A Short Review on Neonicotinoids; Use in Crop Protection and Issues on Honeybee and Hive Products
Abstract
Neonicotinoids are a relatively new class of insecticides. Due to higher field efficacy, flexible application procedure and claim of low vertebrate toxicity neonicotinoid became a promising insecticide candidate, surpassed 2.6 billion USD in 2009 global scale and in the consecutive years had highest market share among all insecticides. But also, growing volume of evidence suggested that neonicotinoids affect honeybee adversely primarily via impairments on learning and memory, and ultimately foraging success leading to colony collapse. In order to protect honeybee and bee diversity, European Union, Korea banned the use of some neonicotinoids, such as clothianidin, thiamethoxam, imidacloprid. Undoubtedly the restriction of the use of these neonicotinoids on nectar and pollen-rich crops could be expected to reduce a potential threat to bees and at the same time, the policy allowed time for investigation in a field-realistic manner. In this present review, we focus on the concerns of neonicotinoids related to honeybee and hive contamination.
Keywords:
Neonics, Honeybee, Learning and memory, Foraging success, Policy, ToxicityINTRODUCTION
Plant pests including plant-feeding invertebrates, pathogens and weeds are significant constraints on crop production. Pesticide has become an indispensable component of present day’s agriculture in order to feed the rapidly growing global population. A sizable portion of pesticide industry has been occupied by insecticide. Insecticides are natural and synthetic chemicals used to control insect pests (Table 1). At the same time, synthetic insecticides are often received attention for appearing as an environmental pollutant, insect resistance development and especially non-target toxicities. It stands true for neonicotinoids, a class of relatively new insecticides introduced in the 1990s. The use of this class is of particular concern due to their potential non-target effect primarily on insect pollinators, honey bee in particular. At first, information came out based on observation of huge bee colony loss in 2006 (EPA, 2017a) and research on bees. Since the approval and marketing of neonicotinoids, two decades almost passed. During this period, many investigations have come up with evidence of the toxic effect of neonicotinoid on many different terrestrial and aquatic animals other than bees (Gibbons et al., 2015). Another concern is the long-term persistence of neonicotinoids in the environment. Despite having a voluminous dataset on the lethal and sub-lethal effect of neonicotinoids on bees and other terrestrial and aquatic organisms, information on the actual field-based usage of the insecticide are limited. As responsive policy few countries impose time-limited restriction on the use of three active ingredients of neonicotinoids namely clothianidin, thiamethoxam, and imidacloprid. However, the scientific debate continues. In this present review, we focus on the concerns of neonicotinoids related to honeybee and hive contamination and on-going regulatory debates for risk assessment and management relative to pollinator protection.
Use of neonicotinoids in crop protection
Insect control with chemical began about 2000 years ago and most natural products dominated as controlling agents. In the year 1940, first synthetic insecticide has been introduced as dichlorodiphenyl trichloroethane (DDT) and the journey of synthetic insecticide began. Neonicotinoids, synthetic derivatives of nicotine, are relatively new class of insecticides used worldwide to control a number of insect pests, particularly plant-chewing, -piercing and -sucking insects primarily belonging to hemipterans including aphids, leafhoopers, planthoopers, thrips and whiteflies (Elbert et al., 1991; Tomizawa and Casida, 2005). It is also effective managing some insect pests belonging to lepidopteran, dipteran and coleopteran insects (Elbert et al., 1998). According to IRAC (Insecticide Resistance Action Committee, 2017), there are seven active ingredients like imidacloprid, nitenpyram, acetamiprid, thiamethoxam, thiacloprid, clothianidin, dinotefuran. Out of these seven active ingredients, imidacloprid, thiamethoxam, clothianidin and dinotefuran belong to nitroguanidines; nitenpyram belongs to nitromethylenes; acetamiprid and thiacloprid belong to cyanamidines (Fig. 1). The physicochemical properties, pest spectrum, and application have been represented in Table 2.
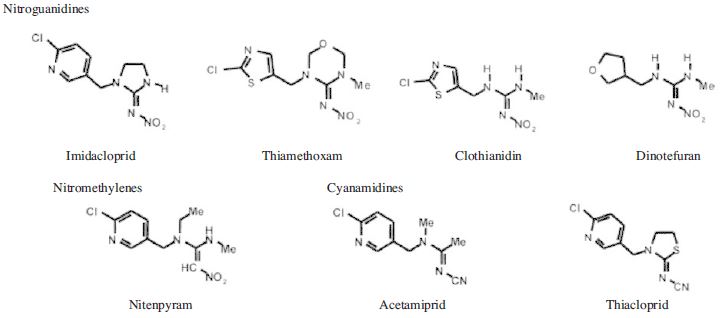
Chemical structures of seven known active ingredients of neonicotinoids relative to 3 chemical categories of nitroguanidines, nitromethylenes, and cyamidines.

