
Controlling Sacbrood Virus Disease in Apis cerana Colonies with Biological Methods in Korea
Abstract
As Sacbrood virus (SBV), a causative agent of larval death and colony collapse in Apis cerana honey bee, is prevalent and poses one of the most significant threats to the Korean apiculture, development of methods to counter this viral disease is urgently needed. In this study we tested some SBV controlling methods, such as requeen, shook swam, adding Apis mellifera and spraying yogurt to SBV inoculated colony. Colony size measured by number of sealed brood and adult were evaluated every 15 days until two months while instances of recurrence were recorded up to five months after applying treatment methods. We also test the effects of yogurt on healthy and SBV-infected larvae at both in vitro reared larvae and colony level. Our result showed that all SBV controlling methods had similar success rates with respect to elimination of SBV clinical symptom up to 30 days post treatment. Mix-species and spraying yogurt method had similar pattern of sealed brood and adult number and higher than that of other SBV controlling methods up to 45 days post treatment. These two groups also showed the lower percentage of SBV recurrence (50% and 66.7%) at 120 days post treatment than other group that mostly colony had clinical symptom. Result on in vitro reared larvae challenged with yogurt showed that yogurt have neither harmless on healthy larvae nor remedial effect on SBV infected larvae. However, at colony level, colony in group received yogurt treatment removed significantly more SBV-infected larvae, SBV-killed larvae, and even healthy larvae in comparison to the control, suggested that yogurt could trigger the hygienic behavior of nurse bee. Our results recommended that it is practical in beekeeping by adding A. mellifera and spraying yogurt to control SBV in A. cerana colony.
Keywords:
Apis cerana, Apis mellifera, Honey bee, Shook swarm, Yogurt, Mix-species, HygienicINTRODUCTION
Honey bees (Hymenoptera: Apidae) are vital pollinators for wildlife plants and agriculture crops (Klein et al., 2007). In Korea, the Asiatic hive bee, Apis cerana Fabricius, is an indigenous honey bee species whereas the European honey bee, Apis mellifera Linnaeus, is exotic species imported since 1900s (Jung and Cho, 2015). Both A. cerana and A. mellifera species are raised to produce high value bee-products. A. mellifera has specific advantages over Apis mellifera, including its higher ectoparasite-mite resistance (Varroa destructor), stronger tolerance in extreme climates (cold/hot weather), and lower cost of supplemental food (Peng et al., 1987; Oldroyd and Wongsiri, 2006; Verma and Attri, 2008; Xu et al., 2009). In contrast to such characteristics, Korean A. cerana population is decreasing and appears close to extinction in recent years (Choi et al., 2010; Theisen-Jones and Bienefeld, 2016). The prime reason for such A. cerana colony losses was due to the lethal Sacbrood virus (SBV) disease which caused more than 90% of mortality since the first observation in 2008 (Choe et al., 2012; Jung and Cho, 2015).
SBV is a linear positive single-stranded RNA virus belonging to the genus Iflavirus (Baker and Schroeder, 2008). The virus primarily affects the brood stage of the honeybees with a high viral replication causing significant morphological alterations and ultimately resulting in larval death (Bailey et al., 1964; Chen et al., 2004). Furthermore, the infection of SBV in adult worker bees modify their behaviour such as accelerating progression from brood tending to foraging and aversion to collecting pollen, and even can reduce their life span (Bailey and Fernando, 1972; Anderson and Giacon, 1992; Anderson, 1995).
Different methods have been introduced in attempts to control SBV in A. cerana, which included feeding SBV-specific dsRNA sequences during the larval stages to block the expression of the viral gene (Liu et al., 2010; Zhang et al., 2016), using silver ions or herbal medicines to treat SBV infected colony (Ahn et al., 2015; Aruna et al., 2017) and feeding SBV-infected colony with specific immunoglobulin Y (egg yolk antibodies) (Sun et al., 2018). Unfortunately, no effective method for field application has been achieved yet.
As social insects, honeybees have evolved novel physiological, behavioral and organizational adaptations as individual and social immunity to combat diseases (Cremer and Sixt, 2009). Colony member can perform both individual defences (when alone) and collective defences (when interacting with its colony members) to promote advantage of fitness for entire colony (Evans et et., 2006; Cremer et al., 2018). Among the social immunity of honeybees, hygienic behavior plays an important role in reducing the risk of pathogens (Rothenbuhler, 1964; Spivak and Gilliam, 1998; Spivak and Reuter, 2001). Hygienic behaviour involves the ability of adult workers to detect and remove abnormal brood (e.g. diseased, dead, and sometimes parasitized brood) from the colony, and thus reduces the loads and transmission rates of pathogens. Therefore, hygienic trait is colony level adaptive pathogen resistance mechanism to defend against spectrum of brood diseases (Arathi et al., 2000; Spivak and Reuter, 2001; Palacio et al., 2010; Leclercq et al., 2017).
SBV can infect both A. mellifera and A. cerana colonies, but the infection appears to be less detrimental to A. mellifera colonies than to A. cerana colonies (Anderson, 1995). In addition, although the larva and adult of A. mellifera are susceptible to the infection of both SBV serotypes isolated from SBV-infecting A. cerana and SBV-infecting A. mellifera, it is unusual for SBV infection in A. mellifera colonies to cause colony death or even the obvious symptom whereas SBV were reported to be a lethal disease in A. cerana (Ai et al., 2012; Gong et al., 2016) This may be due to the different levels of resistance of the bees to the virus under different species (e.g. differential hygienic responses), suggesting that it is potentially useful for beekeeping practices by adding the A. mellifera bee to boost the resistant ability of A. cerana colony against SBV disease. Moreover, researches on some biological characteristic of interspecifically mixed species showed that adult workers of A. mellifera were accepted as members of the mix-species colonies headed by A. cerana queen and appeared to behave normally by performing house cleaning, brood provisioning, foraging, thermoregulation, and participating in the retinue surrounding the A. cerana queen (Yang et al., 2010). In this study, we tested the possibility of using the A. mellifera honey bee to assist A. cerana colony defence against SBV disease.
