Attractiveness of the Small Hive Beetle (Aethina tumida) to Volatiles from Honey bee (Apis mellifera) and Beehive Materials
Abstract
Small hive beetle (SHB) is considered as an important honeybee pest, native to sub-Saharan Africa and reproduce in association with honeybees and beehive produced materials in various regions throughout the world. The likelihood of further spread to additional territories and continents where Apis species are endemic is expected. SHB has become a global threat to both apiculture and wild bee populations, but in Korea our knowledge of this pest is still limited, creating demand for more research to prevent and control them. Various studies have reported that the small hive beetle is attracted to volatiles from hive products and those of the yeast Kodamaea ohmeri. This study investigated the attractiveness of adult worker bees (Apis mellifera) and natural honeybee hive produced material (pollen dough) and to the SHB as the pollen dough was altered by the action of beetle larvae and fermentation by K. ohmeri for 13 and 22 days in comparison with fresh honey and a fresh pollen patty. Additionally, we evaluated the attractiveness of 20 day-fermented honey without involvement of yeast K. ohmeri here for the first time. We used Y-tube olfactometric bioassay and GC-MS to investigate attractiveness of samples and their composition analysis, respectively. Slime (13 day- and 22 day-fermented pollen dough), the fermented honey and the workers bees are strong attractants (> 72%) to both males and females SHB. However fresh pollen patty and fresh honey are not attractive (< 50%) to the beetles. Volatile oils constitute octadecanoic acid (22.8%) and (Z,Z,Z)-9,12,15-octadecatrienoic acid, ethyl ester (18.7%) in the 13 day-fermented pollen dough; hexadecanoic acid, ethyl ester (22.9%) and (Z,Z,Z)-9,12,15-octadecatrienoic acid, ethyl ester (17.1%) in 22 day-fermented pollen dough; (Z)-9-octadecenamide (5.6%), 7-hexyl-docosane, (4.8%); and tetracosane (4.8%) in 20 day-fermented honey as major components. Major components reported here might contribute to developing effective and safe methods for SHB management.
Keywords:
Small hive beetle, Attractive, Fermented pollen dough, Y-tube olfactometric bioassay, GC-MSINTRODUCTION
The small hive beetle (SHB, Aethina tumida Murray; Coleoptera: Nitidulidae) is considered as an important honeybee pest, native to sub-Saharan Africa (Neumann et al., 2016). SHB has distributed into many parts of the world (Neumann and Elzen, 2004; Neumann and Ellis, 2008; Giangaspero and Turno, 2015) such as USA in 1996, Australia in 2002, Canada in 2002, Jamaica in 2005, Portugal in 2005, Mexico in 2007, Philippines in 2014, Italy in 2014 (Palmeri et al., 2015; Giangaspero and Turno, 2015) and South Korea in 2016 (Lee et al., 2017). In the native distribution range, SHB does not impose heavy threat to honeybees partly because of the behavioral resistance of African honey bee subspecies (Hood, 2004). This pest is an invasive species, and it can have a significant impact on apiculture as well as on wild and feral bee populations in the new area (Neumann et al., 2016). In the United States and Australia it becomes a devastating pest of European honey bees, and consequently, is a threat to European honey bees pollinated crops, worth $14 billion per annum in the United States (Torto et al., 2007). In USA, SHB has been reported to damage both weak and strong colonies (Sanford, 1998). Damage to honey bee colonies is caused mainly by beetle larvae. The attacks of a beehive by the larvae cause fermentation of honey and making it unsuitable for human consumption. The resulting fermented honey (or ‘slime’) is rejected by honeybees and cannot be marketed by the beekeeper (Hayes et al., 2015). Additionally the SHBis attracted to volatiles from hive products and those of the yeast Kodamaea ohmeri. The attractiveness of the fermenting hive products (‘slime’) increased as fermentation progressed, and volatile profiles became more complex (Hayes et al., 2015). Understanding the attractiveness of SHB to beehive is the prime interest to prevent invasion or to attract for monitoring. Several works are undergoing globally to address this issue. It was reported that odors from hive products plus adult bees were found to be significantly attractive to flying adult beetles, as evidenced in baited trap studies. Hive products alone or bees alone were not attractive to the beetles (Elzen et al., 1999).
The honey bee’s alarm pheromones such as isopentyl acetate (IPA), 2-heptanone, and methyl benzoate serve a negative function because they are potent attractants for SHB. Interestingly, the sting response and alarm pheromone are the key components of the honey bee defense system against predators and parasites (Torto et al., 2007). These workers also reported the beetles from both Africa and the United States vector a strain of Kodamaea ohmeri yeast produces the same honey bee alarm pheromones when grown on pollen in hives. Adult SHB is attracted to volatiles of European honeybees and the attraction is mediated by a blend of components dominated by the honey bee’s alarm pheromones (Torto et al., 2007). The response of SHB to pollen volatiles was dose-dependent, while the response to volatiles from worker bees increased with both the number and age of the bees (Suazo et al., 2003).
