least preferred by A. mellifera and B. terrestris, when compared to ‘Manpungbae’ and ‘Whasan’. Therefore, future studies should investigate methods of attracting bees to ‘Niitaka’ blooms. Several previous studies have improved low rates of crop pollination using queen mandibular pheromone (QMP; Winston and Slessor, 1993). Naumann et al. (1994) investigated the effect of synthetic pheromone on the fruiting quality of pears, and Akai et al. (1995) reported that bee pheromone attractants (Bee-Scent; Scentry, Billings, MT, USA) increased fruit set in the Japanese pear cultivars ‘Hangsu’ and ‘Pungsu’. In addition, a mixture of synthetic honeybee pheromone and highly attractive sugars has been developed and applied to watermelons, ridge gourd, and sunflowers (Ellis and Delaplane, 2009; Jayaramappa et al., 2011; Jayaramappa and Bhargava, 2015).
In the present study, we also compared the average foraging rates of A. mellifera and B. terrestris in ‘Fertile’ and ‘Sterile’ pear cultivars and found that bees generally exhibited higher foraging rates on ‘Fertile’ flowers. In particular, the foraging rate of B. terrestris was 2.8 times greater on ‘Fertile’ flowers than on ‘Sterile’ flowers indicating the importance of pollen in determining foraging by B. terrestris. In contrast, the foraging rate of A. mellifera was greater on ‘Sterile’ flowers; however, the difference was much less pronounced and was not significant. For example, the foraging rate of A. mellifera on the ‘Sterile’ cultivar ‘Whangkeumbae’ was 1.3~3 times greater than the foraging rate of A. mellifera on ‘Fertile’ cultivars. Therefore, the availability of both pollen and nectar, as well as nectar composition, must influence the foraging activity of bees, especially A. mellifera. Indeed, the chemical components contributing to a flower’s fragrance also play an important role in the attractiveness of flowers to A. mellifera and B. terrestris (Matile and Altenburger, 1988; Loughrin et al., 1991; Henning et al., 1992). Additional, pollen volatile profile also plays an important role in flower selection by A. mellifera and B. terrestris (Fewell and Winston, 1992). Previous studies have reported that A. mellifera foragers prefer pollen that contains phenylalanine, valine, leucine, and isoleucine (Inouye and Waller 1984; Cook et al., 2003). Therefore, future studies should investigate the correlation between the preferences of commercial pollinators and the amount and composition of both nectar and pollen.
In addition, artificial pollination yielded higher fruit sets than bee pollination. However, in terms of flower cluster fruit set, which is an actual harvest indicator, the two methods yielded similar results. For example, pollination by both bee species produced fruit set rates in the ‘Niitaka’ cultivar that were over 60% the fruit set achieved through artificial pollination, and in other cultivars, the fruit set of bee-pollinated plants was comparable to that of artificially pollinated ones. Furthermore, pollination had no significant effect on fruit quality. Therefore, the pollination by either A. mellifera or B. terrestris is a good option instead of artificial pollination in various cultivars except ‘Niitaka’ cultivar. That being said, it is important to note that the present study was conducted in the field, where various cultivars of pear pollinizers were grown.
Furthermore, to pollinate by bees, pollinizer was essential. However, pear growers often cultivate only single cultivars which were high economic efficiency without pollinizer in Korea (Nam et al., 2014). Pollinizers can be incorporated using a variety of methods, such as planting a certain percentage of pollinizers, grafting pollinizers onto ‘Sterile’ target cultivars, and hanging pollinizer branches in target cultivars (Shim et al., 2000). Therefore, in order to maximize the effect of insect pollination in pears, further study is needed to determine the optimal arrangement and application of pollinizers.
Acknowledgments
This work was supported by a grant from the National Institute of Agricultural Sciences, Rural Development Administration, Republic of Korea (Project No.: PJ010829022017).
LITERATURE CITED
- Akai, T., Y. Saoyama, and A. Miki, (1995), Evaluation of bee attractants for japanese pear pollination, Bull. Tokushima. Hort. Sta, 23, p18-26.
- Benedek, P., and J. Ruff, (1998), Flower constancy of honeybee and its importance during pear pollination, Acta. Hort, 475, p427.
- Cho, K. S., S. S. Kang, D. S. Son, S. B. Jeong, J. H. Song, Y. K. Kim, M. S. Kim, K. H. Hong, H. M. Cho, and G. C. Koh, (2007), ‘Jinhwang’, a New mid-season pear with high quality and good appearance, Korean J. Hortic. Sci. Technol, 25, p133-137.
- Cook, S. M., C. S. Awmack, D. A. Murray, and I. H. Williams, (2003), Are honeybees' foraging preferences affected by pollen amino acid composition? Ecol, Entomol, 28, p622-627.
- Delaplane, K. S, and D. F. Mayer, (2000), Crop pollination by bees, In CABI Publishing, New York, USA.
-
Ellis, A., and K. S. Delaplane, (2009), An evaluation of Fruit-BoosTM as an aid for honey bee pollination under conditions of competing bloom, J. Apic. Res, 48(1), p15-18.
[https://doi.org/10.3896/ibra.1.48.1.04]