Chronology of development of neonicotinoid pesticides with physico-chemical and biological properties
Foliar application of neonicotinoids is generally targeted against pests attacking crops such as cereals, maize, rice, potatoes, vegetables, sugar beet, cotton, tobacco and deciduous fruits (Elbert et al., 1998). It has been shown that 2.5~5 ppm a.i. (active ingredient) application in the soil controlled typical soil insect pests such as Agriotes sp., Diabrotica balteata or Hylemyia antiqua (Elbert et al., 1991). A new horizon of crop protection strategy i.e. seed treatment has been opened up with the development of neonicotinoids. Seed dressing, film coating, pelleting or multilayer coating allow for environmentally safe and perfect protection of crops against insect pests (Elbert et al., 2008). Neonicotinoids are widely used in seed coatings of a variety of crops such as cotton, corn, cereals, sugar beet, soybean, oilseed rape and are taken up systemically by growing plants and distributed to all tissues (Elbert et al., 2008; Rundlöf et al., 2015). Table 2 represents the pest spectrum and biological profile (foliar use, soil use, and seed treatment) of neonicotinoids.
Due to higher field efficacy, flexibility in application methods and most importantly in comparison to alternative insecticides like organophosphate lower vertebrate toxicity neonicotinoids became a promising insecticide candidate, surpassed 2.6 billion USD in 2009 global scale (Jeschke et al., 2011; Tomizawa and Casida, 2005 cf. LaLone et al., 2017). In the consecutive year, neonicotinoids have replaced organophosphate and became the largest category of insecticides with the market share of 25% of total insecticides globally. This rise continued for next few years. In 2014, the global neonicotinoids market value reached USD 3.9 billion, up by 7.1% compared with that of 2013.
However, there are some other concerns about resistance development and nontarget impact on beneficial invertebrates. Several insect pests have developed resistance to neonicotinoids insecticides (Table 3) with known resistance mechanisms. Apart from the list, neonicotinoid resistance has also been reported in several other insect pests such as white-backed planthopper Sogatella furcifera (Horvath), small brown planthopper Laodelphax striatellus (Fallèn), Asian citrus psyllid Diaphorina citri (Kuwayama), codling moth Cydia pomonella L., and western flower thrips Frankliniella occidentalis (Pergande) (Bass et al., 2015). Increasing list of neonicotinoid resistant insects (Bass et al., 2015), evidence suggesting the adverse primarily sub-lethal effects of neonicotinoids on terrestrial and aquatic vertebrate wildlife (Gibbons et al., 2015) are found to be threats to continue neonicotinoid applications.
Exposure of neonicotinoids to honeybee
In 2006 large number of worker honeybees disappeared from honeybee colony in the US. Sudden loss of colony’s worker bees led to losses of 30 to 90% of beehives in the US during 2006-2007. This phenomenon was called ‘colony collapse disorder’ (CCD) which has been characterized by the disappearance of majority of the worker bees, leaving behind a queen, plenty of food and a few nurse bees to care for remaining broods and immature bees and queen. Worldwide 35% of the crop production depends on pollinators (Klein et al., 2007) and reduction in honeybee colony would negatively effect on human nutrition (Chaplin-Kramer et al., 2014; Smith et al., 2015). Thus there was growing concern and initiatives to investigate the possible causes of the CCD and it included pesticide, habitat degradation, malnutrition, pathogens and Varroa infestations as possible factors.
Since the introduction of neonicotinoids in the global market and wide use in a variety of crops, several approaches also have been taken in order to investigate their lethal and sub-lethal (including foraging behavior, memory, locomotion, navigation or orientation) effects on honeybees (Auteri et al., 2017). The attention of the scientific communities has been observed by the increasing trend of publications (Fig. 2). Google scholar platform has been used to search the results. Year wise hit number was considered against ‘neonicotinoids and honeybee’ and ‘insecticides and honeybee’ and proportion of ‘neonicotinoid and insect’ in ‘insecticides and honeybee’ was calculated for the respective year. Growing volume of evidence suggested that neonicotinoids affect honeybee adversely primarily via impairments on learning and memory, and ultimately foraging ability (LaLone et al., 2017). Table 4 demonstrates the acute toxic level of neonicotinoids on honeybee. It has found that clothianidin and thiamethoxam possessed comparatively higher toxicity to honeybee. Metabolites of neonicotinoids also contribute to the toxicity. However, N-demethyl acetamiprid, 6- chloro-pyridilmethyl alcohol and 6-chloro-nicotinic acid (metabolites of acetamiprid) possesses less toxicity with a higher value of LD50 (>50000 ng bee-1) (Iwasa et al., 2004). Several studies have been carried out on different metabolites of imidacloprid viz. olefin (LD50 36 ng bee-1), 5-OH-imidacloprid (LD50 159 and 153 ng bee-1), Di-OHimidacloprid (LD50 > 49 ng bee-1), urea-metabolite (LD50 > 100000 ng bee-1) and 6-chloronicotinic acid (LD50 122000 ng bee-1) (Nauen et al., 2001; Decourtye et al., 2003). Genetic differences also exist in the response to neonicotinoids toxicity in honeybee (Laurino et al., 2013). A significant difference in LD50 value was found in case of imidacloprid and thiamethoxam for A. mellifera ligustica, A. mellifera mellifera and A. mellifera carnica and in case of clothianidin for A. mellifera ligustica and A. mellifera mellifera (Laurino et al., 2013). This is true for indirect contact toxicity i.e. LC50 also. However, existing reports on semi-field condition shows variable results. Test on Bombus terrestris foraging on plants grown from imidacloprid treated sunflower seed with 0.7mg seed-1 with chronic exposure did not demonstrate any foraging and colony vitality (Tasei et al., 2001).
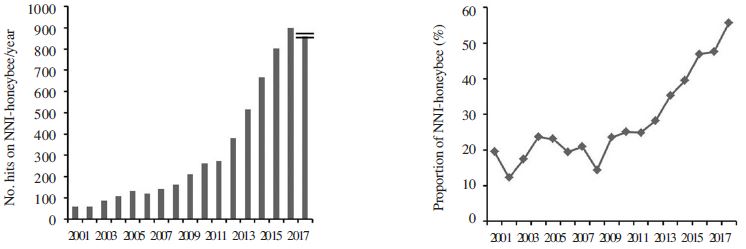
Search results in Google Scholar on ‘Neonicotinoids and honeybee’ (left), and proportion (%) of ‘Neonicotinoids and honeybee’ among ‘Insecticides and honeybee’.
In contrast, chronic exposure of 11 weeks with 200mg l-1 to 10μg l-1 imidacloprid contaminated sugar syrup with or without foraging resulted in LC50 59μg l-1 (ppb) and 20μgl-1 (ppb) respectively (Mommaerts and Smagghe, 2011). Effects on learning and memory under filed based condition are yet to be demonstrated and the small volume of existing data under variable conditions is not enough to draw conclusive remarks.
