
Effects of Larval Grafted Age for Artificial Queen-rearing on Queen Reproductive Potential and Growth of Apis cerana Colony
Abstract
Queen honey bees (Apis cerana) can be reared artificially on demand by the use of grafting technique. The technique consists of grafting young worker larvae into queen cell cups and raising in a queenless strong colony. As the age of grafted larvae for queen rearing exhibited several aspects related to quality and reproductive potential of queen, this study were conducted to investigate the influence of age of grafted larva on morphological characteristics and lifespan of queen, and the growth of colony she headed. Our results demonstrated that queens reared from young worker larvae (i.e., less than 1-day old larvae) were significantly larger in size (i.e., body weight and thorax width) than that of queens reared from 2-day old worker larvae. Moreover, queens reared from younger worker larvae initiated egg-laying earlier, stored more spermatozoa in spermatheca and had a longer lifespan compared to queens raised from older worker larvae. We also found a significant positive effect of queen grafting age on the production of worker and drone brood, adult worker population in colonies headed by queens reared from younger larvae. These findings suggested that rearing queens from brood grafted at the earliest possible age could increase the reproductive potential of queen as well as fitness of colony she head.
Keywords:
Apis cerana, Queen rearing, Reproduction, Colony growth, Queen quality, Natural matingINTRODUCTION
Honey bees colony reveals a strong reproductive division of labour with three types of individual groups as queen, worker and drone. Caste determination is a consequence of environmental conditions during development, during which female larvae may become either queens or workers depending on their larval diet (Wheeler, 1986; Linksvayer et al., 2011). The queen, a sole fertilized-egg layer within a colony, holds the most important role in a colony for both genetic and social reasons to keep colony fitness and productivity. In addition, the queen continuously produces a suite of pheromones to prevent the workers from both raising new queens and developing their ovaries (Winston, 1991). Therefore, understanding the factors affecting reproductive potential of honey bee queen will provide valuable insights for improving queen quality and honey bee colony fitness.
The reproductive potential of honey bee queens, served as proxies for queen reproductive quality, is directly linked to many aspects of a queen’s reproductive phenotype including morphological measures, such as body weight, thorax width (Dedej et al., 1998; Hatch et al., 1999; Kahya et al., 2008), reproductive success or fecundity (Woyke, 1971; Nelson and Gary, 1983). In addition, quality of queen is not only a function of her own reproductive potential but also related with number of stored sperm in the spermatheca (Woyke, 1975; Shah and Shah, 1980; Al-Lawati et al., 2009). A mated queen of Apis cerana typically stores approximately 1.3~2.7 million spermatozoa in spermatheca that she uses to fertilize eggs over her lifetime (Ruttner et al., 1972; Woyke, 1975).
Queen body size has been found to be directly associated with mating frequency, spermatheca size, ovariole number and ovary weight (Woyke, 1971; Dedej et al., 1998; Hatch et al., 1999; Delaney et al., 2011; Tarpy et al., 2011). Given that the sexually mature queen with a higher reproductive potential might be likely to produce colonies that exhibit higher growth and more productivity. Nelson and Gary (1983) found that both honey and brood production were significantly associated with queen size. However, there were little known the effects of A. cerana queen size on longevity and reproduction potential of queen and the aspects of colony growth (e.g., worker population, amount of drone and worker brood produced).
Rearing honeybee queens naturally occurs when the colony is in the process of swarming, supersedure or when the queen has been accidentally lost or killed (Winston, 1991). In beekeeping practice, queen can be artificially reared on demand by the use of various queen-rearing methods. Beekeepers commonly transfer (graft) young worker larvae into artificial queen cell cups, and introduce these into a queenless colony for acceptance and initial feeding. Previous studies demonstrated how queens raised from worker larvae of increasing age had diminished reproductive potential, as measured by weight, ovariole number, spermatheca diameter, and stored sperm counts (Woyke, 1971; Woyke, 1973; Abrol et al., 2005; Rangel et al., 2013). However, it was unclear how different age of grafted larvae affected to the quality of A. cerana queen.