Some synthetic chemicals were reported to be used to control SHB but such chemicals are not safe unless used with precaution. If larvae are present in the colony, the soil around the hive can be treated with a permethrin drench to prevent the larvae from pupating, killing them in the soil. However permethrin is highly toxic to bees and it is corrosive and can cause irreversible eye damage (Zawislak, FSA-7075.). The residues of coumaphos present in wax (Mullin et al., 2010) can contaminate honey and the product does not seem to work well in cooler temperatures (Mostafa and Williams 2000). Moreover, SHB may develop resistance to any chemical used for their control because of their high mobility and fecundity (De Guzman et al., 2011). Since its invasion into Milyang city, GyoungNam province, South Eastern part of Korea in 2016, the infested apiary was completely collapsed, but its impact seemed restricted in the local ranges (Hong and Jung, 2017). However, effective sampling protocols with high efficiency attraction trap would benefit the effective monitoring of its distribution and movement to contain population spread, as are the cases for invasive pests (Jung et al., 2009; Jung, 2012).
Even though there are different controls and prevention methods reported so far against this pest, our knowledge of biology, diagnosis, control, and prevention of SHB still limited, creating demand for more research (Neumann et al., 2016). In order to fill this gap here, we aimed at investigating the responses of SHB to beehive materials and volatiles of adult workers bees to search for safe to the user and the environment in controlling this pest. The result of our study will be important for effective control to treat A. tumida within the hive without endangering the resident bee population.
MATERIALS AND METHODS
Material collection and hydro-distillation
Fresh pollen patty was purchased from local beekeeping supplier, Hyeongjey beekeeping, Eui-seong, Gyeongbuk, Korea. 10g of fresh honey was added to 100g fresh pollen patty and kept feeding to 50 adult small hive beetles of mixed sexes and their larvae for 13 days in a cage (13 day-fermented pollen dough). Another 100g fresh pollen patty was added with 100 ml of 50% sugar solution and kept feeding to 50 adult SHB of mixed sex and their larvae for 22 days in a cage (22 day-fermented pollen dough). Fresh honey and adult worker bees (Apis mellifera) were collected from May 15 to June 25, 2017, from Bee Lab, Andong National University, South Korea. 20 day-fermented honey was prepared by dissolving 20g of fresh honey in 20ml of water and allowing fermenting at 28°C for 20 days. Volatiles were collected from workers bees by putting 35 adult workers into two beakers each (2 liters, 15cm i. d.). The beakers were then placed upside down in a plastic container for 6 h while feeding the bees with 50% sugar solution. The beakers were rinsed with CHCl3 and the mixture was hydro-distilled for 3h. Similarly, the given amount of other hive material was also hydro-distilled for 3h. The distillate was placed in a separatory funnel. Then chloroform was added to extract oil from the aqueous layer. The chloroform layer was then dried using anhydrous sodium sulfate (1g), filtered using Whatman No. 1 filter paper and concentrated on rotary evaporator to yield the given amount of oil (Table 1). Chloroform (extra pure) was purchased from Daejung chemicals and metals Co., LTD, Korea and sodium sulfate anhydrous (extra pure) was purchased from Duksan pure chemical Co. LTD, Korea.
Insects
A colony of the SHB was reared in acrylic cages (38×38×34cm) at 25±2°C and 60±5% relative humidity on a 12:12 hr L/D photoperiod. The beetles were provided with pollen patty-honey diet (Pollen dough) and water in insect laboratory, Andong National University. Pupation sand (7cm) was added to the cage and regularly humidified by adding water on tissue papers and on sand. The pollen dough was prepared from commercially packaged pollen patty. The genitalia was exposed by applying slight pressure to the ventral side of the abdomen to determine their sex (Suazo et al., 2003). After sexing and prior to bioassays, male and female beetles were kept in separate cages. Beetles were deprived of food and water for 1 day before bioassays. 5-7 days old mated adult beetles were used for the bioassay.