Most of the risk assessments of NNI on honeybee or pollinators were conducted in the laboratory or semi-field environments, which make the policy implementation difficult because of the lack of evidence in a realistic situation. Recently a country-specific field-based study has come up with evidence of negative effects of neonicotinoids on honeybee, bumblebee and solitary wild bees linked to neonicotinoid residues across the landscape (Woodcock et al., 2017). Winter-sown oilseed rape was grown commercially with either seed coating containing neonicotinoids (clothianidin or thiamethoxam) or no seed treatment (control) across three countries namely Hungary, Germany, and the UK. The investigation revealed variations in response among countries. Clothianidin resulted in significant reduction of overwintering colony size in Hungary and UK, in contrast, no effect was found in Germany. Further, reproduction of B. terrestris and Osmia bicornis was negatively correlated with neonicotinoid residues. In another field-based study in Canada demonstrated honeybee colonies located in neonicotinoid treated cornfield had higher Varroa loads than those in untreated corn fields (Alburaki et al., 2017).
Mechanisms on lethal and sub-lethal effect on honeybee
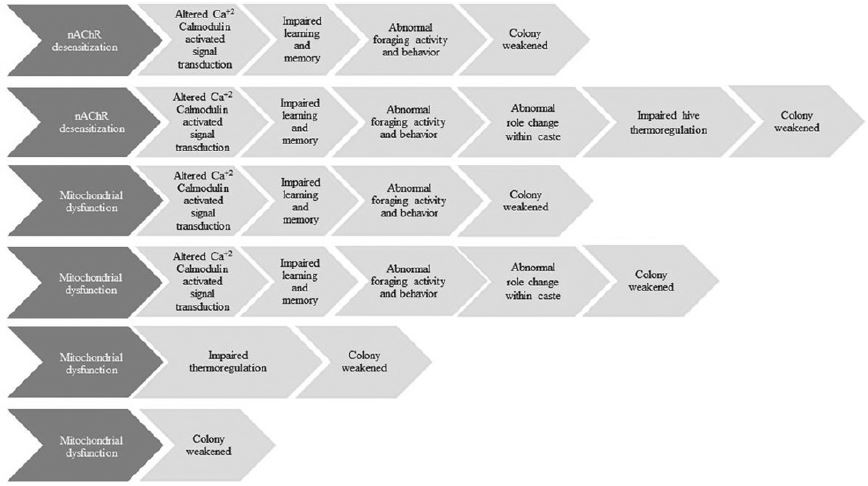
Neonicotinoids are neurotoxins affecting the nervous system of the organisms. Fig. 3 represents the plausible adverse outcome pathways (LaLone et al., 2017). Nicotinic acetylcholine receptors (nAChR) belong to the cys-loop superfamily of ligand-gated ion channel, responsible for rapid neurotransmission and are conserved across vertebrates and invertebrates (Karlin, 2002; Jones and Sattelle, 2010). Because of diverse functional architecture, the toxicological responses differ. Neonicotinoids are nAChR agonists i.e. a substance that initiates a physiological response when combined with a receptor. Upon prolonged and repeated exposure to neonicotinoids, desensitization occurs at the receptors resulting initial opening of the ion channel, ion exchange across cell membrane followed by rapid channel closure, effectively inhibiting neurotransmission (Quick and Lester, 2002; cf. LaLone et al., 2017). This is true for the case of honeybee, alterations in gene transcripts such as increasing abundance of nAChRα1 subunit in brain, vitellogenin, and genes related to immune and memory formation in response to?neonicotinoids (Christen et al., 2017). In another study (Li et al., 2017) comparing between Apis mellifera and A. cerana, it was found that A. cerana was more sensitive to imidacloprid and clothianidin. The same pattern was found for imidacloprid (Lee et al., 2016). However, they possess distinct mechanisms to mount an innate immune response against neonicotinoid exposure. In contrary to upregulation of carboxylesterase, prophenol oxidase and acetylcholinesterase activities in A. cerana, they were found significantly downregulated in A. mellifera after 48 hours imidacloprid treatment whereas during clothianidin exposure AChE was downregulated and glutathione S-transferase activity was upregulated in both the species. Different response was observed in different developmental stages of honeybee, increasing activities of glutathione S-transferase and carboxylesterase para were found in pupal stage in response to thiamethoxam exposure (Tavares et al., 2017). Strong downregulation of gene coding for major jelly proteins (MRJPs) was observed in response to imidacloprid exposure which weakens bee colony (Wu et al., 2017).
Another key mechanism is mitochondrial dysfunction. Mitochondria play a critical role in cellular respiration resulting in the production of adenosine triphosphate (ATP), biological energy currency. Further, mitochondria are also associated with several processes such as Ca+2 storage and release for cell signaling, heat production, mediates cell growth and cell death. Thus mitochondrial dysfunction manifests perturbations in these processes. However, uncertainty exists as to the presence of the nA-ChR in invertebrate mitochondria (LaLone et al., 2017). Studies with honeybee and bumblebee (Bombus terrestris) demonstrated adverse impacts on mitochondria while exposed to nAChR agonists (Moffat et al., 2015; Nicodemo et al., 2014). Moffat et al. (2015) described mitochondrial depolarization (i.e. loss of membrane potential) in bumblebee neurons upon 48 hours 1nM imidacloprid exposure. Change in foraging behavior of bumblebee in response to clothianidin has been confirmed by one recent study (Arce et al., 2017). Not only managed bee population but the study suggests that sub-lethal effects of neonicotinoids could also scale up causing loss of wild bees diversity (Woodcock et al., 2016).
Contamination of hive products
The laboratory experiments demonstrated well about the mechanism of how neonicotinoids affect honeybees. With the increasing volume of evidence, the doubt no longer exists about the negative effect of neonicotinoids on honeybee. However, one question still remains unanswered. The amounts detected in nectar and pollen was found often less than 10 ppb (Chauzat et al., 2006; 2009) whereas at least 40 ppb dose is necessary for abnormal honeybee foraging behavior, 0.5 ppm for missing bee and 3 ppm for 100% failure to return to a source of sugar offered to them (Yang et al., 2008). Guttation was found the overlooked source that contained a higher amount of neonicotinoids. Guttation, a physiological phenomenon occurring in many vascular plants especially grasses, is a formation of drops of xylem sap on the tips or along the edges of leaves. As neonicotinoids are systemic insecticides, it dissolves with water entering roots, mixing in the xylem sap and be exuded through hydathodes of leaf margins. In a study by Girolami et al. (2009) showed that leaf guttation drops of corn plants germinated from neonicotinoid-coated seeds contained insecticides constantly higher than 10mg/l (=10 ppm) with maxima up to 100mg/l (=100 ppm) for thiamethoxam and clothianidin and up to 200mg/l (=200 ppm) for imidacloprid. However, the analyses of environmental neonicotinoid pesticide residual level in plant, bees and bee products become an essential tool for risk assessment of the insecticides. Although environmental neonicotinoid residues were found to be lower than acute or chronic toxic level, it does not outskirt the possibility of being a source of bee poisoning (Blacquière et al., 2012; Codling et al., 2016). Because of their systemic property, neonicotinoids translocate from seed treatment to different plant parts and could be the cause of bee intoxication. Thus, there is increasing interest to examine the concentration of neonicotinoids in bee-collected materials such as pollen, nectar and bee products like honey and bee wax and bee itself to measure the exposure of the neonicotinoids (Mullin et al., 2010; Blacquière et al., 2012; Barganska et al., 2013; Kasiotis et al., 2014; Gbylik-Sikorska et al., 2015). However, the results revealed wide variations. In one study investigating the neonicotinoid residues in honey from beehive with proximity to canola, alfalfa, dandelions, and willow found that clothianidin and thiamethoxam were most frequently detected neonicotinoids found in 68 and 75% of honey samples with the mean concentration of 8.2 and 17.2 ng g-1 (ppb) respectively (Codling et al., 2016). 