SBV prevalence was reported to be seasonal variation as it’s incidence in bee population increases dramatically from spring to summer, peaks in mid-summer and declines in fall following natural brood cycles (Tentcheva et al., 2004; Desai and Currie, 2016). Tentcheva et al. (2004) reported that SBV infections occurred persistently in bee populations despite the lack of clinical symptoms, suggesting that colony disease outbreaks might result from health condition of colony and environmental factors that lead to activation of viral replication in bees. Thus, SBV might be an opportunistic pathogen that is favoured when colonies are succumbing to stresses of brood rearing burden in reproductive season. Previous study demonstrated that breaking brood cycle of SBV-infected colony by requeening with a queen cell or caging the laying queen to temporarily suppress brood production appeared to be a promising method in controlling SBV disease (Toan et al., 2014). Furthermore, other approaches with breaking brood cycle, such as shook swarm technique, is widely practiced to control brood diseases in A. mellifera colony, such as American foulbrood and European foulbrood (Hansen and Brodsgaard, 2003; Waite et al., 2003; Thompson et al., 2006). The shook swarm treatment emulates the natural behaviour of the honey bee to move nest site (i.e. absconding) when disease pressure is high, thereby reducing the build-up of disease agents. Comb replacement into a clean box presents a disease free environment, and forces a break in the brood cycle that also provides a break in the disease cycle (Guler, 2008). In this study, we utilized the shook swam technique to test whether forcing a break in the brood cycle by transferring all adult of SBV-infected colony to a disease-free hive without brood combs would assist colony to overcome the disease infection.
Lactic Acid Bacteria (LAB) are Gram-positive, non-spore forming beneficial bacteria that are found in food matrices such as dairy products (e.g. yogurt) (Dicks and Botes, 2009). LAB are closely associated with food and feed processing and production as safe to use as probiotics for human and animal consumption (Rashid and Sultana, 2016). The abundance of LAB species are also found in honey bee colony and bee products such as honey, pollen and bee bread (Vásquez and Olofsson, 2009; Wu et al., 2013). LAB were reported to have inhibitory effects on honeybee bacterial pathogens (Forsgren et al., 2010; Killer et al., 2014). Previous studies demonstrated that the oral administration of LAB decreased the mortality rate of A. mellifera larvae infected with Paenibacillus larvae and Melissococcus plutonius (agents of American and European foulbrood diseases, respectively) in larval bioassay under laboratory conditions (Forsgren et al., 2010; Vásquez et al., 2012). However, it is unknown whether applying LAB to A. cerana has general positive effect (i.e. initiatory or remedial effect) on larvae infected with SBV. As LAB are dominant in fermented milk product (Crowley et al., 2013), therefore we utilized the product of LAB, the yogurt, to treat the SBV-infected larvae of A. cerana reared in vitro and at colony level.
The aim of this study was to test biological methods, such as requeen, shook swam, adding A. mellifera (i.e. mix-species) and spraying yogurt to SBV infected colony to evaluate effectiveness in controlling SBV disease in A. cerana colony. In addition, we also tested the adverse influence and remedial effects of yogurt on healthy and SBV-infected larvae, respectively.
MATERIALS AND METHODS
Honey bee sources
A. cerana and A. mellifera honey bees were used in our study. The A. cerana colonies were of indigenous origin (Korean A. cerana), derived from an apiary in Tongyeong (south Gyeongsang province). The A. mellifera colonies were derived from unselected and heterogeneous stocks of Western honey bee imported to Korea more than a century ago (Jung and Cho, 2015). As the comb and hive of A. mellifera and A. cerana colony were different, experimental colonies of A. mellifera were transferred to hive type of A. cerana in a modified Langstroth 10-frame hive-box (41×30×25cm) in April 2017. Queens of both species were raised from a singer mother colony of their own species, thus queen were sisters to each other. Queen rearing were practiced using grafting method in April 2017. Larvae of similar age, which were younger than 24 hrs old, were physically transferred (“grafted”) from worker cells in a source colony to cell-cups containing 10μL of fresh royal jelly, and then suspended vertically in a queenless nursery colony to be raised as queens. The resulting queens developed inside their respective cell in nursery colony. Ten days after grafting, queen cell was introduced to five-comb mating hive. Virgin queens of both species were allowed to mate naturally. As the queens initiated egg-laying about one week, colonies of both A. cerana and A. mellifera were respectively equalized to four bee combs fully covered with workers (approximately 5.000 workers in each colony of both species) by removing excess bees and combs. These colonies were used as sources of bees for further experiments. All of the colonies then were located in a single apiary at National Institute of Agricultural Sciences, Rural Development Administration, Jeonju City, Korea (36°49’30” N, 127’2°3” E) from April 2017 to June 2018. During the experiment, colonies were received similar supplement feeding of sugar syrup (50% sugar) and pollen supplement at dearth times.
SBV diagnosis
The presence of the SBV in experimental colony was confirmed by RT-PCR. Ten larvae of 4th instar, which showed overt symptoms of SBV-infection (Gong et al., 2016), were individually collected and total RNAs were extracted from each individual larva using TRIzol reagent (Invitrogen, Carlsbad, USA) by following the manufacturers’ instructions. Extracted RNA was eluted in 100μL of diethyl-pyrocarbonate (DEPC) RNase-free water. Reverse transcription reactions for cDNA synthesis were performed using PrimeScript II 1st strand cDNA Synthesis kit (Takara, Japan) using approximately 2μg of extracted RNA in 20μL final volume following the manufacturer recommendations. PCR amplifications for the detection of SBV were performed using AccuPower Hot-start PCR premix (Bioneer, Korea). The reaction according to the manufacturer’s protocol was performed in a total volume of 25μL containing 1μL of synthesized cDNA as template to amplify the SBV and using 1μL forward primer 5’-GACCAAGAAGGGAATCAGC-3’, 1μL reverse primer 5’CATCTTCTTTAGCACCAGTATCCA-3’ as a gene specific primer of Korean SBV (Choi et al., 2010). The PCR assays consisted of an initial 5-min pre-denaturation at 94°C, followed by 33 cycles of denaturation at 94°C for 1 min, annealing 50°C for 1 min, elongation at 72°C for 1 min, and final post-extension at 72°C for 5 min. Resultant PCR products were electrophoresed on a 1% agarose gel containing 0.5μg/ml ethidium bromide and visualized under an ultraviolet (UV) light.
SBV inoculation
SBV lysate was obtained by collecting honey bee larvae with overt symptom of SBV infection from a SBV-infectious A. cerana colony. Ten SBV-infected larvae were ground in 10ml sterile phosphate-buffered solution (PBS: 137mM NaCl, 2.7mM KCl, 10mM Na2HPO4, 1.8mM KH2PO4, pH 7.4) with a sterile grinder and centrifuged for 40 min at 10,000 rpm at 4°C The supernatant was collected and penetrated to 0.45mm cell filter. To provoke SBV infection in experimental colony, both larvae and adults were orally inoculated. Briefly, five ml of SBV suspension was used to spray to an area of comb section contained 500 young larvae (1st-3rd instar), and then the comb were placed to the experimental colony. Adults of experimental colony also were fed with 200 ml sugar syrup (50% sugar) mixed with 2ml SBV suspension. These procedures were repeated twice in 24 hrs. Five days after SBV inoculation, ten larvae with clinical symptoms of SBV disease were sampled from each hive and subsequently diagnosed to confirm the present of SBV by RT-PCR according to the procedure described above (in SBV diagnosis session).