This study focused on the effects of queen reproductive potential on colony growth in long-term experiment. In doing so, we measured the morphological characteristics, insemination success and lifespan of queens reared from different larval ages, and determined the effects of queen’s reproductive quality on growth of colony she head. We showed that age of larva to rear queen had effects on queen’s reproductive potential, and that this directly influenced on colony’s phenotype.
MATERIALS AND METHODS
Honey bee colonies, indigenous Korean A. cerana, used as sources of bees in this study were hived in modified Langstroth 10-frame hive-box (41×30×25cm) containing four bee combs, and located in an apiary at National Institute of Agricultural Sciences, Rural Development Administration, Jeonju City, Korea (36°49’30” N, 127°2’3” E) from May 2017 to April 2018. Drones were raised from five colonies on 20 April 2017 prior to queen rearing about one month. All the experimental queens were raised from a single mother colony, and thus they were sisters to each other. Queens were reared from known ages of worker larvae using grafting method. To obtain agecontrolled larvae for queen rearing, all comb containing open brood of the source colony were eliminated and the queen were confined to a well-drawn comb with empty cells using a queen excluder. The queen was allowed to lay eggs on that comb for six hours, and then she was eliminated from the hive. Six hours after larval eclosion, or 78 hrs after oviposition, the comb was retrieved from the source colony and newly emerged larva (now < 0-day old larva) was physically transferred (“grafted”) from the cell of comb into queen cup containing 10μl of fresh royal jelly, and then suspended vertically on a queen cell frame containing 25 queen cups in a queenless nursery colony to be raised as queens. Since the first cohort of larvae was grafted, the larval comb was immediately placed back to its hive. The following day, or approximately two days after larval eclosion, another cohort of second instar larvae (i.e., now > 2-day-old larvae) were grafted and queen cups were raised in a separate queenless nursery colony. Strong colonies crowded with nurse bees were selected as queen-rearing colony (i.e., “nursery colony”) to rear grafted larvae. The resulting queens developed inside their respective cell in nursery colony and were later transferred to individual queen-cages (3.5×10×1.5cm) at day tenth after grafting. Queen cells were then kept in incubator (Growth chamber, Vision VS-1203PFHLN, Korea) at 34°C and 60% relative humid until emergence. Virgin queens, soon after emerging from queen cells, were either used to measure morphological characters or introduced to mating hive containing three brood combs (39×23cm) covered with approximately 3,000 adult worker bees. Two cohorts of queen, 50 queen cells for each group, were artificially reared on 28 and 29 May 2017.
Queens reared from first and second instar larvae were respectively termed as high-quality and low-quality queen group. Twelve newly emerged queens of each group were anesthetized by narcotizing them with CO2 for about five minutes or until they were immobilized to characterize their body size. They were weighed individually to the nearest 0.1mg on a digital scale, and then thoracic widths were measured using digital caliper to the nearest 0.01mm. Another cohort of twenty newly emerged queens (i.e., virgin queen) of each group was individually released to mating hives, where they were allowed to roam freely for mating flight. Mating hives were checked daily from day seventh after queen emergence to determine which queens had mated successfully by verifying that they initiated egg-laying. After queens started in laying eggs, six queens of each group were confined to separated queen cages, and then were dissected in phosphate-buffered solution (PBS: 137mM NaCl, 2.7mM KCl, 10mM Na2HPO4, 1.8mM KH2PO4, pH 7.4) to count the number of spermatozoa stored in their spermatheca. The spermatheca of each queen was transferred to a 1.5ml Eppendorf tube and number of sperm cells were counted by the standard method described by Cobey et al. (2013). Other ten egg-laying queens in each group were introduced to new established colonies for further investigation of colony growth and queen supersedure.
