Antimicrobial Activities of Tanzania Honey Bees in Relation to Vegetation Types
Abstract
The effect of area and vegetation types on antimicrobial activity of stinging honey bees in Tanzania was studied. Twelve potential honey producing areas with various vegetation types were selected and 36 beekeepers were randomly sampled and involved in the study. Areas selected included Itigi, Nyakanazi, Gairo, Inyonga, Bukombe, Dodoma rural, Dodoma urban, Manyoni, Morogoro, Kisarawe and Tabora. Honey samples were taken direct from the bee hives, three samples were collected from the same area and same vegetation type, bulked to obtain one representative sample. The honey sugar and water content were determined, antimicrobial activities using four pathogenic micro organisms namely Staphylococcus aureus, Salmonella typhi, Escherichia coli and Candida albicans were tested. Results showed that all honey were acidic with pH ranging from 4.05 to 4.8. Area, vegetation types and their interactions significantly affected antimicrobial activities of honey. Gairo honey (A. digitata and A. malvaceae) significantly inhibited growth of both bacteria (E. coli 26.5mm, S. aureus 35.5mm and S. typhi 27.5mm) and fungus (C. albicans 30.5mm). Similarly, honey from Manyoni (Baphia massaiensis, Baphia burtu, Raphia pruinoides and Pseudoprosopsis fischeri), Morogoro (Eastern Arc forests), Kisarawe (E. bicompacta), Itigi (Baphia massaiensis, Baphia burtu, Raphia pruinoides and Pseudoprosopsis fischeri ) and Dodoma (Brachystegia spp., Julbernadia spp. and Isoberlinia spp.), Dodoma (sunflower and A. digitata) resisted bacteria and fungus growth. It was concluded from the study that honey quality and composition depends much on vegetation types available in an area. Human activities that results into deforestation for any reason affects honey composition and quality.
Keywords:
Medicinal, Properties, Stinging, Bees, Variation, AreasINTRODUCTION
Honey is an organic, naturally sweet substance, produced from nectar and sugary exudation of plants by honey bees like Apis mellifera and Trigona meliponini (Codex Standard, 1996). Nectar of flowers is gathered, modified, mixed with some enzyimes and regurgitated and transformed by honey bees into honey cell (Stefan, 2009).
Currently consumption of honey is increasing because of its beneficial biological and physical chemical properties including antibacterial activities. Tanzania produces an estimate of 10,000 tons of honey annually (UNDP, 2014). more than half of the honey produced in Tanzania is consumed locally as food and medicine. The rest is sold to other countries particularly European Union member countries such as UK, Netherlands and German. Other countries are Oman, United Arab Emirates, Iran, Uganda, Kenya, and Rwanda (Mwakatobe and Mlingwa, 2004).
Honey being a natural product with many qualities that are beneficial for human beings, it is considered to be important in human nutrition and health (Alis et al., 2012). For thousands of years (since 2100 BC), ancient Greeks used honey as traditional food and healing agent (Alisi et al., 2012). Its properties make it potential to serve as a natural food with high sugar, hydrogen peroxide and high acidity which are responsible for its medicinal properties. In addition to inhibiting pathogenic microbial growth, some of honey components have a role to play in controlling inflammation and promoting the healing process through the modulation of cytokines, fibroblast proliferation and angeo-genesis (Tonks et al., 2003). It has powerful immune system booster and carbohydrates that provide strength and energy to the body. Presence of enzymes in honey helps to improve digestive system, and it decreases muscle fatigue of the body (Rodriguez, 2004).
Honey differs in composition and quality between types, batches, production methods, husbandry and area where honey emanates (French et al., 2005).This variations also results into variation of antimicrobial properties of honey. It has been reported elsewhere that ability of honey to inhibit pathogenic microbial growth vary with area where honey emanates (Kumar et al., 2010; Jones, 2001). However, available studies in Tanzania have reported on physico-chemical properties of Tanzanian honey (Gidamis et al., 2004; Masoud, 2014). There is scanty information on antimicrobial activities of Tanzanian honey. Therefore, the objective of this study was to investigate the influence of geographical area, vegetation types where bees are foraging on antimicrobial properties of Tanzanian honey and recommend the best honey in the country based on antimicrobial activity.