Y-tube olfactometer
The purpose of using this device was to investigate a preference of individual insects exposed to a dual choice of odors. The set-up was placed in a dark room at 25°C. The Y-tube was placed in the center of a black box (20×20×30cm), covered inside with black paper in order to avoid visual stimuli. A halogen lamp, attached to the ceiling of the box, illuminated the Y-junction of the olfactometer with 160 lux light intensity. For this set-up, we recorded the parameter preference as the capability of insects to choose between one of two odor sources. The end tubes of the Y were connected to two plastic adapters, having attached to silicone tubing connected to two jars. The airflow was pumped into the odor chambers from the humidifier jar and then to the Y-tube olfactometers at a rate of 400±10ml/min at the entrance. The test chamber consisted of a Y-shaped glass tube, with each arm 8cm long and 1.8cm in diameter. As an odor chamber, we used a glass jar (18cm long, 6cm diameter) consisting of two parts with a ground glass joint. The odor chamber contained a hive material, while the control chamber remained empty. A score line was drawn on the two arms of the olfactometer at 4 cm from the joint. For the bioassay, individual SHB were released at the open end of the common arm of the Y-tube. A choice was recorded when the insect crossed the score line within 1 min from release and stayed in the portion of the arm behind the score line for at least 30 seconds. A no-choice was recorded when the insect remained in the common arm for more than 2 min. Y-tubes were cleaned with detergent and ethanol after testing each insect. The position of odor sources was switched after testing 5 insects to minimize positional bias.
GC/MS analyses
The GC-MS analysis was done by using a GC (7890B, Agilent Technologies, USA) coupled with an MS (5977A Network, Agilent Technologies). The GC had an HP 5MS column (non-polar column, Agilent Technologies), 30m×250μm internal diameter (i.d.) and 0.25μm film thickness. The carrier gas was helium flowing at a rate of 1mL/ min. The injector temperature was 230°C and the injection mode was a split mode with split ratio 4:1. The initial oven temperature was 40°C held for 3 min. It was raised to 70°C at 4°C/min held at this temperature for 3 min. The oven temperature was then raised 80°C at 1°C/min with no holding time, 100°C at 4°C/min with no holding time then to 180°C at 10°C/min then with no holding time 20°C/min until it reached 280°C and held at this temperature for 2 min. The total run-time was 43 min. Mass spectra were recorded in EI mode at 70 eV, scanning the 33-500 m/z range. The identification of the volatile compounds was performed by comparing the mass spectra of the compounds with those in the database of NIST11 and literature data.
Statistical analysis
One-way ANOVA was used to compare the attractiveness of hive materials and honeybees volatiles toward SHB. Means were compared at the P=0.05 level, and Tukey’ test was used to separate means (SPSS 16.0 statistical software).
RESULTS AND DISCUSSION
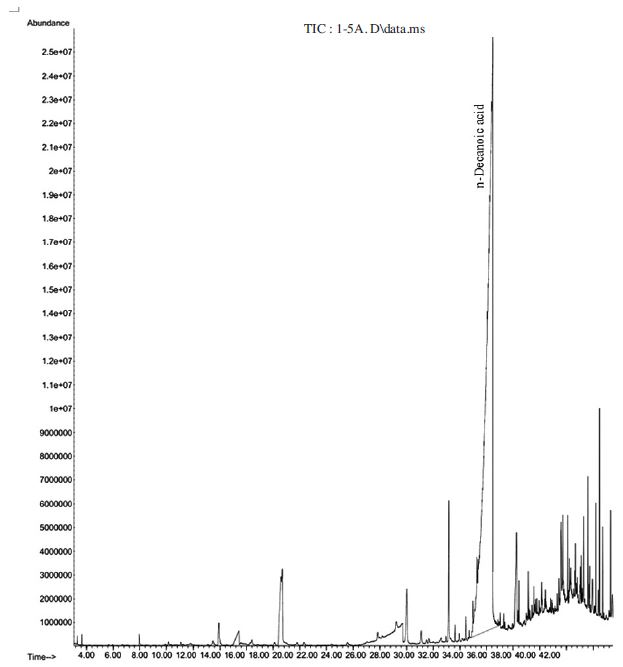
Fresh pollen patty was selected for investigation because it is the main ingredient in the feed of SHB. Y-tube olfactometer bioassay showed fresh pollen patty has a low attractiveness to both adult males and females SHB (Fig. 1 and Fig. 2). GC-MS analysis of fresh pollen patty indicated it contains 99% of n-decanoic acid (Table 2 and Fig. 3). All other components were found at a trace level. Thus the essential oil of pollen patty can be used as a source of n-decanoic acid. Based our bioassay and GC-MS analysis result we believe n-decanoic acid does not attract these pests.
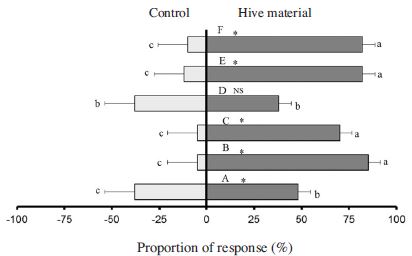
Mean proportion olfactometer responses of 5-7 days old male SHB to hive-produced volatiles. Hive material dark bars and control grey bars. A: Fresh pollen patty; B: 13 day-fermented pollen dough; C: 22 day-fermented pollen dough; D: Fresh honey; E: 20 day-fermented honey; F: Worker bees. Asterisks indicate a significant preference of the SHB for a particular odor source and NS stands for no significant preference. Error bars represent standard errors. Bars with the same small letters indicate no significant difference in mean proportions. Bars with different small letters indicate a significant difference in mean proportions (SPSS: P<0.05).
