
Eyes and Vision of the Bumblebee: a Brief Review on how Bumblebees Detect and Perceive Flowers
Abstract
Bumblebees have apposition compound eyes (one on either side of the head) of about 6,000 ommatidia and three small single-lens ocelli on the frons of their head capsule. The surface of the eye is smooth and interommatidial hairs, as in the honeybee, are not developed. Each ommatidium (approx. 26 μm in diameter) is capped by a hexagonal facet and contains in its centre a 3 μm wide, columnar light-perceiving structure known as the rhabdom. Rhabdoms consist of thousands of regularly aligned, fingerlike microvilli, which in their membranes contain the photopigment molecules. Axons from each ommatidium transmit the information of their photic environment to the visual centres of the brain, where behavioural reactions may be initiated. Since bumblebee eyes possess three classes of spectrally different sensitivity peaks in a ratio of 1:1:6 (UV=353 nm, blue=430 nm and green=548 nm) per ommatidium, they use colour vision to find and select flower types that yield pollen and nectar. Ommatidial acceptance angles of at least 3° are used by the bumblebees to discriminate between different flower shapes and sizes, but their ability to detect polarized light appears to be used only for navigational purposes. A flicker fusion frequency of around 110 Hz helps the fast flying bumblebee to avoid obstacles. The small ocelli are strongly sensitive to ultraviolet radiation and green wavelengths and appear to act as sensors for light levels akin to a photometer. Unlike the bumblebee’s compound eyes, the ocelli would, however, be incapable of forming a useful image.
Keywords:
Bombus hortorum, Honey Bee, Photoreception, Visual physiology, Visual behaviour, Spectral sensitivity, Polarization sensitivity, Ocellus, Flicker fusionBUMBLEBEE PHOTORECEPTORS: A BRIEF OVERVIEW
Vision plays an important role in the lives of most insects except for those that are cave dwellers. All adult insects as well as the nymphs of hemimetabolous species possess compound eyes as adults and bumblebees are no exception (Gullan and Cranston, 2000). They and other members of the order Hymenoptera such as, for instance the honeybees, not only possess compound eyes, one on either side of the head, but also three additional small single lens eyes known as ocelli on the frons of their head capsule. Despite being similar with regard to their position and shape (Fig. 1A, B), the eyes of the bumblebee (and its approximately 6,000 facets: Campan et al., 1965) are very slightly larger than those of the honeybee and, moreover, lack the long and numerous interfacetal hairs on the eye’s surface that are so characteristic for the honeybee. Why the hairs should be missing from the bumblebee’s eye but are so prominent in that of the honey bee is something yet to be fully understood.
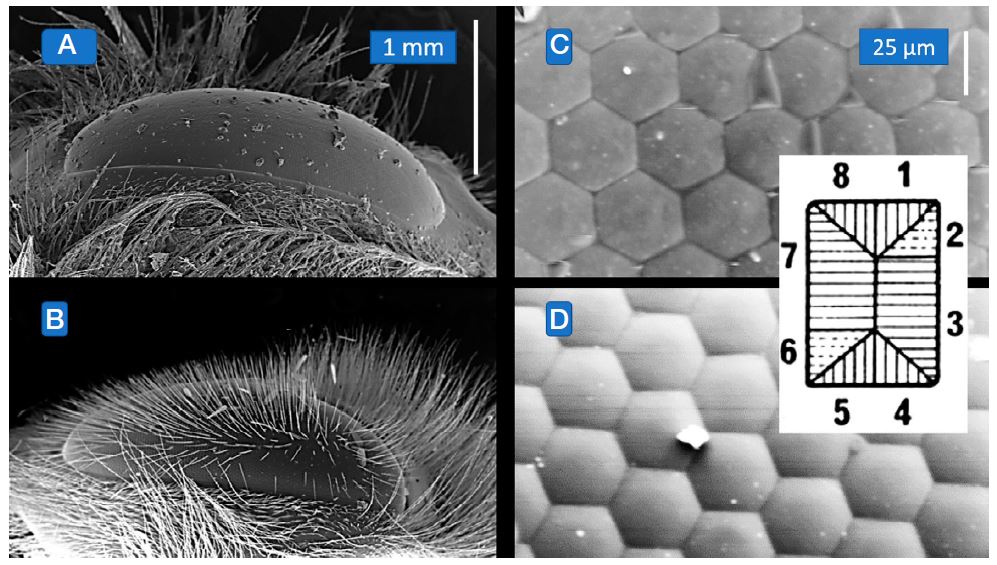
(A) and (B): Scanning electron micrographs of the compound eyes of bumblebee (A, top) and honeybee (B, bottom) at identical magnifications. Note the dense cover of interommatidial hairs on the eye of the honeybee. (C) and (D): The scanning electron micrographs show the facets covering the eye of the bumblebee (C, top) and that of the honeybee when the hairs have been brushed off (D, bottom). The inset shows the orientation of the visual membranes in the rhabdom and cellular contribution present in both bumblebee and honeybee eyes.