In order to evaluate effect of queen grafting age on colony growth, egg-laying queens of each group were individually introduced to new established colonies contained approximately 4,000 adults adhering on four brood combs (39×23cm) on 2 July 2017. Newly established colonies headed by experimental queens were located in an apiary and randomly arranged with the distance of at least three meters to each other to limit adults drifting among colonies.
Colony growth measured by number of sealed brood (i.e., drone and worker brood) and adult worker in experimental colony were evaluated monthly from 2 August 2017 (i.e., one month after colony establishment) through 30 March 2018 (i.e., the last day of data collection, or day 270) using a wire-gridded wooden frame consisting of 28, 4.6× 4.6cm square-compartments, For the estimation of total number of sealed brood and adult worker, we first counted number of adult workers in all square-compartment of gridded frame overlaid on both sides of comb, and summed both sides to have number of adult worker on a comb. Then, we counted all square-compartment containing sealed brood on both sides of comb, and extrapolated the resulting number of sealed brood for each comb by multiplying of total square-compartments by 100 (approximately 100 worker cells of A. cerana per a square-compartment) (Chinh et al., 2005). The measure process was repeated for every comb in a hive to have total number of sealed brood and adult worker. In addition, any episodes of queen supersedure were recorded to determine whether queen reproductive potential had an effect on the likelihood that a colony superseded its queen. A supersedure event was defined as the presence of queen cells (or new virgin queen) and the absence of the experimental queen and eggs on the cells of comb (e.g., oviposition stopped). Any queen lost with unknown reason was assumed to be queen supersedure. In the case of queens that died during the winter (i.e., from November 2017 to January 2018), the date of death represented in the first colony inspection in February 2018.
Data analysis
Data on weight, thorax width, number of spermatozoa in spermatheca and number of day to initiate egg-laying of queen, and number of sealed brood (i.e., drone and worker) and adult worker in colonies headed by either queen that were raised from newly emerged larvae (i.e., less than 1-day old larvae) or those raised from 2-day old larvae were analyzed using Student’s t-test for independent samples. The proportion of queen supersedure over time was compared using Kaplan-Meier estimates followed by log-rank (Mantel-Cox) post hoc tests. The time that queen survived in colony was defined by rounding up to nearest month. For all testing, differences between groups at P<0.05 were considered statistically significant. All data sets were analyzed using IBM SPSS statistic version 22 for Windows (IBM Corp., NY, USA).
RESULTS AND DISCUSSION
The size of virgin queens, measured by wet weight and thorax width, was significantly larger for the queens raised from newly emerged larvae (high-quality queens, mean±SE: 159.8±2.8mg, 3.98±0.04mm, respectively), compared to low-quality queens raised from 2-day old larvae (Student’s t-test, wet weight: t=8.01, P<0.01, Fig. 1A; thorax width: t=3.93, P<0.01, Fig. 1B). Previous findings were corroborated our results that rearing queens from brood grafted at the earliest possible age could increase the body size of queen (Woyke, 1971; Tarpy et al., 2011; Rangel et al., 2013). Moreover, body size of queen honey bee could be used as a factor for judging the quality of queen as a larger queen had more ovarioles, larger spermathecae and more spermatozoa in the spermathecae, and thus positively correlated with aspects of queen fecundity and egg laying (Woyke, 1971; Nelson and Gary, 1983; Dedej et al., 1998; Hatch et al., 1999).
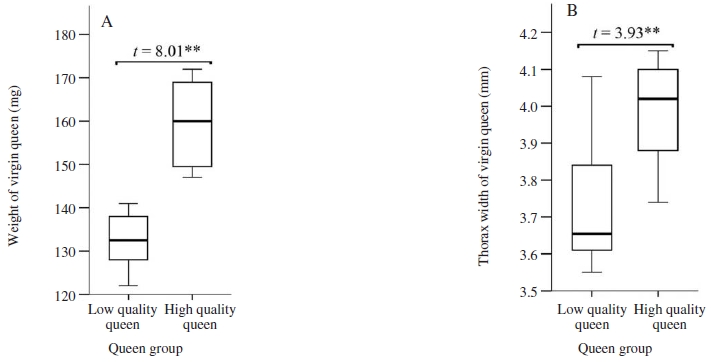
Wet weight (A) and thorax width (B) of virgin queen. Boxplots demonstrate the lower quartile, median (vertical lines inside the box), and upper quartile, and whiskers represent 1.5 times the interquartile range. Independent-samples t-test of mean comparison fo1lowed by a P value shown as; **: significant difference at the 0.01 level.