239 honey samples collected from 24 apiaries in different parts of France analyses found that 17.6 and 21.8% samples were positive with 6-chloronicotinic acid (a metabolite of imidacloprid) and imidacloprid with maximum 10.2 and 1.8μg kg-1 (ppb) concentration respectively (Chauzat et al., 2011). One monitoring study in Belgium published in 2007 showed only 4.6% of honey samples were found positive for imidacloprid contamination (Pirad et al., 2007). Another study in 2009 revealed, out of 48 honey samples collected different areas of Belgium in the proximity of maize field treated with 0.05 to 2.48% imidacloprid, 8.4% samples were found positive with mean concentration 0.275μg kg-1 (Nguyen et al., 2009). However, 91 honey samples from 73 apiaries in North West Spain analyses did not find any sample positive with neonicotinoid residues (Garcia-Chao et al., 2010). In the most recent study of 198 honey samples from all over the world demonstrated that overall, 75% of samples contained quantifiable neonicotinoid residues (Mitchell et al., 2017). However, the proportion varied considerably, being highest in North American honey (86%) followed by Asian (80%), European (79%) and the least for South American (57%) with an average of 1.8 ng g-1 (ppb) neonicotinoid in contaminated honey and reached to a maximum 56 ng g-1 (ppb) which lies in the range bioactive range causing deficits in learning behavior (Mitchell et al., 2017). 33 and 40.5% pollen samples out of 187 samples in Chauzat et al. (2011) study were detected positive with 6-chloronicotinic acid and imidacloprid with highest concentration of 9.3 and 5.7μg kg-1 (ppb) respectively. In contrast, Higes et al. (2010) and Bernal et al. (2010) did not find any neonicotinoid residues in pollen samples collected from different vegetation in Spain. To measure the exposure of the neonicotinoid, the best way is to determine the concentration in honeybees. In contrast to many findings with negative results (Pirad et al., 2007; Nguyen et al., 2009; Mullin et al., 2010). Chauzat et al. (2011) study found 18.7 and 11.2% honey bee samples with positive with the mean concentration of 1 and 1.2μg kg-1 (ppb) for 6-chloronicotinic acid and imidacloprid respectively. However, in majority, the concentration of neonicotinoid residues is lower than MRL (maximum residue limits) set by EC (European Commission) (EU MRLs: 50 ng/g for acetamiprid, imidacloprid, and thiacloprid and 10 ng/g for clothianidin and thiamethoxam cf. Tanner and Czerwenka, 2011).
Regulatory decision
The loss of honeybee colony has become a concern for the present and future food security and environmental sustainability although neonicotinoids share a sizable global economy. This provides the scope of scientific debate. Mounting evidence not funded by pesticide industry indicates even low level of exposure affects the ability of honeybee to communicate, can suppress their immune system, becomes more susceptible to viruses and Varroa mites (Vidau et al., 2011; Sánchez-Bayo et al., 2016; Brandt et al., 2016; Gregorc et al., 2016). In spring 2008, serious colony losses were reported in Italy and the Italian authority suspended the use of clothianidin, thiamethoxam, imidacloprid along with fipronil treated maize seed on the temporary basis (Auteri et al., 2017). Considerable amount investigations examining the impact of neonicotinoids on honeybee colony survival and development produced diverging data. Based on scientific reports (Whitehorn et al., 2012 on imidacloprid; Henry et al., 2012 on thiamethoxam; Schneider et al., 2012 on clothianidin) and data obtained from APENET during 2009 to 2011 in June 2012 European Food Safety Authority (EFSA) urged for further experimental data in order to draw a definite conclusion. In May 2013 European Commission declared restriction on the use of clothianidin, thiamethoxam, and imidacloprid in seed treatment. Subsequent to the ‘ban regulation’, in July 2013 EFSA also mandated to perform the risk assessment for foliar application and all uses other than seed treatment in these 3 neonicotinoids. In response to the EU decision, the Rural Development Administration (RDA) in Korea imposed a limited-time ban on the further inclusion in the list of application of those 3 chemicals in February 2014 (RDA, 2014). Following the open call in 2015, EFSA carried out an updated risk assessment in 2015 and 2016. In March 2017 EC presented its draft regulation to ban neonicotinoids to the Member States and later the deadline for this evaluation has been proposed 30 November 2017 (Pesticide Action Network, Europe 2017; EURAC TIVE.com). EFSA is currently working on the new mandate to review the risk assessment on neonicotinoids and the recent investigations with the field-based data are of immense importance to draw a conclusion which fosters the sustainable use of the chemical. Similarly U.S. Environmental Protection Agency (EPA) 2017 assessment also finds that imidacloprid possesses a risk to aquatic organisms. However, EPA keeps clothianidin, thiamethoxam and dinotefuran out the list of neonicotinoid possesses significant risk. The agency is scheduled to make the final decision in 2018 (EPA, 2017b). Canada’s Pest Management Regulatory Agency (PMRA) has targeted completion of the pollinator risk assessment for the neonicotinoids by December 2017 and consultation has been scheduled in early 2018 (Health Canada, 2017).
Acknowledgments
This study is partly supported from RDA Agenda research program (PJ01303701) on Economic valuation of pollination service.
LITERATURE CITED
-
Alburaki, M., B. Cheaib, L. Quesnel, P.-L. Mercier, M. Chagnon, and N. Derome, (2017), Performance of honeybee colonies located in neonicotinoid-treated and untreated cornfields in Quebec, J. Appl. Entomol, 141, p112-121.
[https://doi.org/10.1111/jen.12336]