Various pathogenic microorganisms such as bacteria including Escherichia coli, Salmonella typhi, Staphylococcus aureus and fungus such as Candida albicans are detrimental to health and food as they can cause diseases, food spoilage and food poisoning (Blackburn, 2006). Escherichia coli cause sickness, food poisoning and are potential indicator organisms to test environmental hygiene for contamination (Feng et al., 2002). Bacteria such as Salmonella typhi cause illnesses such as typhoid fever, and food poisoning (Ryan and Ray, 2004). Staphylococcus aureus is a common causative of boils, impetigo, cellulites, toxic shock syndrome and food poisoning (Levinson, 2010). In the same way fungus such as Candida albicans is a causal agent of oral and genital infections in humans. All these pathogens, cause food borne diseases and become a cause of major health concerns. Therefore, alternative use of natural food products such as honey with biological properties can help in suppression and prevention of these pathogenic microorganisms.
MATERIALS AND METHODS
Honey sampling procedure
A purposive sampling protocol was used to select high honey producing areas and vegetation types. Twelve available areas were selected. Each 3 beekeepers were selected randomly belonging to each area and vegetation type, making a total of 36 beekeepers who were involved in the study. Areas selected and vegetation types are as shown in Table 1.
Honey was harvested directly from the beehives and bulked according to the area and vegetation type, packaged in sterilized plastic bottles (330ml) then transported and stored at ambient temperature (25±2°C) at laboratory of Micro-Biology at the Nelson Mandela Institute of Science & Technology, Arusha-Tanzania.
Antimicrobial sensitivity test
Antimicrobial sensitivity test of the honey samples was determined by Agar Wells Diffusion method as described by Clinical Laboratory Standard Institute (2009). Where Mueller-Hinton Agar (composed of 2g beef extract, 17.5g acid hydrolysate of casein, 1.5g starch and 17g agar) medium was used for antimicrobial susceptibility test. The medium was prepared following the manufacturer’s instructions. After autoclaving (121°C for 15min) the medium was left to cool to 50°C. Then 25ml per plate (15 ×100mm) was measured and pouring in a way that level pouring surface within the petridish was uniform to a depth of 4 mm and was incubated in an incubator (35±2°C) for 24 h.
The test organisms included bacteria (Escherichia coli (ATCC 25922), Salmonella typhi (ATCC14023) and Staphylococcus aureus (ATCC 25923) and fungus (Candida albicans-isolated from clinical samples), were streaked on to a non-inhibitory agar medium (broth agar) to obtain isolated colonies. After incubation at 35°C overnight, 4 to 5 colonies were picked and inoculated into broth (Mueller- Hinton broth) and incubated at 35°C for 24 h. A sterile cotton swab was dipped into the suspension, pressed firmly against the inside wall of the tube just above the fluid level, then streaked over the entire surface of the medium rotating the plate approximately 60 degree after each application to ensure an even distribution of the inoculums.
Finally cotton were placed all around the edge of the agar surface. Small holes of 5 mm were made on the petri dishes with agar by using sterile loops, and then 100μl of honey sample was placed in the agar hole using sterile micropipette. The plates were inverted and incubated at 37 ±1°C for 24 h for tested microorganisms. After incubation period the diameter (mm) of the zones of complete inhibition (including the diameter of the disk) was measured and recorded. The measurements were made with ruler on the undersurface of the plate without opening the lids.
Honey pH determination
pH of honey samples was determined using a digital portable pH meter (JENWAY, UK 3305P) in accordance with International Honey Commission (2009). In between the readings of different samples, the electrode was washed with distilled water and dried with tissue paper, and inserted into prepared honey samples and recorded. The experiment was done in triplicates.
Honey total sugar and water content
The honey sugar and water content wasdetermined using a digital portable honey refractometer that was held on a flat surface and a drop of honey was inserted on a glass prism that determines refractive index. The measurement key was pressed and results were displayed on the backlit LC-Display. In between the readings of different samples, the surface of the glass prism was washed with distilled water and dried with tissue paper before another honey sample was inserted.
Statistical data analysis
Data obtained from antimicrobial analysis were analyzed using SAS software. Analysis of Variance (ANOVA) was used to determine the influence of main independent variables, the factor influence was considered to be significant when p<0.05. Mean comparison between area and vegetation types was done using Duncan Multiple Range Test (DMRT) and results were presented as Mean±SD.