- Farkas, A., Z. Orosz-Kovacs, and L. Szabo, (2002), Insect attraction of flowers in pear cultivars, Acta. Hort, 596, p773-776.
-
Fewell, J. H., and M. L. Winston, (1992), Colony state and regulation of pollen foraging in the honey bee, Apis mellifera L, Behav. Ecol. Sociobiol, 30, p387-393.
[https://doi.org/10.1007/bf00176173]

- Free, J. B., (1993), Insect pollination of crops, 2nd ed., Academic Press, London, UK.
- Graham, J. M., (1993), The hive and the honeybee, Hamilton, Dadant and Sons, Illinois, USA.
- Henning, J. A., Y. S. Peng, M. A. Montague, and L. R. Teuber, (1992), Honey bee (Hymenopeftra: Apidae) behavioral response to primary alfalfa (Rosales fabaceae) floral volatiles, J. Econ. Entomol, 85, p233-239.
-
Inouye, D. W., and G. D. Waller, (1984), Responses of honey bees (Apis mellifera) to amino acid solutions mimicking floral nectars, Ecology, 65, p618-625.
[https://doi.org/10.2307/1941424]

- Itai, A., (2007), Fruits and nuts, Springer, Berlin Heidelberg, Germany, p157-170.
-
Jacquemart, A. L., A. Michotte-Van der Aa, and O. Raspe, (2006), Compatibility and pollinator efficiency tests on Pyrus communis L. cv. ‘Conference’, J. Hort. Sci. Biotech, 81, p827-830.
[https://doi.org/10.1080/14620316.2006.11512145]

- Jang, H. I., H. H. Seo, and S. J. Park, (2002), Strategy for fruit cultivation research under the changing climate, Korean J. Hortic. Sci. Technol, 20, p270-275.
- Jayaramappa, K. V., and H. R. Bhargava, (2015), Enhancement of crop productivity in sun Flower (Helianthus annusl) by the influence of honeybee attractants, Wor. Appl. Scie. Jou, 33, p673-678.
- Jayaramappa, K. V., P. Mahesh, and H. R. Bhargava, (2011), Influence of bee-attractants on yield parameters of Ridge Gourd (Luffa acutangula L.) Cucurbitaceae, Wor. Appl. Scie. Jou, 15, p457-462.
- Kim, Y. S., J. W. Cho, M. Y. Lee, and M. L. Lee, (2003), The pollination of honeybee on peach blossom planted in vinyl house and its valuation of the fruits after harvest, Korean J. Apiculture, 18, p23-28.
-
Konzmann, S., and K. Lunau, (2014), Divergent rules for pollen and nectar foraging bumblebees - a laboratory study with artificial flowers offering diluted nectar substitute and pollen surrogate, PLoS one, 9, e91900.
[https://doi.org/10.1371/journal.pone.0091900]

-
Lee, K. Y., S. H. Yim, H. J. Seo, S. Y. Kim, and H. Y. Yoon, (2016), The influence of insect pollination and artificial pollination on fruit quality and economic profit in the ‘Niitaka’ pear (Pyrus pyrifolia Nakai), Korean J. Org. Agric, 24, p759-771.
[https://doi.org/10.11625/kjoa.2016.24.4.759]