Larvae challenged with yogurt
To determine whether yogurt treatment had adverse impact on healthy larvae or remedial effect on SBV-infected larvae, healthy and SBV-infected larvae were challenged by feeding yogurt to evaluate their survivorship. Experiments were conducted with individual larva reared in vitro and at colony level.
Yogurt preparation
Yogurt was prepared following protocol described by (Machado et al., 2017). Briefly, a 50g of skim milk powder was added to 100ml of distilled water, mixed well and then subjected to heat treatment (85°C/10 min). Next, the milk was cooled 45°C, and the cultures were inoculated by adding 10ml of commercial yogurt to supplement thermophilic lactic acid bacteria for the starter culture. Fermentation was performed in an incubator at 45°C, and the end point of yogurt fermentation was at pH value of 4.2~4.5 in 8 hr. Finally, clot was broken by manual stirring with a glass rod, and then the product was cooled to 34°C before use.
In vitro rearing larvae
Larvae were reared individually in 48-well tissue culture plates with a larval diet (LD) consisting of 50 grams of fresh royal jelly (50%), 6 grams of D-glucose (6%), 6 grams of D-fructose (6%), 1 gram of yeast (1%) extract, and 37ml sterile deionised water (Toan et al., 2014). First instar worker larvae taken from the combs of colony were carefully grafted with a grafting tool into individual wells, each containing 20μL of pre-warmed LD (Crailsheim et al., 2013). The culture plates were placed within a humid desiccator containing 10% sulfuric acid solution in water to ensure a high relative humidity at 95% and thereby avoiding larval dehydration.in, and then kept in incubator (Growth chamber, Vision VS-1203PFHLN, Korea) at 34°C. From day third after grafting, each larva was fed once a day for 4 consecutive days, with increasing amounts of daily diet (20μL, 30μL, 40μL and 60μL LD/larva, respectively).
To evaluate the influence of yogurt on survivorship of healthy larva and curative effect on larva infected with SBV, in vitro rearing larvae were respectively retrieved from two different A. cerana colonies: the SBV-free (healthy) and SBV-infected colony, and distributed into four groups (termed groups from 1 to 4) with each group containing 24 larvae. Group 1 and 2 were SBV-uninfected larvae grafted from healthy colony. Group 3 and 4 reared the SBV-infected larvae grafted from SBV-inoculated colony (see above in SBV inoculation). Group 1 and 3 served as yogurt-free controls (which were fed only with LD) for assessing the background larval survivorship. Larvae in group 2 and 4 were challenged with yogurt by feeding the mixture of 5μL yogurt and 20μL LD in the first two days (i.e. first and second instar larvae). The present and absent of SBV were confirmed by RT-PCR (described above in SBV diagnosis) before experiment. The larvae were observed every 24 h to examine their feeding activity and mortality. The death of the larvae could be confirmed when they had no response after stimulating with a soft tip, and by the color change of their bodies (from white to yellow or even dark grey).The above assay was performed in triplicate on May 2017.
Colony application of yogurt was performed in twelve A. cerana colonies distributed into two groups. Six colonies (group 1) were infected with SBV that were SBV-inoculated prior to the experiment one week (see above in SBV-inoculation) and six others (group 2) were colonies without SBV infection (healthy). In both groups, larvae were challenged with yogurt twice at the first and second instar larvae. Other cohorts of six SBV-infected and six healthy colonies, that their larvae received distilled water instead of yogurt treatment, were served as controls (termed as group 3 and 4, respectively). To obtain agecontrolled larvae, all comb containing open brood were eliminated and the queen was confined on a well-drawn comb using queen excluder, and left to lay eggs for 12 hrs. Approximately 4 days after oviposition, the comb was retrieved from the colony. One hundred first instar larvae were trickled with 5μL yogurt to the bottom of the cell for the colonies in group 1 and 2. Locations of 100 cells on experimental comb containing yogurt-trickled larvae were marked on a transparent plastic sheet overlaying the comb for the further relocalization of the treated larvae, and then the comb was placed back to its colony. The following day, each larva was received the second 5μL yogurt trickling. Colonies in control groups (group 3 and 4) received similar treatment but 5μL distilled water was used instead of yogurt. Each experimental comb was examined daily for six consecutive days to determine the changes in individual cells. Empty cells, death or partly removed larvae by adults were taken into account of mortality. After all remaining open brood corresponded with marked cell was capped, percentages of brood removed daily were calculated by comparing the recorded brood markers to that of the previous day. The experiment was conducted twice, using the same bee colonies and experimental procedure.
SBV-killed larval removal
To determine the effects of hygienic behavior on SBV-killed larval removal, the larval comb containing SBV-dead larvae of A. cerana and A. mellifera retrieved from SBV-inoculated A. cerana and A. mellifera colonies were respectively introduced to experimental colonies of two species. In addition, the yogurt was used to spray in the SBV-killed larval comb to evaluate its effect on hygienic responses of bee. The experiment was conducted with four groups: (group 1) six A. mellifera colonies, (group 2) six colonies of A. cerana contained worker bee of A. mellifera (mix-species) and (group 3) six A. cerana, and all colonies received one comb containing SBV-killed larvae respectively retrieved from SBV inoculated colonies of each species (see above description of SBV inoculation). The mix-species group received a comb of SBV-killed larvae of A. cerana. One group with six colonies of A. cerana received a A. cerana SBV-killed larval comb which was sprayed with yogurt before introduction to the hive (group 4: yogurt). In order to mix A. mellifera bee to A. cerana colony, three week prior to the experiment, a A. mellifera comb with cohort of approximately 1,500 sealed brood was introduced to the host A. cerana colony of group 2 (mix species) by exchanging with a similar sealed brood comb of host colony so that the proportion of A. mellifera adult was about 30% at the time of experiment. To verify the hygienic removal of SBV-killed larvae, a representative of 100 individual cells containing dead larvae on each experimental comb were marked on a transparent plastic sheet overlaying on surface of comb before introduction. Each comb containing SBV-dead larvae (i.e. experimental comb) was then placed in the center of the brood nest of each experimental colony and was examined daily for three consecutive days to determine the changes in individual cells. The empty cells and cells with completely SBV-killed larvae removed daily were calculated by comparing the recorded cell markers to that of the previous day. Marked position of cell that the bees completely removed SBV-killed larva or empty cells were taken into account of hygienic removal. Experiment was conducted twice from April to June 2018.
Trial set-up for SBV treatment regimens