-
Arce, A.N., T.I. David, E.L. Randall, A.R. Rodrigues, T.J. Colgan, Y. Wurm, and R.J. Gill, (2017), Impact of controlled neonicotinoid exposure on bumblebee in a realistic field setting, J. Appl. Ecol, 54, p1199-1208.
[https://doi.org/10.1111/1365-2664.12792]

-
Auteri, D., M. Arena, S. Barmaz, A. Ippolito, A. Linguadoca, T. Molnar, R. Sharp, C. Szentes, B. Vagenende, and A. Verani, (2017), Neonicotinoids and bees: the case of the European regulatory risk assessment, Sci. Total Environ, 579, p966-971.
[https://doi.org/10.1016/j.scitotenv.2016.10.158]

- Bargańska, Z., M. Slebioda, and J. Namieśsnik, (2013), Pesticide residues levels in honey from apiaries located of Northern Poland, Food Control, 31, p196-201.
-
Bass, C., C.T. Zimmer, J.M. Riveron, C.S. Wilding, C.S. Wondji, M. Kaussmann, L.M. Field, M.S. Williamson, and R. Nauen, (2013), Gene amplification and microsatellite polymorphism underlie a recent insect host shift, Proc. Natl. Acad. Sci. USA, 110, p19460-19465.
[https://doi.org/10.1073/pnas.1314122110]

-
Bass, C., I. Denholm, M.S. Williamson, and R. Nauen, (2015), The global status of insect resistance to neonicotinoid insecticide, Pestic. Biochem. Physiol, 121, p78-87.
[https://doi.org/10.1016/j.pestbp.2015.04.004]

-
Bernal, J., E. Garrido-Bailon, M.J. del Nozal, A.V. Gonzalez-Porto, R. Martin-Hernandez, J.C. Diego, J.J. Jimenez, J.L. Bernal, and M. Higes, (2010), Overview of pesticide residues in stored pollen and their potential effect on bee colony (Apis mellifera) losses in Spain, J. Econ. Entomol, 103, p1964-1971.
[https://doi.org/10.1603/ec10235]

- Blacquière, T., G. Smagghe, C.A.M. van Gestel, and V. Mommaerts, (2012), Neonicotinoids in bees: a review on concentrations, side-effects and risk assessment, Ecotoxicol:, 21, p973-992.
-
Bonmatin, J-M., C. Giorio, V. Girolami, D. Goulson, D. P. Kreutzweiser, C. Krupke, M. Liess, E. Long, M. Marzaro, E. A. D. Mitchell, D. A. Noome, N. Simon-Delso, and A. Tapparo, (2015), Environmental fate and exposure; neonicotinoids and fipronil, Environ Sci. Pollut. Res, 22, p35-67.
[https://doi.org/10.1007/s11356-014-3332-7]

-
Brandt, A., A. Gorenflo, R. Siede, M. Meixner, and R. B?chler, (2016), The neonicotinoids thiacloprid, imidacloprid, and clothianidin affect the immunocompetence of honey bees (Apis mellifera L.), J. Insect Physiol, 86, p40-47.
[https://doi.org/10.1016/j.jinsphys.2016.01.001]

-
Chaplin-Kramer, R., E. Dombeck, J. Gerber, K.A. Knuth, N.D. Mueller, M. Mueller, G. Ziv, and A-M. Klein, (2014), Global malnutrition overlaps with pollinator-dependent micronutrient production, Proc. R. Soc. B, 281, 20141799.
[https://doi.org/10.1098/rspb.2014.1799]

-
Christen, V., S. Bachofer, and K. Fent, (2017), Binary mixtures of neonicotinoids show different transcriptional changes than single neonicotinoids in honeybees (Apis mellifera), Environ. Pollut, 220, p1264-1270.
[https://doi.org/10.1016/j.envpol.2016.10.105]

-
Chauzat, M-P., A-C. Martel, N. Cougoule, P. Porta, J. Lachaize, S. Zeggane, M. Aubert, P. Carpentier, and JP. Faucon, (2011), An assessment of honeybee colony matrices, Apis mellifera (Hymenoptera: Apidae) to monitor pesticide presence in continental France, Environ. Toxicol. Chem, 30, p103-111.
[https://doi.org/10.1002/etc.361]

- Chauzat, M-P., P. Carpentier, A-C. Martel, S. Bougeard, N. Cougoule, P. Porta, J. Lachaize, F. Madec, M. Aubert, and J-P. Faucon, (2009), The influence of pesticide residues on honey bee (Hymenoptera: Apidae) colony health in France, Environ. Entomol, 38, p514-523.
- Chauzat, M-P., J-P. Faucon, A-C. Martel, J. Lachaize, N. Cougoule, and M. Aubert, (2006), A survey on pesticide residues in pollen loads collected by honeybees (Apis mellifera) in France, J. Econ. Entomol, 99, p253-262.
- Codling, G., Y.A. Naggar, J.P. Giesy, and A.J. Robertson, (2016), Concentration of neonicotinoids in honey, pollen and honey bees (Apis mellifera L.) in central Saakatchewan, Canada, Chemosphere, 144, p2321-2328.
-
Decourtye, A., E. Lacassie, and M.H. Pham-Delegue, (2003), Learning performance of honeybees (Apis mellifera L.) are differentially affected by imidacloprid according to the season, Pest Manag. Sci, 59, p269-278.
[https://doi.org/10.1002/ps.631]

-
Decourtye, A., and J. Devillers, (2010), Ecotoxicity of neonicotinoid insecticides to bees, p85-95, in Insect neonicotinic acetylcholine receptors S.H. Thany ed. By, 1st ed., Springer, New York.
[https://doi.org/10.1007/978-1-4419-6445-8_8]

-
EFSA (European Food Safety Authority), (2015a), Conclusion on the peer review of the pesticide risk assessment for bees for the active substance clothianidin considering all uses other than seed treatment and granules, EFSA J, 13, p4210.
[https://doi.org/10.2903/j.efsa.2015.4210]

-
EFSA (European Food Safety Authority), (2015b), Conclusion on the peer review of the pesticide risk assessment for bees for the active substance imidacloprid considering all uses other than seed treatment and granules, EFSA J, 13, p4211.
[https://doi.org/10.2903/j.efsa.2015.4211]

-
EFSA (European Food Safety Authority), (2015c), Conclusion on the peer review of the pesticide risk assessment for bees for the active substance thiamethoxam considering all uses other than seed treatment and granules, EFSA J, 13, p4212.
[https://doi.org/10.2903/j.efsa.2015.4212]

- Elbert, A., B. Becker, J. Harwtig, and C. Erdelen, (1991), Imidacloprid - anew systemic insecticide, Pflanzenschutz- Nachrichten Bayer (German edition), 44, p113-136.
-
Elbert, A., M. Haas, B. Springer, W. Thielert, and R. Nauen, (2008), Applied aspects of neonicotinoid uses in crop protection, Pest Manag. Sci, 64, p1099-1105.
[https://doi.org/10.1002/ps.1616]