- Lee, U. Y., (2014), Improvement of fruiting and fruit shape by pollen source, thinning, and plant groeth regulators in ‘Niitaka’ pears, A thesis for the doctor degree of Chungnam National University, p1-8.
-
Lee, U. Y., Y. K. Kim, I. S. Shin, K. S. Oh, O. K. Jung, and J. P. Chun, (2015), Comparison of fruit development and quality indices according to blossom thinning on earlyseason ‘Hanareum’ and mid-season ‘Niitaka’ Pears, Korean J. Hort. Sci. Technol, 33, p486-491.
[https://doi.org/10.7235/hort.2015.15030]

- Loughrin, J. H., T. R. Hamilton-Kemp, R. A. Anderson, and D. F. Hildebrand, (1991), Circadian rhythm of volatile emission from flowers of Nicotiana sylvestris and N. suaveolens, Physiol. Plant, 83, p492-496.
-
Matile, P., and R. Altenburger, (1988), Rhythms of fragrance emissions in flowers, Planta, 174, p242-247.
[https://doi.org/10.1007/bf00394777]

- Mayer, D. F., K. D. Patten, and R. P. Mcfarlane, (1994), Pear pollination with managed bumblebee (Hymenoptera: Apidae) Colonies, Melandria, 50, p20-23.
- Nam, K. W., M. K. Han, and D. H. Yoon, (2014), Control effect of sodium dichloroisocyanurate for pear scab (Venturia nashicola) on Niitaka pear during flowering Period, Korean J. Organic. Agr, 22, p347-357.
-
Naumann, K, M. L. Winston, K. N. Slessor, and M. J. Smirle, (1994), Synthetic honey bee (Hymenoptera: Apidae) queen mandibular gland pheromone applications affect pear and sweet cherry pollination, J. Econ. Entomol, 87, p1565-1599.
[https://doi.org/10.1093/jee/87.6.1595]

-
Potts, S. G., J. C. Biesmeijer, C. Kremen, P. Neumann, O. Schweiger, and W. E. Kunin, (2010), Global pollinator declines: Trends, impacts and drivers, Trends Ecol. Evol, 25, p345-353.
[https://doi.org/10.1016/j.tree.2010.01.007]

- Rural Development Administration (RDA), (2016), Pear cultivation, RDA press, Jeonju, Korea, p68.
- Seo, H. H., and H. S. Park, (2003), Fruit quality of ‘sugaru’ apples influenced by meteorological elements, Korean J. Agric. For. Meteor, 5, p218-225.
- Shim, K. K., Y. M. Ha, and K. H. Chung, (2000), Selection of Korean native dwarf pear (Pyrus calleryana var. fauriei) as pollinizers for single-cultivar ‘Niitaka’ pear orchard, Hort. Environ. Biotechnol, 41, p281-288.
- Statistics Korea, (2016), Crop production statistics, http://kosis.kr/.
- Tanaka, M., S. Hayashida, A. Morita, and K. Nakakurae, (2007), Establishment of technology for labor saving culture system on japanese pear ‘Niitaka’, Bull. Nagasaki Fruit Tree Exp. Stn, 10, p41-51.
- Van den Eijnde, J., (1995), Pollination of pear by bumblebees (Bombus terrestris L.) and honeybees (Apis mellifera L.), In II Workshop on Pollination, 423, p73-78.
- Webster, A. D., (2002), Factors influencing the flowering, fruit set and fruit growth of european pears, Acta. Hort, 596, p699-709.
- Wu, W. Q., Y. Guo, J. S. Shen, W. H. Ma, B. B. Guo, and Y. Q. Shao, (2011), Present situation investigation of bee pollination for dangshan pear, Apicul. China, 62, p40-44.
-
Yoon, H. J., K. Y. Lee, H. S. Lee, M. Y. Lee, Y. S. Choi, M. L. Lee, and G. H. Kim, (2017), Survey of insect pollinators use for horticultural crops in Korea, 2016, J. Apiculture, 32, p223-235.
[https://doi.org/10.17519/apiculture.2017.09.32.3.223]