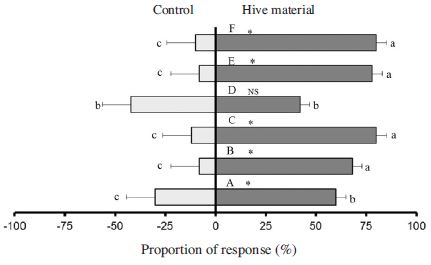
Mean proportion olfactometer responses of 5-7 days old female SHB to hive-produced volatiles. Hive material dark bars and control grey bars. A: Fresh pollen patty; B: 13 day-fermented pollen dough; C: 22 day-fermented pollen dough; D: Fresh honey; E: 20 day-fermented honey; F: Worker bees. Asterisks indicate a significant preference of the SHB for a particular odor source and NS stands for no significant preference. Error bars represent standard errors. Bars with the same small letters indicate no significant difference in mean proportions. Bars with different small letters indicate a significant difference in mean proportions (SPSS: P<0.05).

The composition major components identified in the volatiles of hive materials and adult workers bees
Previous reports indicated the volatiles emanating from the hive products as they were broken down by the action of SHB larvae and yeast were highly attractive to adult SHB and became more attractive with time (Hayes et al., 2015). In this study, we prepared slime by fermenting pollen dough, progressively altered by the action of small hive beetles and yeast over time for 22 days and evaluated responses of SHB to this material after 13 and 22 days. Both these fermented materials have a very strong attraction to both sexes of adult beetles (Fig. 1 and Fig. 2) compared with fresh pollen patty (SPSS 16, P<0.05). No significant difference between males and female response were found with both fermented pollen dough (P>0.05).
126 compounds were identified in the oil of 13 day-fermented pollen dough and octadecanoic acid (22.8%) and (Z,Z,Z)-9,12,15-octadecatrienoic acid, ethyl ester (18.7%) were the major compounds in the oil and the sum of these two compounds comprise 42.0% (Table 2 and Fig. 4). A total of 133 compounds were identified in the volatile oil of 22 day- fermented pollen dough out of which hexadecanoic acid, ethyl ester (22.9%) and (Z,Z,Z)-9,12,15-octadecatrienoic acid, ethyl ester (17.1%) were the most abundant compounds (Table 2 and Fig. 5). 22 day-fermented pollen dough and 13 day-fermented pollen dough constitute (Z,Z,Z)-9,12,15-octadecatrienoic acid, ethyl ester 17.1% and 18.7% respectively in common. Hexadecanoic acid, ethyl ester which was found 22.9% in 22 day-fermented pollen dough was absent in and 13 day-fermented pollen dough even in a trace level.
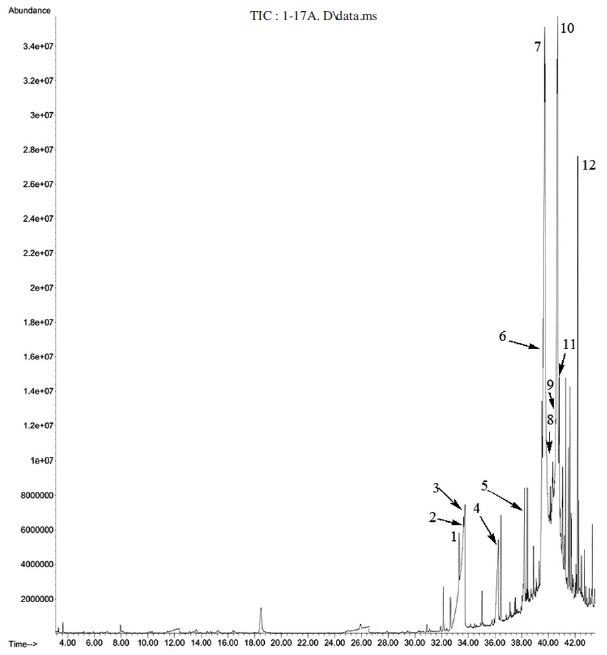
Gas chromatogram of hydro-distilled oil of 13 day-fermented pollen dough 1: Tetradecane (2.8%); 2: 1-(4,5-dihydro-2-thiazolyl)-ethanone (4.4%); 3: n-Decanoic acid (1.8%); 4: Dodecanoic acid (2.1%); 5: Tetradecanoic acid (2.1%); 6: cis-9-Hexadecenoic acid (4.9%); 7: Octadecanoic acid (22.8%); 8: 1-Eicosene (2.2%); 9: n-Hexadecanoic acid (4.2%); 10: (Z,Z,Z)-9,12,15-Octadecatrienoic acid, ethyl ester (18.7%); 11: Hexadecanamide (4.4%); 12: Heneicosane (2.9%).