RESULTS AND DISCUSSION
pH, sugar and water contents of stinging bee honey
Table 2 shows the composition and chemical properties of honey collected from various areas with various vegetation types. The pH of honey samples used in this study ranged from 4.05 to 4.8 which means that all honey samples were acidic.The pH rangeobserved in sampled honeys falls within the range reported by Gidamis et al. (2004) while working with Tanzanian honey collected from Dodoma, Tanga, Morogoro, Same, Arusha and Tabora, reported a pH range of 4.4 - 4.87. Also, complies with the range reported by United Republic of Tanzania - Ministry of Natural Resources and Tourism (2007) who reported a pH range of 3.42 to 6.10 for Tanzanian honey. However, the observed pH range in this study was higher than that reported by Masoud (2014) working with 26 honey producing areas in Tanzania who reported a pH range of 2.6 to 4.4. The difference could be due to difference in soil type and vegetation growing in areas where the samples were collected. Despite the slight differences in pH the honey samples in the present study might have potential medicinal properties as its acidic nature could have resulted from organic acids which remarkably create an acidic micro-environment that threatens pathogenic microbial growth (Aparna and Rajalakshmj, 1999).

pH, sugar and water contents of stinging bee honey collected from different places in and vegetation types in Tanzanian
The total sugar content that ranged from 74~83% (Table 2) was within a range observed by other researchers in Tanzania. Masoud (2014) working on the quality of Tanzanian honey collected from 26 honey potential producing areas reported a sugar content range of 64.2 to 84.8%. Furthermore, it is above the minimum standards of Tanzanian honey of not less than 65% (URT, 2007). The sugar contained in studied honey was sufficient enough to produce high osmotic effect that could dehydrate microbial cells (Molan, 1992 and Bogdanov et al., 1997). Gluconic acids which remarkably create an acidic micro - environment prevents the growth of many microorganisms (Cooper, 2007).
There was an inverse relationship between sugar and water contained in honey. The minimum value observed in this study was 17% with a maximum value of 24%. Some honey in this study had higher water content than the recommended range of 20~22% (URT (2007) for Tanzanian honey. High water content contributes to reduced shelf life of honey that undergoes fermentation and spoil more easily (Cortopassi et al., 2006). However, despite the high water content of some few honey samples, the results of this study suggest that Tanzanian honeys could be among the best honeys that can be used not only as food but also as remedies to various microbial infections.
Effect of area, vegetation and their interaction on microbial growth
Generally, area from which honey was collected, vegetation type on which honey bees foraged, test microorganisms and its interaction influenced significantly (df=47, F=454, P=0.0001) the antimicrobial properties of honey.
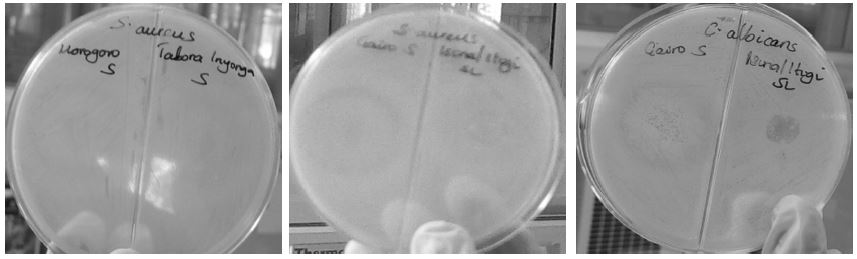
It is evident from Table 3 that honey from Gairo where bees foraged on Astripomoea malvaceae and Adansonia digitata was the best honey (df=5, F=101.30, P=0.0001) as it had relatively strongest ability to inhibit microbial growth than other honeys. The Manyoni thicket honey was the second best honey as followed by Morogoro Eastern Arc forest honey that did not differ in inhibiting microbial growth with that from Kisarawe where bees mostly foraged on Euphobia bicompacta. Honey from Inyonga exhibited significantly (df=11, F=316.18, P=0.0001) lowest ability to inhibit microbial growth, generally most of honey from Miombo woodland including Nyakanazi and Tabora had unexpectedly lower ability to inhibit microbial growth (Table 3, Plate 1).
The difference in ability to resist microbial growth could probably be due to differences in vegetation types between these areas (Kumar et al., 2010). Different vegetation types contain different floral types that vary in pollen and nectar which influence the honey medicinal qualities and composition (Jones, 2001).
The conclusive selection of the best honey can not only be judged by looking at the overall resistance on microbial growth alone. The ability of honey to inhibit multiple microorganisms or to have a broader spectrum antimicrobial ability could be the best property to be looked at.