The trial compared the four different treatments within a singer apiary to limit external factors such as availability of forage and weather conditions; these should be uniform for colonies in the same apiary. To take part in this trial, all colonies were SBV-inoculated prior to the experiment one week. The present of SBV in each experimental colony were confirmed by the overt symptoms of infected larvae and by RT-PCR. Colonies were also of similar size contained approximately 4,000 to 4,500 adults adhering on four brood combs (39×23cm).
There were four groups received four different treatments
Group 1 (termed as requeen method): Queens of ten colonies were eliminated and a ready emerging queen cell was introduced to each colony (see above description for artificial queen rearing). Virgin queen was allowed to mate naturally. As the inherent proportion of queen loss during their mating flights, this group was carried out with ten colonies at the beginning, and then six colonies headed by successful mated queen were randomly selected as experimental colonies.
Group 2 (named shook-swarm): Shook swarm treatment consisted of removal of adult bees to a clean hive containing three new wax foundation frames and a honey comb without SBV infection retrieved from healthy colony, followed by feeding as required. Adult population from the infected brood frames was carefully shaken inside new hive. Queen was confined in queen cage (3.5×10×1.5cm) and kept in middle of bee cluster for one day to avoid absconding. Shaken colonies were fed with sugar syrup (50% by volume in water) until all combs were drawn.
Group 3 (mix-species): Six colonies were added a brood comb of A. mellifera with approximately 1,500 sealed brood for each colony before the experiment 3 weeks.
Group 4: Six colonies receive three treatments of yogurt. Yogurt was sprayed to every comb three times in three days interval with a dose of 50 ml for a comb in one spray.
Control group was a cohort of six colonies received no treatment. Treatments were carried out on May 2017. In order to evaluate the effectiveness of treating method in controlling SBV, the growth of experimental colony (e.g. number of adult and sealed worker brood) was estimated after SBV inoculation every 15 days until two months. Number of adult and sealed worker brood was visually estimated using a gridded wooden frame consisting of 28, 4.6×4.6cm squares (see above for detailed description). Experimental colonies were also inspected for the recurrence of SBV disease every month from June to October 2017 or until SBV symptom appeared in all colonies.
Data analysis
Data on number of sealed brood and adult bees were analyzed using multivariate measures analysis of variance (ANOVA) tests followed by Fisher’s Least Significant Difference (LSD) post hoc tests for pairwise comparison of means. Each larva challenged with yogurt (in vitro and in colony tests) and SBV-killed larva in experiment of hygienic removal was treated as an individual in a survival analysis. Kaplan-Meier plots and log-rank (Mantel-Cox) post hoc tests were used to describe and compare the larval survivorship (in experiments that larva was challenged with yogurt) and SBV-killed larval removal among groups Nguyen Ngoc Vung, Iksoo Kim, Man Young Lee, Hye Kyung Kim, Dong Won Kim and Yong Soo Choi over time. For all testing, differences between groups at P<0.05 were considered statistically significant. All data sets were analysed using IBM SPSS statistic version 22 for Windows (IBM Corp., NY, USA).
RESULTS AND DISCUSSION
As SBV in A. cerana is prevalent in Korea, development of methods for the control of this disease is urgently needed. Our results demonstrated that applying biological methods such as requeening, shook swarm and adding A. mellifera worker bee to SBV-infected colony had curative effect on SBV infection. Treating yogurt (fermented milk) to SBV infected colonies appeared to be a promising method to control this disease because it was efficiency and readiness for beekeeper to apply with not much effort required. However, the general effects of treating yogurt on larvae were unknown. Therefore, in order to evaluate whether yogurt was harmful to healthy larvae or had remedial effect on SBV-infected larvae, both healthy larvae and SBV-infected larvae were challenged with yogurt in in vitro test and at colony level.
Result on in vitro rearing larvae showed that survival rate of both healthy and SBV-infected larvae treated with yogurt was similar with that in control group (Mantel-Cox tests, healthy larvae: χ2=1.76, P>0.05, Fig. 1a; SBV-infected larvae: χ2=3.69, P>0.05, Fig. 1b). Although yogurt might be harmless to larvae, it would have no curative effect on SBV-infected larvae. In contrast to the results of in vitro yogurt treatment, the proportion of larval survivorship in both healthy and SBV-inoculated colonies treated with yogurt were significantly lower than that in colonies of control group (Mantel-Cox tests, healthy colony: χ2=65.51, P<0.01, Fig. 2a; SBV-inoculated colony: χ2=26.57, P<0.01, Fig. 2b). However, both healthy and SBV-infected larvae were found to be prominently removed at the stages of second and third instar larvae (Log rank test, P<0.01). Treatment of yogurt to A. cerana colony might trigger the hygienic response of nurse bee to remove the treated larvae. The hygienic behaviour of honey bee was found to be largely relied upon cues. Previous studies found that the hygienic bee used various kinds of cues to determine the abnormal brood. These included chemically mediated cues, pheromones or metabolic products of diseased or dead brood with characteristic odour that was perceived by the bees to be abnormal to trigger hygienic removal of abnormal brood. The expression of hygienic behavior therefore largely depended on the perception of worker bees (Spivak and Gilliam, 1993; Swanson et al., 2009; Palacio et al., 2010). Yogurt treatment might change the odour of larva or probably because the larvae were perceived as being “abnormal” by the nurse bees, and thus the hygienic removal was triggered as in the case of brood cannibalism (Webster et al., 1987).
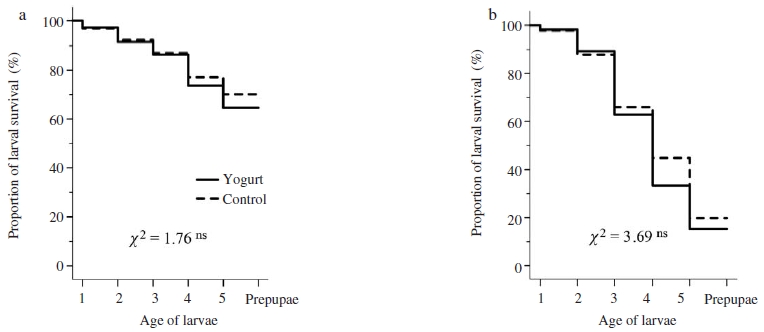
Survivorship of in vitro reared larvae grafted from Apis cerana colony without SBV-infection (a) and SBV-infected A. cerana colony (b). Kaplan-Meier estimates expressed the proportion of larval survival over time from first day instar larvae to prepupae. Chi-square (χ2) test of overall comparison fo1lowed by a P value shown as; P>0.05 ns: not significant difference at the 0.05 level.
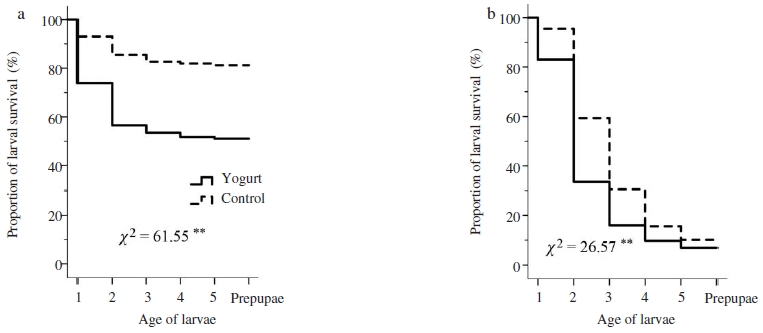
Laval survivorship in Apis cerana colony without SBV-infection (a) and in SBV-inoculated colony (b). Kaplan-Meier estimates expressed the proportion of larval survival over time from first day instar larvae to prepupae. Chi-square (χ2) test of overall comparison fo1lowed by a P value shown as **: significant difference at the 0.01 level (P<0.01).