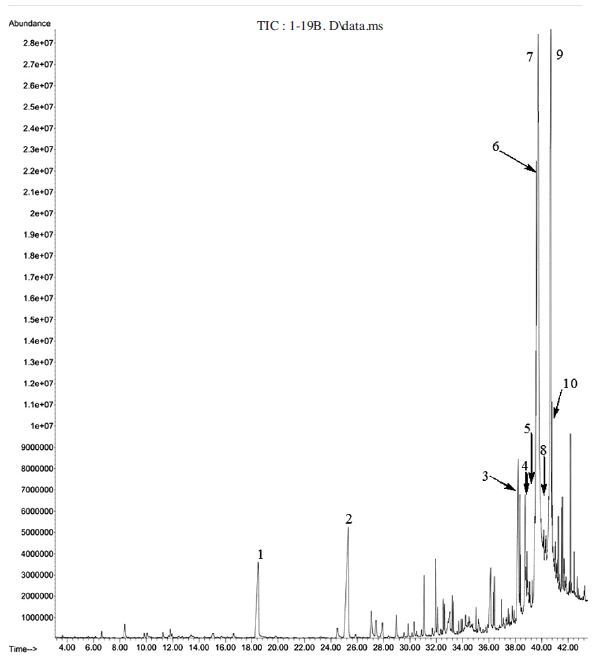
Gas chromatogram of hydro-distilled oil of 22 day-fermented pollen dough 1: Phenylethyl alcohol (2.6%); 2: N-methyl-1,3-propanediamine (4.4%); 3: Tetradecanoic acid (3.3%); 4: Pentadecanoic acid (2.6%); 5: Palmitoleic acid (2.0%); 6: Ethyl 9-hexadecenoate (6.2%); 7: Hexadecanoic acid, ethyl ester (22.9%); 8: n-Hexadecanoic acid (2.4%); 9: (Z,Z,Z)-9,12,15-Octadecatrienoic acid, ethyl ester (17.1%); 10: Octadecanoic acid, ethyl ester (2.7%).
Responses of SHB to fresh honey odor were also assessed and the bioassay result showed fresh honey also does not lure both males and females compared with control (Fig. 1 and Fig. 2). There is no significant difference between the response of both males and females towards fresh honey and fresh pollen patty (P>0.05). Fermentation of honey for 20 days evoked a positive response to males and females (Fig. 1 and Fig. 2). There is no significant difference in response of 20 day-fermented honey and fermented pollen dough (P>0.05). An earlier report indicated the hive materials become attractive to the SHB by the action of SHB larvae and yeast on pollen dough (Hayes et al., 2015). Based on our result we believe in addition to the effect of fermentation process taking place in a beehive by attacking larvae, leakage of honey from hive followed by fermentation with rainwater is also luring these beetles. So it is important for beekeepers to inspect and clean if there is any honey leakage in order to prevent infestation by this pest.
101 components were identified in the essential oil of fresh honey among which phthalic acid, nonyl 2,4,4-trimethylpentyl ester (9.1%) and 1,2-benzenedicarboxylic acid, diheptyl ester (8.8) were predominant (Table 2 and Fig. 6). On the other hand essential oil from 20 day-fermented honey constitutes 98 compounds of which (Z)-9-octadecenamide (5.6%), 7-hexyl-docosane, (4.8%); and tetracosane (4.8%) (Table 2 and Fig.7) were predominant. These major components might be responsible for the positive response fermented honey to SHB and further works remained to find out attractiveness of each of these compounds.