Antimicrobial spectrum of Tanzania stinging bee honey
Table 4 presents the antimicrobial spectrum of honey in relation to the vegetation types that bees foraged on. It is clearly observed that the Gairo honey obtained from bees foraging on Adansonia digitata and Astripomoea malvaceae, significantly (df=32, F=147.56, P=0.0001) inhibited the growth of both, pathogenic fungus (C. albicans 30.5mm) and bacteria (E. coli 26.5mm, S. aureus and S. typhi 27.5mm) followed by honey originating from Manyoni-thicket (C. albicans 14.5mm, E. coli 22.5mm, S. aureus 31.5mm and S. typhi 25.5mm), Morogoro-Eastern Arc forest (C. albicans 16.5mm, E. coli 22.5mm, S. aureus 30.5mm and S. typhi 28.5mm), Kisarawe-Euphobia bicompacta (C. albicans 12.5mm, E. coli 22.5mm, S. aureus 29.5mm and S. typhi 25.5mm) Itigi-thicket (C. albicans 10.5mm, E. coli 10.5mm, S. aureus 21.5mm and S. typhi 28.5mm) and Dodoma-Miombo woodland (C. albicans 14.5mm, E. coli 7.5mm, S. aureus 23.5mm and S. typhi 25.5mm) and Dodoma-Adansonia digitata (C. albicans 11.5mm, E. coli 18.5mm, S.aureus 23.5mm and S. typhi 30.5mm). Other honeys such as Tabora honey from Miombo woodland could not resist the growth of C. albicans, from Miombo woodland (Nyakanazi, Inyonga and Bukombe) and Dodoma honey from sunflower could not resist the growth of E. coli respectively. Based on the criteria of honey to contain relatively broader medicinal properties and thus be broad spectrum antimicrobial, it can be said that the priory mentioned honeys could be the best as far as this study is concerned.

Variation in ability of Tanzanian honey types to inhibit growth of various pathogenic micro-organisms
The general characteristics of honey to prevent bacterial or fungal growth have been explained by various scientists. Garcial et al. (1986); Wahdan (1998); Molan (1999a) and Khan et al. (2007) reported inhibition of pathogenic microbial growth to be due to presence of hydrogen peroxide resulting from the action of glucose oxidase enzyme produced from hypopharyngeal glands of workers bees on glucose in presence of oxygen that inhibits microbial and fungal growth. Presence of physical-chemical properties such as high sugar content (about 80% w/w) that results into high osmotic effect that dehydrate micro-organism has been reported to inhibit microbial growth (Molan, 1992 and Bogdanov et al., 1997). Aparna and Rajalashmj (1999); White (1978) suggested inhibition of microbial growth to be due to presence of diverse organic acids such as gluconic acids that remarkably creates an acidic micro-environment (pH 3-4.5) that prevents growth of many micro-organisms. Apart from hydrogen peroxide as a factor that inhibits microbial growth, Cabrera et al.(2006) elucidated inhibition of microbial growth to be due to presence of non-peroxidic substances such as polyphenols which possess antimicrobial activity. Furthermore, Molan (1992a) reported that in most of honey antimicrobial growth depends on enzymatic generation of hydrogen peroxide to varying degree, but in some honeys there are additional phytochemical antibacterial factors. Cooper et al. (2002) elucidated that antimicrobial agents have been applied to wounds for 1000 years ago but many ancient remedies have been discontinued because the evidence to support their efficacy was anecdotal. He further, said that failure to identify botanical sources of honey used in many of the studies or to determine their antibacterial potency makes comparison of reported sensitivity unreliable. Jones (2001) also reported that it is remarkable that ancient physicians were selective in the honeys they utilized in their remedies. All these observations reported exhibits that there is variation in medicinal contents depending on area and type of vegetation where honey bees forages. This means that the antimicrobial composition depends on whether the plant foraged is a medicinal plant or not.The broader antimicrobial spectrum observed on Gairo honey where bee forages on Adansonia digitata and Astripomoea malvaceae could be attributed not only by acidity, osmolarity and hydrogen peroxide production but also by phytochemical components of that honey. Baobab fruits pulp and seeds are rich in vitamin C and protein and are apparently effective against dysentery and circulatory disease. On the other hand, Astripomoea malvaceae is a medicinal plant, in Tanzania a root decoction is drunk or the ground roots are taken inorder to treat hookworms. In Malawi a poultice of crushed roots is applied to the swellings and inflammations, especially to treat eye infection, also the sap of leaves and flowers is applied to inflammation of eyeball. In Zimbabwe, a root decoction is drunk to treat coughs, female infertility, dizziness and abdominal pain in babies (Schmetzer and Gurib, 2013). Similar arguments also stand for the Kisarawe honey where honey bees were foraging on Euphobia bicompacta. The plant sap of Euphobia bicompacta is used in Kenya to treat East Coast Fever in cattle whereas a decoction of its leaves and stem bark is given to drink to cattle to control ticks (Schmetzer and Gurib, 2013). Furthermore, in Angola the root fusion of Euphobia is drunk to treat pain in hips and madness. An infusion of Euphobia roots is taken to treat stomach ache, dropsy, stitch, urogenital problems, excessive menstruation, Tuberculosis and cardiac palpitation, the latex is used as fish poison (Schmetzer and Gurib, 2013).