High-quality queen initiated egg laying (at 10.83 days after queen emergence) at two days earlier than that in low-quality queen, although the difference between two group was statically significant at the 0.05 level (Student’s t-test, t=2.05, 0.05>P>0.01, Fig. 2).
Once the queens of two groups initiated egg-laying, six queens from each group were sampled to count the sperm cells in their spermatheca. Numbers of sperm cells in spermatheca of queen shown in Fig. 3 revealed that high-quality queens had a statistically higher number of stored spermatozoa in their spermathecae (2.68×106 spermatozoa) in comparison to low-quality queens (1.74×106 spermatozoa, Student’s t-test : t=2.24, 0.05>P>0.01, Fig. 3). Thus, the high-quality queens indeed had not only significantly heavier in weight and larger in thorax size, but also stored more spermatozoa in their spermatheca, compared to low-quality queens. The insemination quality resulting in mating success and number of spermatozoa stored in spermatheca of the queens were inherently affected by various confounding factors. For instance, the differences of local mating environments (e.g., weather and geographic location), drone availability, density and sperm loads among males, or queen decision-making etc., could create significant variation among queens (Woyke, 1975; Lensky and Demter, 1985; da Silva et al., 1995; Schlüns et al., 2003; Koeniger et al., 2005). However, weight and thorax width of queen was found to be positively correlated with both stored sperm number and mating frequency, and hence queen with larger body size mated with higher number of drones and stored more spermatozoa in spermatheca in comparison to smaller queen (Kahya et al., 2008; Delaney et al., 2011; Tarpy et al., 2011). In addition, larger in body size would be a good proxy for the volume of the spermatheca, which would enable larger queens to store a greater number of spermatozoa (Woyke, 1971; Hatch et al., 1999; Delaney et al., 2011). In contrary, studies found in Apis mellifera that flight duration had no effect on mating number or spermatozoa stored in spermatheca (Tarpy and Page, 2000; Koeniger and Koeniger, 2007). Nevertheless, our results found in A. cerana revealed that the larger in body size of queen had positive effect on stored sperm counts, and thus this suggested that the younger in age of grafted larvae for queen rearing, the higher reproductive potential the queen had.
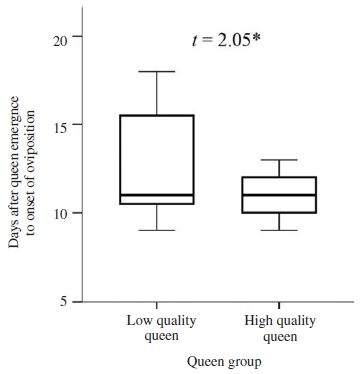
Length of time (day) between emergence until the onset of oviposition in queen. Boxplots demonstrate the lower quartile, median (vertical lines inside the box), and upper quartile, and whiskers represent 1.5 times the interquartile range. Independent-samples t-test of mean comparison fo1lowed by a P value shown as; *: significant difference at the 0.05 level.
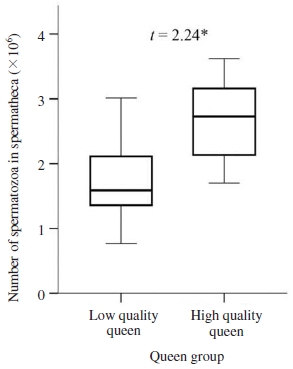
Number of spermatozoa stored in the spermatheca of queens. Boxplots demonstrate the lower quartile, median (vertical lines inside the box), and upper quartile, and whiskers represent 1.5 times the interquartile range. Independent-samples t-test of mean comparison fo1lowed by a P value shown as *: significant difference at the 0.05 level.
The effect of queen reproductive quality on colony growth over time was shown in Fig. 4. The production of drones were found to be earlier in colonies headed by high-quality queen when some strong colonies started in rearing drone in late September 2017 whereas the colonies headed by low-quality queen only started in rearing drone on October 2017 (Fig. 4A). At the two peaks of drone production (i.e., October 2017 and March 2018), colonies headed by high-quality queen reared significantly more drone brood than did the colonies headed by low-quality queen (Student’s t-test, at 90 days after colony establishment: t=5.21, P<0.01; at 270 days after colony establishment: t=3.04, P<0.01; Fig. 4A).
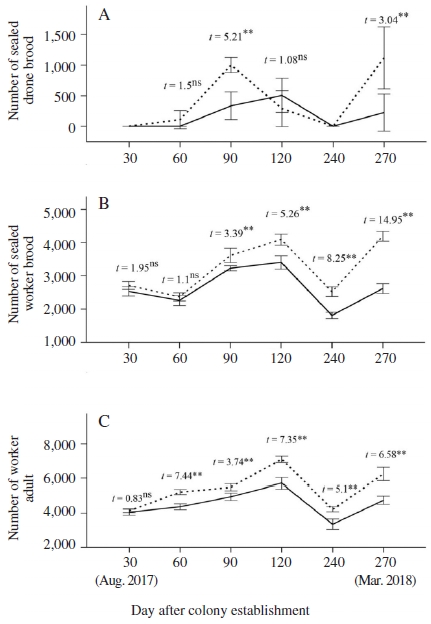
Number of sealed drone brood (A), sealed worker brood (B) and worker adult (C) in colonies headed by high-quality and low-quality queens. Data are presented as the mean±S.E. Independent-samples t-test of mean comparison fo1lowed by a P value shown as; ns: not significant difference, *: significant difference at the 0.05 level and **: significant difference at the 0.01 level.