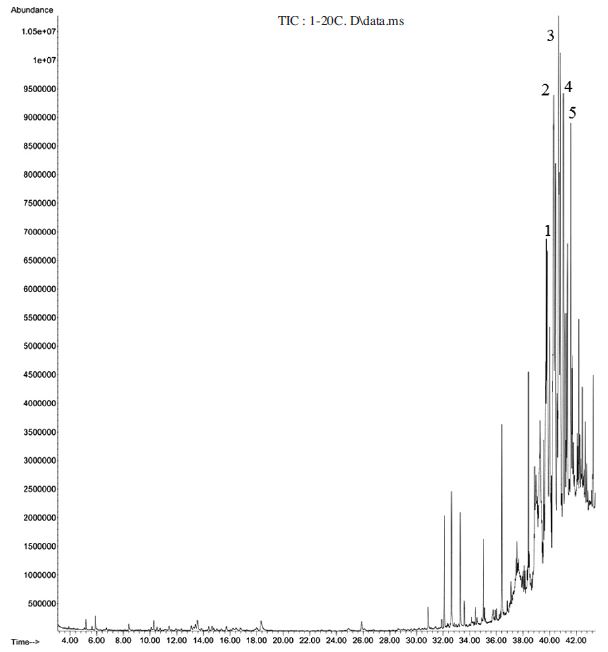
Gas chromatogram of hydro-distilled oil of fresh honey 1: Phthalic acid, 4-methylpent-2- yl nonyl ester (5.1%); 2: Phthalic acid, nonyl 2,4,4-trimethylpentyl ester (9.1%); 3: 1,2-Benzenedicarboxylic acid, diheptyl ester (8.8); 4: Phthalic acid, 3-methylbutyl octadecyl ester (5.3%); 5: 1,2-Benzenedicarboxylic acid, decyl hexyl ester (5.7%).
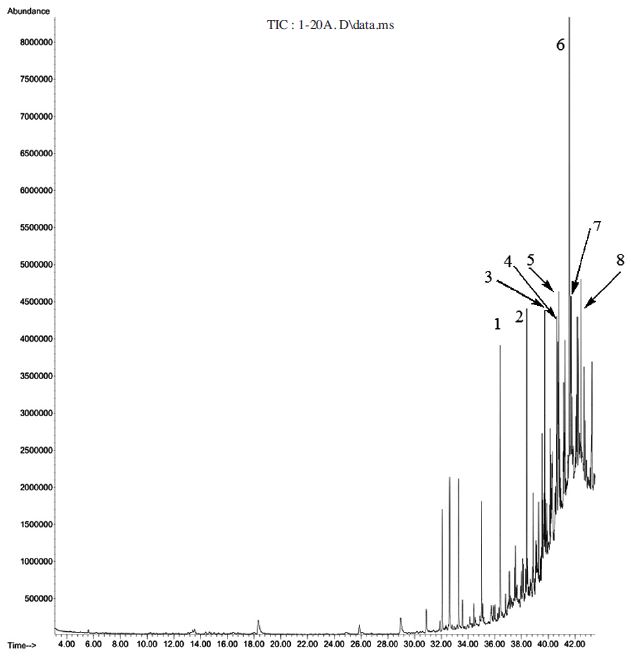
Gas chromatogram of hydro-distilled oil of 20 day-fermented honey 1: Heneicosane (2.8%); 2: 2,6,11-trimethyl-dodecane, (2.8%); 3: Eicosane (3.0%); 4: Ethyl oleate (3.1%); 5: 7-Hexyl-docosane, (4.8%); 6: (Z)-9-Octadecenamide (5.6%); 7: Tetracosane (4.8%); 8: Phthalic acid, 6-ethyloct-3-yl 2-ethylhexyl ester (3.2%).
The response of SHB to adult workers bees was also positive as shown in Fig. 1 and Fig. 2. The bioassay result showed workers bee volatiles are also strong attractant to both males and females beetles. Previous reports also indicated volatiles from adult worker bees attracted small hive beetes in olfactometric and flight-tunnel bioassays and suggested the involvement of bee volatiles mediating host location by SHB (Suazo et al., 2003). The response of SHB toward honey bee volatiles is comparable to those of fermented honey and fermented pollen dough. The strong positive response of both males and females SHB to bee volatiles indicates that the beetles may associate bee volatiles with the presence of food resources in the beehive as they do not feed on adult honey bees (Suazo et al., 2003).
The result of GC-MS analysis indicates the presence of 61 compounds in the volatile oil of adult workers bees among which (Z)-9-octadecenamide (7.2%) and 5-methyl-2-phenyl-1H-indole (7.0%) are the most predominant components (Table 2 and Fig. 8). Previously, the composition of volatiles collected from worker honeybees in an aerated closed system, which did not simulate the natural conditions typical of a honeybee hive was analyzed. The study suggested that honeybees kept under these conditions are usually stressed, as indicated by the high levels of the alarm pheromone, isopentyl acetate, and the odors released by these bees were very attractive to the small hive beetle (Torto et al., 2005). Noteworthy in the present study isopentyl acetate was not detected in the volatile oil of honeybees under the natural condition we collected. Based on our GC-MS result in addition to isopentyl acetate, alarm pheromone other volatile components of workers bees such as (Z)-9-octadecenamide (7.2%) and 5-methyl-2-phenyl-1H-indole (7.0%) might be also responsible to lure the beetles.