In addition, Wagoner et al. (2018) found that olfactory signals of brood play an important role in triggering hygienic behaviour and hygiene level positively correlate to removal rates of abnormal brood. Thus, both brood signal and adult perception contribute to the effective detection and removal of hygienic tasks. Increasing stimulus strength of brood leads to recruitment of a larger number of task performers (i.e., hygienic workers), and thus to greater genotypic variety among workers involved in stimulus response (Beshers and Fewell, 2001). Previous studies demonstrated that increasing brood signals by experimentally increasing quantities of brood cuticular hydrocarbons and brood ester pheromones have been associated with hygienic behaviour (Nazzi et al., 2012; Mondet et al., 2016). Our results showed that survival rate of healthy larvae treated with yogurt at colony level were significantly lower in comparison to control (Fig. 2a), suggested that yogurt might enhance removing signal of treated larvae that perceived by adults as abnormal brood to trigger hygienic removal. Although the treatment of yogurt on larvae in vitro showed no adverse effects (Fig. 1a), yogurt increased the removal rate of treated larvae at colony level and enhanced hygienic performance toward treated brood (Fig. 2).
The hygienic removal of nurse bees expressed by proportion of SBV-killed larval removal among the groups was significantly different (Mantel-Cox tests, χ2=473.64, P<0.01, Fig. 3). The removal of SBV-killed larvae also was observed to be significantly higher in the colony of A. cerana that received the yogurt treated comb containing SBV-killed larval, compared to the control (Log rank test, P<0.01, Fig. 3). In addition, colonies in control groups respectively corresponded for SBV-infected and SBV-killed larvae tests showed lower proportions of SBV-infected and SBV-killed larvae than that in the colonies received yogurt treatment (Log rank test, P<0.01, Fig. 2b; P<0.01 yogurt versus control, Fig. 3, respectively), suggested that the delay in removal of diseased larvae probably because hygienic bees had low sensitivity to olfactory cues associated with diseased larvae to elicit the hygienic removal.
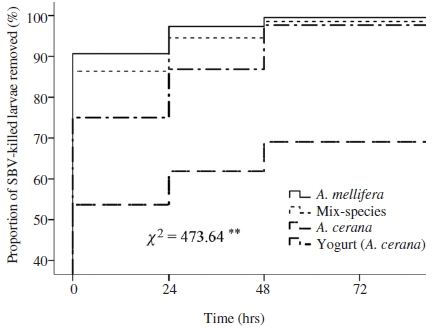
Kaplan-Meier estimates expressed the proportion of SBV-killed larvae removed over time in colonies of different groups. Chi-square (χ2) test of overall comparison fo1lowed by a P value shown as **: significant difference at the 0.01 level (P<0.01).
We also found that colonies of A. mellifera and mix-species showed significantly more SBV-killed larval removal in comparison to A. cerana colonies (Log rank test, P<0.01, Fig. 3). The detection of low odor stimulus could trigger hygienic bees with the lowest response thresholds to begin removing diseased brood rapidly from the colony (e.g. in case of A. mellifera and mix-species, Fig. 3), whereas the slow-hygienic bees (e.g., in case of A. cerana), with less olfactory sensitivity, require a high stimulus concentration to detect diseased brood in the colony (Arathi and Spivak, 2001; Gramacho and Spivak, 2003; Oxley et al., 2010). Early detection and rapid removal of diseased brood in of A. mellifera and mix-species would reduce the burden load and transmission of SBV inside the colony. Therefore, this might explain for the fact that SBV was less detrimental to A. mellifera colony than to A. cerana colony (Anderson, 1995).
Controlling SBV results shown in Fig. 5 revealed that all treating methods had curative effects as the number of sealed brood and adult bee in period from 30 to 60 days after treatment were significantly higher than those in control group (ANOVA test, P<0.01). The mix-species colony had the highest number of sealed brood and adult in the periods of 15, 30 and 45 days post treatment, although population size of among four treated groups were similar at 60 days post treatment (LSD post hoc test, P>0.05 in all pair-wised comparisons). However, at 60 days after treatment, number of sealed brood and adult in colonies of all four treated group were significantly higher than those of control (LSD post hoc test, P<0.05 in all pair-wised comparisons) Number of sealed brood in colonies of requeen group declined at 30 days post treatment compared to that before treatment because virgin due to it required time from emergence of virgin queen to onset of oviposition. Similarly, number of sealed brood in colonies of shook swarm group declined at 15 and 30 days post treatment compared to that before treatment because of the elimination of all brood combs when applying treatment so that colony needed time to compensate the loss. Results obtained in A. mellifera demonstrated that if the bee combs were contaminated with disease, pest, pesticide or heavy metal derived from environment, it can be significantly reduced using of the shook swarm technique (Hansen and Brodsgaard, 2003; Guler, 2008). However, removal of honey, pollen and brood combs from a colony caused significant stress and deficiencies in the energy sharing of the organism, and reduction of colony productivity (Pratt, 2004; Guler, 2008).
The higher hygienic removal in colonies of both mix-species and yogurt treatment group, the better they reduced the burden of viral load, compare to control group (Fig. 3), resulting in their ability to overcome the SBV infection, and thus their population size (i.e. number of adult bee, Fig. 4) did not decline after being SBV-inoculation.
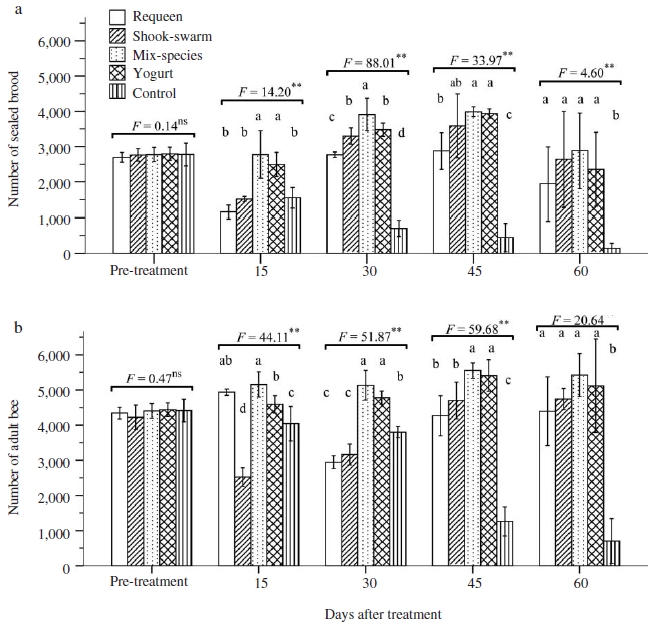
Number of sealed brood (a) and adult bee (b) in Apis cerana colonies treated with different biological methods. Multivariate measures analysis of variance (ANOVA) tests of mean comparison fo1lowed by a P value shown as ns: not significant difference; **: significant difference at the 0.01 level. Fisher’s LSD post hoc test showed as different letters in graphs indicated significant differences at the 0.05 level.
The recurrence of SBV was observed earliest in requeen group with 16.7% of colony had SBV symptom at 30 days post treatment (Fig. 5). Our results were agreed with the previous finding that SBV reoccurred at about 30 days after requeening (Toan et al., 2014). At 45 days post treatment, all group had colonies with SBV symptom. However, the number of SBV recurrent colonies did not increased at the period from 45 to 90 days post treatment. This period was hot summer and dearth period so that the reproduction of colonies were declined, resulting in reduced the stressful of colony from rearing large number of larvae, and thus that would be reduced the susceptibility of colony to SBV infection. However, at 120 days post treatment, almost colony of all group had clinical symptoms of SBV.
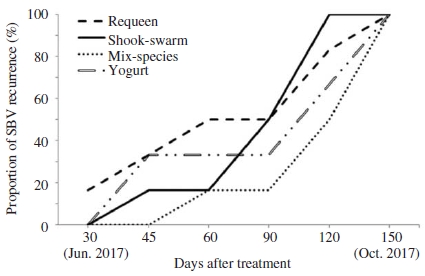
Proportion of Apis cerana colony with SBV recurrence in groups treated with different SBV controlling methods.
In beekeeping practice, the mix-species and spraying yogurt methods appeared to be useful and practical due to they were simple, less disturbance to bee and less effort required, in comparison to other methods.
Acknowledgments
We would like to thank the staffs at Sericultural & Apicultural Materials Division, Department Agricultural Biology, National Institute of Agricultural Sciences, Republic of Korea for their technical assistance in molecular works and beekeeping activities. Financial support came from the PJ012441012018 of national joint research business in National Institute of Agricultural Sciences in Rural Development Administration.
References
- Ahn, A. J., K. S. Ahn, G. H. Suh, J. H. Noh, Y. H. Kim, et al. (2015), Efficacy of silver ions against Sacbrood virus infection in the Eastern honey bee Apis cerana, J. Vet. Sci, 16, p289-295.
-
Ai, H., X. Yan, and R. Han, (2012), Occurrence and prevalence of seven bee viruses in Apis mellifera and Apis cerana apiaries in China, J. Invertebr. Pathol, 109, p160-164.
[https://doi.org/10.1016/j.jip.2011.10.006]