It can be said from findings of this study that honey differs in antimicrobial properties due to differences in constitution and quality of pollen and nectar. Nectar and pollen from medicinal plants is far better to produce honey with broader antimicrobial spectrum. This study supports the study by Tonl et al. (2003) who reported that the Manuka honey produced by bees foraging on Manuka plants in Australia and New Zealand is believed to have superior antimicrobial properties hydrogen peroxide content, phytochemical contents or presence of dihydroacetone in the nectar which is derived from methyglyoxal.
Human activities have been reported to influence so much the honey quality and composition. Schmetzer and Gurib (2013) pointed out that genetic diversity of many plants species in Africa is being eroded sometimes at an alarming rate as a consequence of habitat destruction and over exploitation. The replacement of landraces of cultivated species by modern cultivars by seed companies is another cause of genetic erosion. This was observed in this study that honey from sunflower had relatively low ability in inhibiting microbial growth (C. albicans 8.5mm, E. coli 0.0mm, S. aureus 31.5mm and S. typhi 30.5mm). Surprisingly, honey from Miombo woodland seemed to have lowest medicinal ability (Table 4). This could be attributed by forest encroachment where most of trees are cut down by agro-pastoralists to open up areas for crop farming and creating unfavorable environment for tsetse flies that affects their livestock.
CONCLUSIONS
From the results of this study it can be concluded that low pH and high sugar content of honey creates acidic environment and osmolarity that results into antimicrobial properties of Tanzanian honey. Furthermore, the variation in ability to inhibit microbial growth was exhibited to vary with the vegetation types. Vegetation with medicinal properties foraged by honey bees tended to produce honey with high ability to inhibit microbial. Human encroachment to natural forests impacts on plant genetic diversity by deforestation to create crop lands and chase out tsetse flies for wellbeing of their livestock, affects plants that are foraged by bees, and thus, making bees to forage mainly on cultivated crops with scanty or no medicinal value.
Acknowledgments
The authors are so grateful to the Kyoto University through the Japanese Society for the Promotion of Science (JSPS) that supported financially the study and stay of the first author in Japan. The guidance of Ms. Anna Kawakita in Japan and introduction to various Institutions that enabled me to do this work is highly appreciable. Cooperation of beekeepers and permission to collect samples is exceptionally appreciated. Gratitude goes to Ms. Josephine Mapunda for the use of Microbiology Laboratory of Nelson Mandela African Institute of Science and Technology (NM-AIST) for an excellent microbiology work. Also the sample preparation done by Theodora Ngowi and Winni Makata is acknowledged.
References
-
Alisi, C.S., O.A. Ojiako, C.U. Igwe, C.O. Ujowundu, K. Anugweje, and G.N. Okwu, (2012), Antioxidant Content and Free Radical Scavenging Activity of Honeys of Apis mellifera of Obudu Cattle Ranch, International Journal of Biochemistry Research and Review, 2(4), p164-75.
[https://doi.org/10.9734/ijbcrr/2012/1581]

-
Aparna, A.R., and D. Rajalakshmj, (1999), Honey-Its characteristics, sensory aspects, and applications, Food Review Inernationalt, 15, p455-471.
[https://doi.org/10.1080/87559129909541199]

- Blackburn, C., (2006), Introduction. In food spoilage microorganisms, pxvii-xxii, Blackburn, C Eds. by, 1st ed., 695. Wood head Publishing, Cambridge, England.
- Bogdanov, S., P. Martin, and C. Lullmann, (1997), Harmonised methods of the European Honey Commission, Apidologie, 28, p1-59.