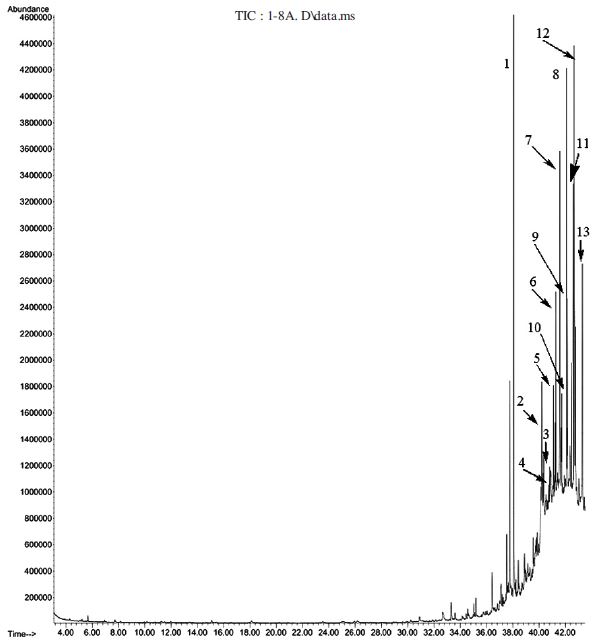
Gas chromatogram of adult workers bees Apis mellifera) volatiles 1: trans-1,1'-(1,2-cyclobutanediyl) bis benzene (5.7%); 2: Oleanitrile (5.3%); 3: Squalene (3.3%); 4: i-Propyl 11-octadecenoate (3.3%) 5: Tributyl acetylcitrate (3.8%); 6: 9-octylheptadecane (3.1%); 7: (Z)-9-Octadecenamide (7.2%); 8: Norgestrel (4.6%); 9: Pentacosane (3.4%); 10: 2-(hexadecyloxy) ethanol (3.7%); 11: Methadone N-oxide (4.0%); 12: 5-methyl-2-phenyl-1H-indole (7.0%); 13: Heneicosane (3.7%).
(Z)-9-Octadecenamide and 5-methyl-2-phenyl-1H-indole which were major compounds in workers bees (Apis mellifera) volatiles were not detected in 13 days and 22 day-fermented pollen dough. (Z)-9-octadecenamide and 7-hexyldocosane are not present in the essential oil of fresh honey. The relative percentage composition of tetracosane was 1.3% in the oil of fresh honey and its composition increased 3.7 times after fermentation.
(Z)-9-Octadecenamide was found 5.6% in 20 day-fermented honey and 7.2% in adult workers bees (Apis mellifera) volatiles in common and might be an attractant to SHB. The presence of this compound in the fermented honey and workers bee volatiles shed light on for further investigation on biological activity toward this compound.
In this study, we found carboxylic acid and esters in a very high concentration of the oils of fermented pollen dough. Amides were found as major volatile compounds in 20 day-fermented honey and adult workers bees. Therefore, carboxylic acids and its derivatives such as esters and amides must be given attention in the research of finding attractants of SHB for their controls. These major compounds identified in the volatiles of hive materials and workers bees either in pure or blended might give a positive response to SHB and further work will be remained in this area. Based on our finding we recommend further studies for utilization of volatile components identified from fermented pollen dough. The attractiveness of SHB toward each compound identified in the fermented pollen dough and fermented honey should be evaluated. This study will have an application in control of SHB using traps treated with volatiles of fermented pollen dough in apiary sites. Knowledge of natural attractants of SHB is a basis for the worldwide efforts to control this invasive pest.
Acknowledgments
The first author was supported by funding from Syngenta on pollinator protection research (Operation pollination Korea) for this study. The authors thank Yang Dahe for helping in the bioassay.
LITERATURE CITED
-
De Guzman, L. I., A. M. Frake, T. E. Rinderer, and R. T. Arbogast, (2011), Effect of Height and Color on the Efficiency of Pole Traps for Aethina tumida (Coleoptera: Nitidulidae), J. Econ. Entomol, 104(1), p26-31.
[https://doi.org/10.1603/ec10300]

-
Elzen, P. J., J. R. Baxter, D. Westervelt, C. Randall, K. S. Delaplane, L. Cutts, and W. T. Wilson, (1999), Field control and biology studies of a new pest species, Aethina tumida Murray (Coleoptera, Nitidulidae), attacking European honey bees in the Western Hemisphere, Apidologie, 30, p361-366.
[https://doi.org/10.1051/apido:19990501]

-
Giangaspero, M., and P. Turno, (2015), Aethina tumida, an exotic parasite of bees, Clin. Microbiol, 4, e128.
[https://doi.org/10.4172/2327-5073.1000e128]

- Hayes, R. A., S. J. Rice, A. Brogan, B. A. Amos, and D. M. Leemon, (2015), Increased attractiveness of honeybee hive product volatiles to adult small hive beetle, Aethina tumida, resulting from small hive beetle larval infestation, Entomol. Exp. Appl, 155, p240-248.