-
Elbert, A., R. Nauen, and W. Leicht, (1998), Imidacloprid, a novel chloronicotinyl insecticide: biological activity and agricultural importance, p50-71, in Insecticides with novelmodes of action I. Ishaaya eds. by, and D. Degheele, 1st ed., p289NArosa Publishing House, New Delhi.
[https://doi.org/10.1007/978-3-662-03565-8_4]

- EPA (Unites States Environmental Protection Agency), (2017a), Colony collapse disorder, www.epa.gov/pollinator-protection/colony-collapse-disorder Accessed on 5th Nov. 2017.
- EPA (United States Environemntal Protection Agency), (2017b), Pollinator protection: Scheduled for review of Neonicotinoid pesticides, https://www.epa.gov/pollinator-protection/schedule-review-neonicotinoid-pesticides Accessed on 11th Nov. 2017.
- EURACTIVE.com, (2017), Commission readies further restrictions to neonicotinoids, www.euractive.com/section/agriculture-food/news/commission-readies-further-restriictions-to-neonicotinoids Accessed on 10th Nov 2017.
- Garcia-Chao, M., M.J. Agruna, G.F. Calvete, V. Sakkas, M. Llompart, and T. Dagnac, (2010), Validation of an off line solid phase extraction liquid chromatographytandem mass spectrometry method for the determination of systemic insecticide residues in honey and pollen samples collected in apiaries from NW Spain, Analytica Chimica Acta, 672, p107-113.
- Gbylik-Sikorska, M., T. Sniegocki, and A. Posyniak, (2015), Determination of neonicotinoid insecticides and their metabolites in honey bee by liquid chromatography tandem mass spectrometry, J. Chromatogr. B, 990, p132-140.
-
Gibbons, D., C. Morrissey, and P. Mineau, (2015), A review of the direct and indirect effects of neonicotinoids and fipronil on vertebrate wildlife, Environ. Sci. Pollut. Sci, 22, p103-118.
[https://doi.org/10.1007/s11356-014-3180-5]

-
Girolami, V., L. Mazzon, A. Squartini, N. Mori, M. Marzaro, A. D. Bernardo, M. Greatti, C. Giorio, and A. Tapparo, (2009), Translocation of neonicotinoid insecticides from coated seeds to seedling guttation drops: A novel way of intoxication for bees, J. Econ. Entomol, 102, p1808-1815.
[https://doi.org/10.1603/029.102.0511]

- Gregorc, A., E.C.M. Silva-Zacarin, S.M. Carvalho, D. Kramberger, E.W. Teixeira, and O. Malaspina, (2016), Effects of Nosema ceranae and thiamethoxam in Apis mellifera: comparative study in Africanized and Carniolan honey bees, Chemosphere, 147, p328-336.
-
Henry, M, M. Beguin, F. Requier, O. Rollin, J-F. Odoux, P. Aupinel, J. Aptel, S. Tchamitchian, and A. Decourtye, (2012), A common pesticide decreases foraging success and survival in honey bees, Sciencexpress, 1215039.
[https://doi.org/10.1126/science.1215039]

- Health Canada, (2017), Update on neonicotinoid pesticide, 29 June 2017, https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/pesticides-pest-management/fact-sheet-sother-resources/neonicotinoid-pesticides-bee-health/update-2017.html Accessed on 11th Nov 2017.
-
Higes, M., R. Martin-Hernandez, A. Martinez-Salvador, E. Garrido-Bailon, A.V. Gonzalez-Porto, A. Meana, J.L. Bernal, M.J. del Nozal, and J. Bernal, (2010), A preliminary study of the epidemiological factors related to honey bee colony loss in Spain, Environ. Microbiol. Rep, 2, p243-250.
[https://doi.org/10.1111/j.1758-2229.2009.00099.x]

- Hojland, D.H., K.M.V. Jensen, and A. Kristensen, (2014), A comparative study of P450 gene expression in field and laboratory Musca domestica L, Strains. Pest Manag. Sci, 70, p1237-1242.
- IRAC (Insecticide Resistance Action Committee), (2017), Mode of Action (MoA) classification Version 8.3, July 2017.
-
Iwasa, T., N. Motoyama, J.T. Ambrose, and M.R. Roe, (2004), Mechanism for the differential toxicity of neonicotinoid insecticides in the honey bee, Apis mellifera, Crop. Prot, 23, p371-378.
[https://doi.org/10.1016/s0261-2194(03)00230-8]

-
Jeschke, P., R. Nauen, M. Schindler, and A. Elbert, (2011), Overview of the status and global strategy for neonicotinoids, J. Agric. Food Chem, 59, p2897-2908.
[https://doi.org/10.1021/jf101303g]

- Jones, A.K., and D.B. Sattelle, (2010), Diversity of insect nicotinic acetylcholine receptor sub-units, Adv. Exp. Med. Biol, 683, p25-43.
-
Karatolos, N., Y. Pauchet, P. Wilkinson, R. Chauhan, I. Denholm, K. Gorman, (2011), Pyrosequencing the transcriptome of the greenhouse whitefly, Trialeurodes vaporariorum reveals multiple transcript encoding insecticide targets and detoxifying enzymes, BMC Genomics, 12, p56.
[https://doi.org/10.1186/1471-2164-12-56]

- Karlin, A., (2002), Emerging structure of the nicotinic acetylcholine receptors, Nat. Rev. Neurosci, 3, p102-114.
-
Karunker, I., E. Morou, D. Nikou, R. Nauen, R. Sertchook, B.J. Stevenson, M.J. Paine, S. Morin, and J. Vontas, (2009), Structural model and functional characterization of the Bemisia tabaci CYP6CM1vQ, a cytochrome P450 associated with high levels of imidacloprid resistance, Insect Biochem Mol. Biol, 39, p697-706.
[https://doi.org/10.1016/j.ibmb.2009.08.006]

-
Karunker, I., J. Benting, B. Lueke, T. Ponge, R. Nauen, E. Roditakis, J. Vontas, K. Gorman, I. Denholm, and S. Morin, (2008), Over-expression of cytochrome P450 CYP6CM1 is associated with high resistance to imidacloprid in the B and Q biotypes of Bemisia tabaci (Hemiptera: Aleyrodidae), Insect Biochem. Mol. Biol, 38, p634-644.
[https://doi.org/10.1016/j.ibmb.2008.03.008]