Colonies headed by high-quality queens produced significantly more amount of sealed worker brood than those colonies headed by low-quality queens in the period from 90 days after colony establishment to the end of experiment (Student’s t-test, from 90 to 270 days after colony establishment, P<0.01, Fig. 4B). At the two peaks of brood production at 120 and 270 days after colony establishment, colonies headed by high-quality queen had respectively 20.1 and 60% more sealed worker brood, compared to colonies headed by low-quality queens. Due to the lack of floral resources (e.g., natural pollen and nectar) in dearth period of summer season, although colonies were supplemental fed (e.g., sugar syrup and pollen supplement), they did not prosper and the areas of brood being measured were small. Therefore, the number of sealed brood in colonies between two groups was not significantly different from August to September 2017 (Student’s t-test, P>0.05, Fig. 4B).
Colony size expressed by number of adult bees was found to be larger in colonies headed by high-quality queens over time after colony establishment 60 days, compared to colonies headed by low-quality queens (Student’s t-test, from 60 days after colony establishment to the end of experiment: P<0.01, Fig. 4C). At the two peaks of colony size at 120 and 270 days after colony establishment, colonies headed by high-quality queen had respectively 24.1 and 31.4% more adult worker, compared to colonies headed by low-quality queens. Our study showed that A. cerana colonies headed by high-quality queens grew larger than those headed by low-quality queens. Findings in A. mellifera were also demonstrated that queen reproductive potential was directly associated with various aspects of colony fitness including positive effect on parameters of colony growth and honey production (Nelson and Gary, 1983).
Queen supersedure occurred at 150 days after colony establishment with low-quality queen (Fig. 5). Only one of the ten low-quality queen survived (10% survival) at the end of experiment (i.e., 270 days after colony establishment) whereas the number of queen in group of high-quality queen were recorded with eight queens survived (80% survival to the end of experiment). Proportion of queen supersedure was significantly different between two groups (Mantel-Cox tests, χ2=7.33, P<0.01, Fig. 5). Queens in group of low-quality queen were found to be supersedured mainly in spring of 2018 (from February to March 2018), addressed a question of whether adult workers within a colony decided to replace their queen due to her low reproductive quality. Previous studies found in A. mellifera demonstrated that lower insemination success and depletion of spermatozoa in spermatheca inextricably linked to queen longevity (Winston, 1987; Dedej et al., 1998; Richard et al., 2007; Al-Lawati et al., 2009). In addition, the insemination quantity of the queen have been found to be associated with ovary activation and pheromone production and chemical composition (Richard et al., 2007; Kocher et al., 2009). However, it was unkn-own whether there were any differences of pheromone and chemical profiles of A. cerana queen that perceived by workers to induce queen supersedure.
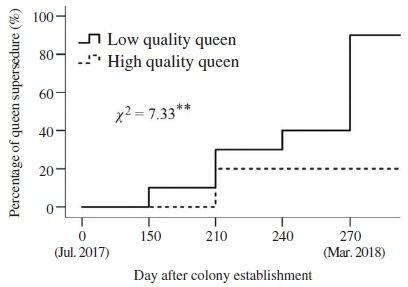
Kaplan-Meier estimates expressed the percentage of queen supersedure over time in colonies headed by high-quality and low-quality queens. Chi-square (χ2) test of overall comparison fo1lowed by a P value shown as; **: significant difference at the 0.01 level.
All the aforementioned studies, including ours, demonstrated that queens artificially reared from young larvae showed a higher reproductive potential, compared to queens reared from older larvae, and subsequently directly affected on the growth of the colony they headed.
Acknowledgments
We would like to thank staff at Sericultural & Apicultural Materials Division, Department Agricultural Biology, National Institute of Agricultural Sciences, Republic of Korea for their technical assistance in beekeeping activities. Financial support came from the PJ012036022018 of national joint research business in National Institute of Agricultural Sciences in Rural Development Administration.
References
-
Abrol, D. P., R. Bhagat, and D. Sharma, (2005), Mass rearing of Apis cerana F. queen, Journal of Asia-Pacific Entomology, 8, p309-317.
[https://doi.org/10.1016/s1226-8615(08)60251-4]