-
Hood, W. M., (2004), The small hive beetle, Aethina tumida: a review, Bee World, 85(3), p51-59.
[https://doi.org/10.1080/0005772x.2004.11099624]

-
Hong, S. M., and C. Jung, (2017), Spatial distribution patterns of a newly invaded honeybee pest, Aethina tumida Murray. 1867 (Coleoptera: Nitidulidae) in an apiary where it was first detected, J. Apic, 32, p163-170.
[https://doi.org/10.17519/apiculture.2017.09.32.3.163]

- Jung, C., (2012), Spatial expansion of an invasive hornet, Vespa velutina nigrithorax buysson (Hymenoptera: Vespidae) in Korea, Kor. J. Apic, 27, p87-93.
- Jung, C., D. W. Kim, H. S. Lee, and H. Baek, (2009), Some biological characteristics of a new honeybee pest, Vespa velutina nigrithorax Buysson. 1905 (Hymenoptera: Vespidae), Kor. J. Apic, 24, p61-65.
- Lee, S., K. J. Hong, Y. S. Cho, Y. S. Choi, M. S. Yoo, and S. Lee, (2017), Review of the subgenus Aethina eric-hsons. str. (Coleoptera: Nitidulidae: Nitidulinae) in Korea, reporting recent invasion of small hive beetle, Aethina tumida, J. Asia-Pac. Entomol, 20, p553-558.
- Mostafa, A. M., and R. N. Williams, (2000), New record of the small hive beetle in Egypt and notes on its distribution and control, Bee. World, 83, p99-108.
-
Mullin, C. A., M. Frazier, J. L. Frazier, S. Ashcraft, R. Simonds, D. vanEngelsdorp, and J. S. Pettis, (2010), High levels of miticides and agrochemicals in North American apiaries: Implications for honey bee health, plos one, 5(3), e9754.
[https://doi.org/10.1371/journal.pone.0009754]

-
Neumann, P., and J. D. Ellis, (2008), The small hive beetle (Aethina tumida Murray, Coleoptera: Nitidulidae): distribution, biology and control of an invasive species, J. Apic. Res, 47(3), p181-183.
[https://doi.org/10.1080/00218839.2008.11101453]

-
Neumann, P., and P. J. Elzen, (2004), The biology of the small hivebeetle (Aethina tumida, Coleoptera: Nitidulidae): gaps in our knowledge of an invasive species, Apidologie, 35(3), p229-248.
[https://doi.org/10.1051/apido:2004010]

-
Neumann, P., J. S. Pettis, and M. O. Schafer, (2016), Quo vadis Aethina tumida? Biology and control of small hive beetles, Apidologie, 47, p427-466.
[https://doi.org/10.1007/s13592-016-0426-x]

-
Palmeri, V., G. Scirto, A. Malacrino, F. Laudani, and O. Campolo, (2015), A scientific note on a new pest for European honeybees: first report of small hive beetle Aethina tumida, (Coleoptera: Nitidulidae) in Italy, Apidologie, 46(4), p527-529.
[https://doi.org/10.1007/s13592-014-0343-9]

- Sanford, M. T., (1998), Aethina tumida: a new beehive pest in the Western hemisphere, Apis, 16, p1-5.
-
Suazo, A., B. Torto, P. E. A. Teal, and J. H. Tumlinson, (2003), Response of the small hive beetle (Aethina tumida) to honey bee (Apis mellifera) and beehive-produced volatiles, Apidologie, 34, p525-533.
[https://doi.org/10.1051/apido:2003043]

- Thomas, M. C., (1998), Pest alert: small hive beetle, Am, Bee J, 138, p565.
-
Torto, B., D. G. Boucias, R. T. Arbogast, J. H. Tumlinson, and P. E. A. Teal, (2007), Multitrophic interaction facilitates parasite-host relationship between an invasive beetle and the honey bee, PNAS, 104, p8374-8378.
[https://doi.org/10.1073/pnas.0702813104]

-
Torto, B., A. Suazo, H. Alborn, H. James, J. H. Tumlinson, and P. E. A. Teal, (2005), Response of the small hive beetle (Aethina tumida) to a blend of chemicals identified from honeybee (Apis mellifera) volatiles, Apidologie, 36, p523-532.
[https://doi.org/10.1051/apido:2005038]

- Zawislak, J., Managing small hive beetles. University of Arkansas, United States Department of agriculture and county governments cooperating, FSA-7075, (Available online at https://www.uaex.edu/publications/PDF/FSA-7075.pdf)/ Accessed 10 October 2017.