- Cabrera, L., E. Cespedes, R. Nava, and G. Ojeda de Rodriguez, (2006), Antibacterial activity with honey, Journal of Antimicrobial Agents, 16, p556-5563.
- Clinical and Laboratory Standards Institute, (2009), Performance standards for antimicrobial disk susceptibility tests, p238-488.
- Codex Standard for Honey, C.A.C, (1996), FAO Agricultural Services Bulletin, Value Added Products from Beekeeping, Agriculture and Consumer Protection Department, Rome.
- Cooper, R., (2007), Honey in wound care: Antibacterial properties, GMS International Journal of. Antimicrobial Agents, 2, p1-3.
- Feng, P., S. Weagant, and M. Grant, (2002), “Enumeration of Escherichia coli and the Coliform Bacteria”, In Bacteriological Analytical Manual 8th Ed., p115-124.
-
French, V.M., R.A. Cooper, and P.C. Molan, (2005), The antibacterial activity of honey against coagulase-negative Staphylococci, Journal of Antimicrobial and Chemotherapy, 56, p228-31.
[https://doi.org/10.1093/jac/dki193]

- Garcia, A., D. Soto, and C. Romo, (1986), Honey composition and properties, Food chemistry, 14, p185-191.
-
Gidamis, A.B., B.E. Chove, B.S. Shayo, S.A. Nnko, and N.T. Bangu, (2004), Quality Evaluation of Honey Harvested from Selected Areas in Tanzania with Special Emphasis on Hydroxymethyl Furfural (HMF) levels, Plant Foods human Nutrition, 59, p129-132.
[https://doi.org/10.1007/s11130-004-0020-7]

- Jones, R., (2001), Honey and healing through the ages, In Honey and healing Edsby Munn, P., and R. Jones, International Bee Research Association IBRA, Cardiff, RC Press, Boca Raton, FL, USA, 239(1), p70-76.
-
Khan, F.R., Z.U. Abadin, and N. Rauf, (2007), Honey nutritional and medicinal value, International Journal of Clinical Practice, 61, p1705-1707.
[https://doi.org/10.1111/j.1742-1241.2007.01417.x]

- Kumar, S.K.P., B. Debjit, and M.R. Chandira, (2010), Medicinal uses and health benefits of Honey:, Journal of Chemistry and Pharmacology Research, 2(1), p385-395.
- Masoud, H.M., (2014), Assessment of Quality of Tanzanian Honey based on Physicochemical Properties, Food Science and Quality Management, 33, p61-72.
- Molan, P.C., (1992), The antibacterial activity of honey, International Journal of Clinical Practice, 73, p5-28.
- Molan, P.C., (1999a), Why honey is effective as a medicine. Its use in modern medicine, Journal of Apicultural Research, 80, p80-92.
- Mwakatobe, A., and C. Mlingwa, (2004), The status of Tanzanian honey Trade-Domestic and International Market, Journal of Apicultural Research, 3, p1-13.
- Rodriguez, O.G., (2004), Characterization of honey produced in Venezuela, Food Chemistry, 84(4), p499-502.
- Ryan, K.J., and C.G. Ray, (2004), Medical Microbiology, Journal of Pharmacological. Research, 4, p8-362.
- Schmetzer, P.H., and F.A. Gurib, (2013), Plant resources of tropical Africa 11(2). In Medicinal plants 2, PROTA Foundation, Wageningen, Netherlands/CTA, Wageningen, Netherlands, p384.
- Stefan, B., (2009), Bee product Science. Chapter 4, CRC Press, New York, USA., p356.
- Tonks, A.J., E. Dudley, N.G. Porter, J. Parton, J. Brazier, E.L. Smith, (2007), Component of Manuka honey stimulates immune cells via TLR4, Journal of Leukalyote Biolology, 82, p1147-1155.
- UNDP, (2014), Honey value chain mapping in Njombe and Siha Districts, https;//info.undp.org/docs/documents/TZA/Reports%20-%20Final%20Honey%20mapping%202014.pdf Sited June 28 2018.
- United Republic of Tanzania-Ministry of Natural Resources and Tourism, (2007), Guideline for Quality Assurance of Bee Products in Tanzania, Forestry and Beekeeping Division, p32.
-
Wahdan, H.A.L., (1998), Causes of the antimicrobial activity of honey, Journal of Food Reearch, 26, p26-31.
[https://doi.org/10.1007/bf02768748]