-
Al-Lawati, H., G. Kamp, and K. Bienefeld, (2009), Characteristics of the spermatheca contents of old and young honeybee queens, Journal of Insect Physiology, 55, p117-122.
[https://doi.org/10.1016/j.jinsphys.2008.10.010]

-
Chinh, T. X., W. J. Boot, and M. J. Sommeijer, (2005), Production of reproductives in the honey bee species Apis cerana in northern Vietnam, J. Apic. Res, 44, p41-48.
[https://doi.org/10.1080/00218839.2005.11101146]

-
Cobey, S. W., D. R. Tarpy, and J. Woyke, (2013), Standard methods for instrumental insemination of Apis mellifera queens, J. Apic. Res, 52, p1-18.
[https://doi.org/10.3896/ibra.1.52.4.09]

-
da Silva, E. C., R. M. da Silva, J. Chaud-Netto, A. C. Moreti, and I. P. Otsuk, (1995), Influence of management and environmental factors on mating success of Africanized queen honey bees, J. Apic. Res, 34, p169-175.
[https://doi.org/10.1080/00218839.1995.11100902]

-
Dedej, S., K. Hartfelder, P. Aumeier, P. Rosenkranz, and W. Engels, (1998), Caste determination is a sequential process: effect of larval age at grafting on ovariole number, hind leg size and cephalic volatiles in the honey bee (Apis mellifera carnica), J. Apic. Res, 37, p183-190.
[https://doi.org/10.1080/00218839.1998.11100970]

-
Delaney, D. A., J. J. Keller, J. R. Caren, and D. R. Tarpy, (2011), The physical, insemination, and reproductive quality of honey bee queens (Apis mellifera L.), Apidologie, 42, p1-13.
[https://doi.org/10.1051/apido/2010027]

-
Hatch, S., D. Tarpy, and D. Fletcher, (1999), Worker regulation of emergency queen rearing in honey bee colonies and the resultant variation in queen quality, Insectes Sociaux, 46, p372-377.
[https://doi.org/10.1007/s000400050159]

-
Kahya, Y., H. V. Gencer, and J. Woyke, (2008), Weight at emergence of honey bee (Apis mellifera caucasica) queens and its effect on live weights at the pre and post mating periods, J. Apic. Res, 47, p118-125.
[https://doi.org/10.1080/00218839.2008.11101437]

-
Kocher, S. D., F.-J. Richard, D. R. Tarpy, and C. M. Grozinger, (2009), Queen reproductive state modulates pheromone production and queen-worker interactions in honeybees, Behavioral Ecology, 20, p1007-1014.
[https://doi.org/10.1093/beheco/arp090]

-
Koeniger, N., and G. Koeniger, (2007), Mating flight duration of Apis mellifera queens: As short as possible, as long as necessary, Apidologie, 38, p606-611.
[https://doi.org/10.1051/apido:2007060]