- Kasiotis, K.M., C. Anagnostopoulos, P. Anastasiadou, and K. Machera, (2014), Pesticide residues in honeybees, honey and bee pollen by LC-MS/MS screeing: reported death incidents in honeybees, Sci. Total Environ, 485-486, p633-642.
-
Klein, A-M., B.E. Vaissiere, J.H. Cane, I. Steffan-Dewenter, S.A. Cunningham, C. Kremen, and T. Tscharntke, (2007), Importance of pollinators in changing landscapes for world crops, Proc. R. Soc. B, 274, p303-313.
[https://doi.org/10.1098/rspb.2006.3721]

- Koo, H.N., J.J. An, S.E. Park, J.I. Kim, and G.H. Kim, (2014), Regional susceptibility to 12 insecticides of melon and cotton aphid, Aphis gossypii (Hemiptera: Aphididae) and a point mutation associated with imidacloprid resistance, Crop Prot, 55, p91-97.
- LaLone, C.A., D.L. Villeneuve, J. Wu-Smart, R.Y. Milsk, K. Sappington, K.V. Garber, J. Housenger, and G.T. Ankley, (2017), Weight of evidence evaluation of a network of adverse outcome pathways linking activation of the nicotinic acetylcholine receptor in honey bees to colony death, Sci. Total Environ. 584-, 585, p751-775.
- Laurino, D., A. Manino, A. Patetta, and M. Porporato, (2013), Toxicity of neonicotinoid insecticides on different honey bee genotypes, Bull. Insectol, 66, p119-126.
-
Lee, C.Y., Jeong, S.M., Jung, C., Burgett, M., (2016), Acute oral toxicity of neonicotinoid insecticides to four species of honey bee, Apis florea, A. cerana, A. mellifera, and A. dorsata, J. Apic, 31, p51-58.
[https://doi.org/10.17519/apiculture.2016.04.31.1.51]

- Li, Z., M. Li, J. He, X. Zhao, V. Chaimanee, W-F. Huang, H. Nie, Y. Zhao, and S. Su, (2017), Differential physiological effects of neonicotinoid insecticides on honey bees: A comparison between Apis mellifera and Apis cerana, Pestic. Biochem. Physiol, 140, p1-8.
- Liu, Z.W., M.S. Williamson, S.J. Lansdell, I. Denholm, Z.J. Han, and N.S. Millar, (2005), A nicotinic acetylcholine receptor mutation conferring target-site resistance to imidacloprid in Nilaparvata lugens (brown planthopper), Proc. Natl. Acad. Sci. USA, 102, p8420-8425.
-
Markussen, M.D.K., and M. Kristensen, (2010), Cytochrome P450 monooxygenase-mediated neonicotinoid resistance in the housefly Musca domestica L, Pestic. Biochem. Physiol, 98, p50-58.
[https://doi.org/10.1016/j.pestbp.2010.04.012]

-
Mitchell, E.A.D., B. Mulhauser, M. Mulot, A. Mutabazi, G. Glauser, and A. Aebi, (2017), A worldwide survey of neonicotinoids in honey, Science, 358, p109-111.
[https://doi.org/10.1126/science.aan3684]

- Moffat, C., J.G. Pacheco, S. Sharp, A.J. Samson, K.A. Bollan, J. Huang, S.T. Buckland, and C.N. Connolly, (2015), Chronic exposure to neonicotinoids increases neuronal vulnerabilityto mitochondrial dysfunction in the bumblebee (Bombus terrestris), Fed. Am. Soc. Exp. Biol, 29, p2112-2119.
-
Mommaerts, V., and G. Smagghe, (2011), Side-effects of pesticides on the pollinator Bombus: an overview, p507-552, in Pesticides of the modern world M. Stoytcheva ed. by, In Tech, Rijeka.
[https://doi.org/10.5772/25254]

-
Mota-Sanchez, D., R.M. Hollingworth, E.J. Grafius, and D.D. Moyer, (2006), Resistance and cross-resistance to neonicotinoid insecticides and spinosad in the Colorado potato beetle, Leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae), Pest Manag. Sci, 62, p30-37.
[https://doi.org/10.1002/ps.1120]

-
Mullin, C.A., M. Frazier, J.L. Frazier, S. Ashcraft, R. Simonds, D. VanEngelsdorp, and J.S. Pettis, (2010), High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health, PloS ONE, 5, e9754.
[https://doi.org/10.1371/journal.pone.0009754]

-
Nauen, R., N. Stumpf, and A. Elbert, (2002), Toxicological and mechanistic studies on neonicotinoid cross resistance in Q-type Bemisia tabaci (Hemiptera: Aleyrodidae), Pest Manag. Sci, 58, p868-875.
[https://doi.org/10.1002/ps.557]

- Nguyen, B.K., C. Saegerman, C. Pirad, J. Mignon, J. Widart, B. Tuirionet, F.J. Verheggen, D. Berkvens, E. De Pauw, and E. Haubruge, (2009), Does imidacloprid seed-treatment maize have an impact on honey bee mortality, J. Econ. Entomol, 102, p616-623.
-
Nauen, R., U. Ebbinghaus-Kintscher, and R. Schmuck, (2001), Toxicity and nicotinic acetylcholine receptor interaction of imidacloprid and its metabolites in Apis mellifera (Hymenoptera: Apidae), Pest Manag. Sci, 57, p577-586.
[https://doi.org/10.1002/ps.331.abs]

-
Nicodemo, D., M.A. Maioli, H.C. Medeiros, M. Guelfi, K.V. Balieira, D. De Jong, and F.E. Mingatto, (2014), Fipronil and imidacloprid reduce honeybee mitochondrial activity, Environ. Toxicol. Chem, 33, p2070-2075.
[https://doi.org/10.1002/etc.2655]

- Pesticide Action Network, Europe, (2017), Press Release on Bee-killing Neonicotinoids: European Commission proposal for a complete ban, www.pan-europe.info/press-release/2017/03/bee-killing-neonicotinoids-european-commission-proposal-complete-ban Accessed on 10th Nov 2017.
- Pirad, C, J. Widart, B.K. Nguyen, C. Deleuze, L. Heudt, E. Haubruge, E. De Pauw, and J.F. Focant, (2007), Development and validation of a multi-residue method for pesticide determination in honey using on-column liquid-liquid extraction and liquid chromatographytandem mass spectrometry, J. Chromatogr. A, 1152, p116-123.
- Puinean, A.M., S.P. Foster, L. Oliphant, I. Denholm, L.M. Field, N.S. Millar, S. Martin, S. Williamson, and C. Bass, (2010), Amplification of a cytochrome P450 gene is associated with resistance neonicotinoid insecticides in the aphid Myzus persicae, PLoS Genet, 6, e1000999.
-
Quick, M.W., and R.A. Lester, (2002), Desensitization of neuronal nicotinic receptors, J. Neurobiol, 53, p457-478.
[https://doi.org/10.1002/neu.10109]