- Anderson, D. L., (1995), Viruses of Apis cerana and Apis mellifera, The Asiatic hive bee: Apiculture, biology, and role in sustainable development in tropical and subtropical Asia, Enviroquest Ltd., Cambridge, Ontario, Canada, p160-171.
-
Anderson, D. L., and H. Giacon, (1992), Reduced pollen collection by honey bee (Hymenoptera: Apidae) colonies infected with Nosema apis and sacbrood virus, J. Econ. Entomol, 85, p47-51.
[https://doi.org/10.1093/jee/85.1.47]

-
Arathi, H., and M. Spivak, (2001), Influence of colony genotypic composition on the performance of hygienic behaviour in the honeybee, Apis mellifera L, Animal Behaviour, 62, p57-66.
[https://doi.org/10.1006/anbe.2000.1731]

-
Arathi, H. S., I. Burns, and M. Spivak, (2000), Ethology of hygienic behaviour in the honey bee Apis mellifera L. (Hymenoptera: Apidae): behavioural repertoire of hygienic bees, Ethol, 106, p365-379.
[https://doi.org/10.1046/j.1439-0310.2000.00556.x]

- Aruna, R., M. R. Srinivasan, and R. Selvarajan, (2017), Efficacy of plant products on sacbrood virus attacking Apis cerana indica Fabricius, J. Entomol. Zool. Stud.
-
Bailey, L., and E. F. W. Fernando, (1972), Effects of sacbrood virus on adult honeybees, Ann. Appl. Biol, 72, p27-35.
[https://doi.org/10.1111/j.1744-7348.1972.tb01268.x]