-
Koeniger, N., G. Koeniger, and H. Pechhacker, (2005), The nearer the better? Drones (Apis mellifera) prefer nearer drone congregation areas, Insectes Sociaux, 52, p31-35.
[https://doi.org/10.1007/s00040-004-0763-z]

-
Lensky, Y., and M. Demter, (1985), Mating flights of the queen honeybee (Apis mellifera) in a subtropical climate, Comparative Biochemistry and Physiology Part A: Physiology, 81, p229-241.
[https://doi.org/10.1016/0300-9629(85)90127-6]

-
Linksvayer, T. A., O. Kaftanoglu, E. Akyol, S. Blatch, G. V. Amdam, et al. (2011), Larval and nurse worker control of developmental plasticity and the evolution of honey bee queen-worker dimorphism, J. Evol. Biol, 24, p1939-1948.
[https://doi.org/10.1111/j.1420-9101.2011.02331.x]

-
Nelson, D. L., and N. E. Gary, (1983), Honey productivity of honeybee colonies in relation to body weight, attractiveness and fecundity of the queen, J. Apic. Res, 22, p209-213.
[https://doi.org/10.1080/00218839.1983.11100589]

-
Rangel, J., J. Keller, and D. Tarpy, (2013), The effects of honey bee (Apis mellifera L.) queen reproductive potential on colony growth, Insectes Sociaux, 60, p65-73.
[https://doi.org/10.1007/s00040-012-0267-1]

-
Richard, F.-J., D. R. Tarpy, and C. M. Grozinger, (2007), Effects of insemination quantity on honey bee queen physiology, PloS one, 2, e980.
[https://doi.org/10.1371/journal.pone.0000980]

- Ruttner, F., J. Woyke, and N. Koeniger, (1972), Reproduction in Apis cerana 1, Mating behaviour. J. Apic. Res, 11, p141-146.
- Schlüns, H., E. A. Schlüns, J. Van Praagh, and R. F. Moritz, (2003), Sperm numbers in drone honeybees (Apis mellifera) depend on body size, Apidologie, 34, p577-584.
-
Shah, F. A., and T. A. Shah, (1980), Early life, mating and egg laying of Apis cerana queens in Kashmir, Bee World, 61, p137-140.
[https://doi.org/10.1080/0005772x.1980.11097796]

-
Tarpy, D., J. Keller, J. Caren, and D. Delaney, (2011), Experimentally induced variation in the physical reproductive potential and mating success in honey bee queens, Insectes Sociaux, 58, p569-574.
[https://doi.org/10.1007/s00040-011-0180-z]

-
Tarpy, D. R., J. J. Keller, J. R. Caren, and D. A. Delaney, (2011), Experimentally induced variation in the physical reproductive potential and mating success in honey bee queens, Insectes Sociaux, 58, p569-574.
[https://doi.org/10.1007/s00040-011-0180-z]

-
Tarpy, D. R., and J. Page, E. Robert, (2000), No behavioral control over mating frequency in queen honey bees (Apis mellifera L.): implications for the evolution of extreme polyandry, The American Naturalist, 155, p820-827.
[https://doi.org/10.2307/3079103]

-
Wheeler, D. E., (1986), Developmental and physiological determinants of caste in social Hymenoptera: evolutionary implications, The American Naturalist, 128, p13-34.
[https://doi.org/10.1086/284536]

- Winston, M., (1987), The biology of the honey bee, Harvard university press.
- Winston, M. L., (1991), The biology of the honey bee, Harvard university press.
-
Woyke, J., (1971), Correlations between the age at which honeybee brood was grafted, characteristics of the resultant queens, and results of insemination, J. Apic. Res, 10, p45-55.
[https://doi.org/10.1080/00218839.1971.11099669]

-
Woyke, J., (1973), Instrumental insemination of Apis cerana indica queens, J. Apic. Res, 12, p151-158.
[https://doi.org/10.1080/00218839.1973.11099743]

-
Woyke, J., (1975), Natural and instrumental insemination of Apis cerana indica in India, J. Apic. Res, 14, p153-159.
[https://doi.org/10.1080/00218839.1975.11099820]