-
Rauch, N., and R. Nauen, (2003), Identification of biochemical markers linked to neonicotinoid cross resistance in Bemisia tabaci (Hemiptera: Aleyrodidae), Arch. Insect Biochem. Physiol, 54, p165-176.
[https://doi.org/10.1002/arch.10114]

-
Rundlöf, M., G.K.S. Andersson, R. Bommarco, I. Fries, V. Hederstrom, L. Herbertsoon, O. Jonsson, B.K. Klatt, T.R. Pedersen, J. Yourstone, and H.G. Smith, (2015), Seed coarting with a neonicotinoid insecticide negatively affects wild bees, Res. Lett.
[https://doi.org/10.1038/nature14420]

- Rural Development Administration (RDA), (2014), Press report. No use of pesticide which harm to honeybee, http://www.rda.go.kr/board/board.doboardId=farmprmninfo&prgId=day_farmprmninfoEntry&currPage=3&dataNo=100000530824&mode=updateCnt&searchSDate=&searchEDate= Accessed on 11th Nov 2017.
- Sánchez-Bayo, F., D. Goulson, F. Pennacchio, F. Nazzi, K. Goka, and N. Desneux, (2016), Are bee diseases linked to pesticides? - A brief review, Environ. Int. 89-, 90, p7-11.
-
Schneider, C.W., J. Tautz, B. Grinewald, and S. Fuchs, (2012), RFID tracking of sublethal effects of two neonicotinoid insecticides on the foraging behaviour of Apis mellifera, Plos ONE, 7, e30023.
[https://doi.org/10.1371/journal.pone.0030023]

- Shi, X.G., Y.K. Zhu, X.M. Xia, K. Qiao, H.Y. Wang, and K.Y. Wang, (2012), The mutation in nicotinic acetylcholine receptor beta 1 subunit may confer resistance to imidacl-oprid in Aphis gossypii (Glover), J. Food Agric. Environ, 10, p1227-1230.
-
Smith, M.R., G.M. Singh, D. Mozaffarian, and S.S. Myers, (2015), Effects of decreases of animal pollinators on human nutrition and global health: a modelling analysis, Lancet, 386, p1964-1972.
[https://doi.org/10.1016/S0140-6736(15)61085-6]

- Tanner, G., and C. Czerwenka, (2011), LC-MS/MS analysis of neonicotinoid insecticides in honey: methodology and residue findkings in Austrain honeys, J. Agric. Food Chem, 59, p12271-12277.
- Tasei, J.N., G. Ripault, and E. Revault, (2001), Hazards of imidacloprid seed coating to Bombus terrestris (Hymenoptera: Apidae) when applied to sunflower, J. Econ. Entomol, 94, p623-627.
-
Tavares, D.A., C. Dussaubat, A. Kretzchmar, S. M. Carvalho, E.C.M. Silva-Zacarin, O. Malaspina, G. B`erail, J-L. Brunet, and L.P. Belzunces, (2017), Exposure of larvae to thiamethoxam affects the survival and physiology of the honey bee at post-embryonic stages, Environ. Pollut, 229, p386-393.
[https://doi.org/10.1016/j.envpol.2017.05.092]

-
Tomizawa, M., and J.E. Casida, (2005), Neonicotinoid insecticide toxicology: mechanisms of selective action, Annu. Rev. Pharmacol. Toxicol, 45, p247-268.
[https://doi.org/10.1146/annurev.pharmtox.45.120403.095930]

-
Vidau, C., M. Diogon, J. Aufauvre, R. Fontbonne, B. Vigues, J.L. Brunet, C. Texier, D.G. Biron, N. Blot, H. El Alaoui, L.P. Belzunces, and F. Delbac, (2011), Exposure to sublethal doses of fipronil and thiacloprid highly increases mortality of honeybees previously infected by Nosema ceranae, PlosONE, 6, e21550.
[https://doi.org/10.1371/journal.pone.0021550]

-
Whitehorn, P.R., S. O’Connor, F.L. Wackers, and D. Goulson, (2012), Neonicotinoid pesticide reduces bumble bee colony growth and queen production, Sciencexpress, 1215025.
[https://doi.org/10.1126/science.1215025]

-
Woodcock, B.A., J.M. Bullock, R. F. Shore, M. S. Heard, M. G. Pereira, J. Redhead, L. Ridding, H. Dean, D. Sleep, P. Henrys, J. Peyton, S. Hulmes, L. Hulmes, M. Sarospataki, C. Saure, M. Edwards, E. Genersch, S. Knabe, and R. F. Pywell, (2017), Country-specific effects of neonicotinoid pesticides on honey bees and wild bees, Science, 356, p1393-1395.
[https://doi.org/10.1126/science.aaa1190]

-
Woodcock, B.A., N.J.B. Isaac, J.M. Bullock, D. B. Roy, D.G. Garthwaite, A. Crowe, and R.F. Pywell, (2016), Impacts of neonicotinoid use on long-term population changes in wild bees in England, Nat. Commun, 7, p12459.
[https://doi.org/10.1038/ncomms12459]

-
Wu, M-C., Y-W. Chang, K-H. Lu, and E-C. Yang, (2017), Gene expression changes in honey bees induced by sublethal imidacloprid exposure during the larval stage, Insect Biochem. Mol. Biol, 88, p12-20.
[https://doi.org/10.1016/j.ibmb.2017.06.016]

-
Yang, E.C., Y.C. Chuang, Y.L. Chen, and L.H. Chang, (2008), Abnormal foraging behavior induced by sublethal dosage of imidacloprid in the honey bee (Hymenoptera: Apidae), J. Econ. Emtomol, 101, p1743-1748.
[https://doi.org/10.1603/0022-0493-101.6.1743]

- Zhao, J.Z., B.A. Bishop, and E.J. Grafius, (2000), Inheritance and synergism of resistance to imidacloprid in the Colorado potato beetle (Coleoptera: Chrysomelidae) populations, J. Econ. Entomol, 93, p1508-1514.