-
Bailey, L., A. J. Gibbs, and R. D. Woods, (1964), Sacbrood virus of the larval honey bee (Apis mellifera Linnaeus), Virol, 23, p425-429.
[https://doi.org/10.1016/0042-6822(64)90266-1]

-
Baker, A. C., and D. C. Schroeder, (2008), The use of RNA-dependent RNA polymerase for the taxonomic assignment of Picorna-like viruses (order Picornavirales) infecting Apis mellifera L. populations, Virol. J, 5, p10.
[https://doi.org/10.1186/1743-422x-5-10]

- Beshers, S. N., and J. H. Fewell, (2001), Models of division of labor in social insects, Ann Rev Entom, 46, p413-440.
-
Chen, Y., Y. Zhao, J. Hammond, H. T. Hsu, J. Evans, et al. (2004), Multiple virus infections in the honey bee and genome divergence of honey bee viruses, J. Invertebr. Pathol, 87, p84-93.
[https://doi.org/10.1016/j.jip.2004.07.005]

-
Choe, S. E., T. T. D. Nguyen, B. H. Hyun, J. H. Noh, H. S. Lee, et al., (2012), Genetic and phylogenetic analysis of South Korean sacbrood virus isolates from infected honey bees (Apis cerana), Veterinary microbiology, 157, p32-40.
[https://doi.org/10.1016/j.vetmic.2011.12.007]

- Choi, Y. S., M. Y. Lee, I. P. Hong, N. S. Kim, H. K. Kim, et al., (2010), Occurrence of sacbrood virus in Korean apiaries from Apis cerana (Hymenoptera: Apidae), Korean J. Apiculture, 25, p187-191.
-
Crailsheim, K., R. Brodschneider, P. Aupinel, D. Behrens, E. Genersch, et al. (2013), Standard methods for artificial rearing of Apis mellifera larvae, J. Apic. Res, 52, p1-16.
[https://doi.org/10.3896/ibra.1.52.1.05]

-
Cremer, S., C. D. Pull, and M. A. Fürst, (2018), Social immunity: Emergence and evolution of colony-level disease protection, Annu. Rev. Entomol, 63, p105-123.
[https://doi.org/10.1146/annurev-ento-020117-043110]

-
Cremer, S., and M. Sixt, (2009), Analogies in the evolution of individual and social immunity, Philos. Trans. R. Soc. London. B: Biol. Sci, 364, p129-142.
[https://doi.org/10.1098/rstb.2008.0166]

-
Crowley, S., J. Mahony, and D. van Sinderen, (2013), Current perspectives on antifungal lactic acid bacteria as natural bio-preservatives, Trends Food Sci Technol, 33, p93-109.
[https://doi.org/10.1016/j.tifs.2013.07.004]

-
Desai, S. D., and R. W. Currie, (2016), Effects of wintering environment and parasite-pathogen interactions on honey bee colony loss in North Temperate regions, PloS one, 11, e0159615.
[https://doi.org/10.1371/journal.pone.0159615]

- Dicks, L., and M. Botes, (2009), Probiotic lactic acid bacteria in the gastro-intestinal tract: health benefits, safety and mode of action, Benef Microbes, 1, p11-29.
-
Evans, J. D., K. Aronstein, Y. P. Chen, C. Hetru, J. L. Imler, et al. (2006), Immune pathways and defence mechanisms in honey bees Apis mellifera, Insect Mol. Biol, 15, p645-656.
[https://doi.org/10.1111/j.1365-2583.2006.00682.x]

-
Forsgren, E., T. C. Olofsson, A. Váasquez, and I. Fries, (2010), Novel lactic acid bacteria inhibiting Paenibacillus larvae in honey bee larvae, Apidologie, 41, p99-108.
[https://doi.org/10.1051/apido/2009065]

- Gong, H.-R., X.-X. Chen, Y. P. Chen, F.-L. Hu, J.-L. Zhang, et al. (2016), Evidence of Apis cerana Sacbrood virus infection in Apis mellifera, Appl. Environ. Microbiol, 82, p2256-2262.
-
Gramacho, K. P., and M. Spivak, (2003), Differences in olfactory sensitivity and behavioral responses among honey bees bred for hygienic behavior, Behav. Ecol. Sociobiol, 54, p472-479.
[https://doi.org/10.1007/s00265-003-0643-y]

-
Guler, A., (2008), The effects of the shook swarm technique on honey bee (Apis mellifera L.) colony productivity and honey quality, J. Apic. Res, 47, p27-34.
[https://doi.org/10.1080/00218839.2008.11101420]

- Hansen, H., and C. J. Brodsgaard, (2003), Control of American foulbrood by the shaking method, Apiacta, 38, p140-145.
- Jung, C., and S. K. Cho, (2015), Relationship between honeybee population and honey production in Korea, Korean J. Apiculture, 30, p7-12.
-
Killer, J., S. Dubná, I. Sedlácek, and P. Svec, (2014), Lactobacillus apis sp. nov., from the stomach of honeybees (Apis mellifera), having an in vitro inhibitory effect on the causative agents of American and European foulbrood, Int. J. Syst. Evol. Microbiol, 64, p152-157.
[https://doi.org/10.1099/ijs.0.053033-0]

-
Klein, A.-M., B. E. Vaissiere, J. H. Cane, I. Steffan-Dewenter, S. A. Cunningham, et al. (2007), Importance of pollinators in changing landscapes for world crops, Proceeding of the Royal Society of London B: Biological Sciences, 274, p303-313.
[https://doi.org/10.1098/rspb.2006.3721]

-
Leclercq, G., B. Pannebakker, N. Gengler, B. K. Nguyen, and F. Francis, (2017), Drawbacks and benefits of hygienic behavior in honey bees (Apis mellifera L.): a review, J. Apic. Res, 56, p366-375.
[https://doi.org/10.1080/00218839.2017.1327938]

-
Machado, T. A. D. G., M. E. G. de Oliveira, M. I. F. Campos, P. O. A. de Assis, E. L. de Souza, et al. (2017), Impact of honey on quality characteristics of goat yogurt containing probiotic Lactobacillus acidophilus, LWT-Food Science and Technology, 80, p221-229.
[https://doi.org/10.1016/j.lwt.2017.02.013]

-
Mondet, F., S. H. Kim, J. R. De Miranda, D. Beslay, Y. Le Conte, et al. (2016), Specific cues associated with honey bee social defence against Varroa destructor infested brood, Sci Rep, 6, p25444.
[https://doi.org/10.1038/srep25444]

-
Nazzi, F., S. P. Brown, D. Annoscia, F. Del Piccolo, G. Di Prisco, et al. (2012), Synergistic parasite-pathogen interactions mediated by host immunity can drive the collapse of honeybee colonies, PLoS Pathog, 8, e1002735.
[https://doi.org/10.1371/journal.ppat.1002735]

- Oldroyd, B. P., and S. Wongsiri, (2006), Asian honey bees: biology, conservation, and human interactions, Harvard University Press.
-
Oxley, P. R., M. Spivak, and B. P. Oldroyd, (2010), Six quantitative trait loci influence task thresholds for hygienic behaviour in honeybees (Apis mellifera), Mol. Ecol, 19, p1452-1461.
[https://doi.org/10.1111/j.1365-294x.2010.04569.x]

-
Palacio, M. A., E. Rodriguez, L. Goncalves, E. Bedascarrasbure, and M. Spivak, (2010), Hygienic behaviors of honey bees in response to brood experimentally pin-killed or infected with Ascosphaera apis, Apidologie, 41, p602-612.
[https://doi.org/10.1051/apido/2010022]

-
Peng, Y.-S., Y. Fang, S. Xu, and L. Ge, (1987), The resistance mechanism of the Asian honey bee, Apis cerana Fabr., to an ectoparasitic mite, Varroa jacobsoni Oudemans, J. Invertebr. Pathol, 49, p54-60.
[https://doi.org/10.1016/0022-2011(87)90125-x]

-
Pratt, S. C., (2004), Collective control of the timing and type of comb construction by honey bees (Apis mellifera), Apidologie, 35, p193-205.
[https://doi.org/10.1051/apido:2004005]

- Rashid, M., and M. Sultana, (2016), Role of probiotics in human and animal health review, J. Prob. Health, 4, p2-4.
-
Rothenbuhler, W. C., (1964), Behavior genetics of nest cleaning in honey bees. IV. Responses of F1 and backcross generations to disease-killed brood, Am. Zool, 4, p111-123.
[https://doi.org/10.1093/icb/4.2.111]

-
Spivak, M., and M. Gilliam, (1993), Facultative expression of hygienic behaviour of honey bees in relation to disease resistance, J. Apic. Res, 32, p147-157.
[https://doi.org/10.1080/00218839.1993.11101300]

- Spivak, M., and M. Gilliam, (1998), Hygienic behaviour of honey bees and its application for control of brood diseases and Varroa: Part II. Studies on hygienic behaviour since the Rothenbuhlerera, Bee world, 79, p169-186.
-
Spivak, M., and G. S. Reuter, (2001), Resistance to American foulbrood disease by honey bee colonies Apis mellifera bred for hygienic behavior, Apidologie, 32, p555-565.
[https://doi.org/10.1051/apido:2001103]

-
Sun, L., M. Li, D. Fei, J. Wang, L. Li, et al. (2018), Preparation and application of egg yolk antibodies against Chinese sacbrood virus infection, Frontiers in microbiology, 9, p1814.
[https://doi.org/10.3389/fmicb.2018.01814]

-
Swanson, J. A., B. Torto, S. A. Kells, K. A. Mesce, J. H. Tumlinson, et al. (2009), Odorants that induce hygienic behavior in honeybees: identification of volatile compounds in chalkbrood-infected honeybee larvae, J. Chem. Ecol, 35, p1108-1116.
[https://doi.org/10.1007/s10886-009-9683-8]

-
Tentcheva, D., L. Gauthier, N. Zappulla, B. Dainat, F. Cousserans, et al. (2004), Prevalence and seasonal variations of six bee viruses in Apis mellifera L. and Varroa destructor mite populations in France, Appl Environ Microbiol, 70, p7185-7191.
[https://doi.org/10.1128/aem.70.12.7185-7191.2004]

-
Theisen-Jones, H., and K. Bienefeld, (2016), The Asian honey bee (Apis cerana) is significantly in decline, Bee World, 93, p90-97.
[https://doi.org/10.1080/0005772x.2017.1284973]

-
Thompson, H. M., R. J. Waite, S. Wilkins, M. A. Brown, T. Bigwood, et al. (2006), Effects of shook swarm and supplementary feeding on oxytetracycline levels in honey extracted from treated colonies, Apidologie, 37, p51-57.
[https://doi.org/10.1051/apido:2005058]

-
Toan, T. V., M. L. Lee, H. S. Sim, H. K. Kim, G. H. Byuon, et al., (2014), Biological mitigation of Sacbrood disease on Apis cerana colonies, Korean J. Apiculture, 29, p181-186.
[https://doi.org/10.17519/apiculture.2014.09.29.3.181]

-
Toan, T. V., M. L. Lee, H. S. Sim, H. K. Kim, G. H. Byuon, et al., (2014), Initial results of rearing honey bee Apis cerana in vitro, Korean J. Apiculture, 29, p193-197.
[https://doi.org/10.17519/apiculture.2014.09.29.3.193]

- Vásquez, A., E. Forsgren, I. Fries, R. J. Paxton, E. Flaberg, et al. (2012), Symbionts as major modulators of insect health: lactic acid bacteria and honeybees, PloS one, 7, e33188.
- Vásquez, A., and T. C. Olofsson, (2009), The lactic acid bacteria involved in the production of bee pollen and bee bread, J. Apic. Res, 48, p189-195.
- Verma, S., and P. K. Attri, (2008), Indigenous beekeeping for sustainable development in Himachal Himalaya, Indian Journal of Traditional Knowledge, 7, p221-225.
-
Wagoner, K. M., M. Spivak, and O. Rueppell, (2018), Brood affects hygienic behavior in the honey bee (Hymenoptera: Apidae), J Econ Ento.
[https://doi.org/10.1093/jee/toy266]

-
Waite, R. J., M. A. Brown, H. M. Thompson, and M. H. Bew, (2003), Controlling European foulbrood with the shook swarm method and oxytetracycline in the UK, Apidologie, 34, p569-575.
[https://doi.org/10.1051/apido:2003052]

-
Webster, T. C., Y. S. Peng, and S. S. Duffey, (1987), Conservation of nutrients in larval tissue by cannibalizing honey bees, Physiol. Entomol, 12, p225-231.
[https://doi.org/10.1111/j.1365-3032.1987.tb00745.x]

-
Wu, M., Y. Sugimura, N. Takaya, D. Takamatsu, M. Kobayashi, et al. (2013), Characterization of bifidobacteria in the digestive tract of the Japanese honeybee, Apis cerana japonica, J. Invertebr. Pathol, 112, p88-93.
[https://doi.org/10.1016/j.jip.2012.09.005]

-
Xu, P., M. Shi, and X. X. Chen, (2009), Antimicrobial peptide evolution in the Asiatic honey bee Apis cerana, PLoS one, 4, e4239.
[https://doi.org/10.1371/journal.pone.0004239]

- Yang, M.-X., K. Tan, S. E. Radloff, C. W. Pirk, and H. R. Hepburn, (2010), Hetero-specific queen retinue behavior of worker bees in mixed-species colonies of Apis cerana and Apis mellifera, Apidologie, 41, p54-61.