Since vision is generally the dominant sensory modality in connection with a variety of tasks in insects, it is easy to conceive how dependent in particular bumblebees are on the function of their eyes. Their eyes (Meyer-Rochow, 1981), like those of other hymenopteran species, are of the apposition type. In contrast to the so-called superposition eye, which is characteristic of nocturnal insects like moths and many beetles (Meyer-Rochow and Gal, 2004), the photoreceptive cells in the apposition eye of bumblebees (and other mainly diurnally active insect species) are located directly below the dioptric apparatus: there is no screening pigment free ‘clear-zone’ (Horridge, 1971) between receptor cells and dioptric apparatus. The latter consists of the corneal facets covering the eye and the underlying crystalline cones of each ommatidium. The task of the dioptric apparatus is to focus the light onto the photoreceptive elements of the eye, known as the rhabdoms. Photoreception and the resultant nerve responses depend on the interaction of photons with the photopigment in the visual membranes, the latter being microvilli of approximately 60 nm in diameter, stacked and arranged in columns, i.e. the rhabdoms, positioned along the central axis of each ommatidium.
The rhabdoms in bumblebees are formed by 8 retinula cells over most of the rhabdom’s length (a ninth cell is present in honeybees, but contributes little: Skrzipek and Skrzipek, 1974; Eisen and Youssef, 1980) and in cross sections reveal that their constituent microvilli are aligned in a characteristic pattern that is repeated in all ommatidia and allows identifications of the photoreceptive cells that contribute to the rhabdom. Cross sectioned rhabdoms in the bumblebee often reveal somewhat rectangular rather than circular outlines (Fig. 1C, D and inset) and the orientation of the microvilli in the rhabdom can reveal whether the eye possesses the capacity to distinguish the e-vector, i.e. is polarization sensitive (Kirschfeld, 1972; Eguchi, 1999).
There are of course, other sensors that bumblebees and their relatives, the honeybees, make use of in their lives and they include those to distinguish different surface structures (mechanoreceptors), odours (olfactory receptors) and temperature variations (thermoreceptors). However, when observing the behaviour of bumblebees it becomes obvious how extremely important vision is to them: caught in a room with windows on one side they continually bump against the window glass in order to escape from the room. Once outside and free to pursue their normal foraging activity they may seek inflorescences and fly towards them from considerable distances or they may head home to their nest. There is evidence from honeybees that suggests that the more monotonous environment in winter could have led to lighter bee heads and that the richer summer environment with regard to its multitude of stimuli could have been the cause of the summer bees’ heavier heads and possibly more developed and capable brains owing to the significantly greater amount of total RNA in the summer bee heads (Meyer-Rochow, 2002). It would be interesting to test whether such differences also apply to hibernating winter and active spring (or summer) bumblebees.
FUNCTIONAL ASPECTS OF LATERAL EYES AND OCELLI
Painting or covering the lateral (compound) eyes of a bumblebee does not totally incapacitate or inactivate the latter (Chittka et al., 1999), but it inhibits the insect to fly. The inescapable conclusion is that flight without vision is impossible, but movement in two dimensions by crawling is still possible. A bumblebee on the wing uses its compound eyes to detect open spaces, to avoid solid obstacles and snares like spider webs, to evade attacks by birds (or the butterfly net of an entomologist), to detect, select, and land on flowers and to locate its nest entrance as well as finding a partner to mate with. Occasionally a bumblebee in flight may collide with a car or a cyclist if the latter are moving along at too great a speed and that, too, can tell us something about the bumblebee’s vision, namely that the information received by the insect’s eyes about objects that appear in its field of vision is used in the control of its flight muscles to steer and guide the flying insect.
When the number of flashes (or images) registered by the eyes as separate events within a single second can no longer be resolved as separate and begin to fuse, thus creating the impression of a continuous movement, vision researchers talk about the “flicker fusion frequency” (Fig. 2A-C). This flicker fusion frequency depends on the brightness of the ambient light intensity and under daylight conditions has been measured through intracellular electrophysiological recordings from single photoreceptive cells in the eye of the bumblebee Bombus hortorum to be approximately 110 Hz (Campan et al., 1965; Meyer-Rochow, 1981). This figure suits an insect like the bumblebee with its relatively slow flight speed well, but would be unacceptable for “aerial acrobats” like dragonflies, which possess flicker fusion frequencies of up to 300 Hz. In humans flicker fusion occurs at much lower frequencies and even 14 images per second are usually not perceived as separate events, but as movement. As with insects this value drops further under poor lighting conditions.
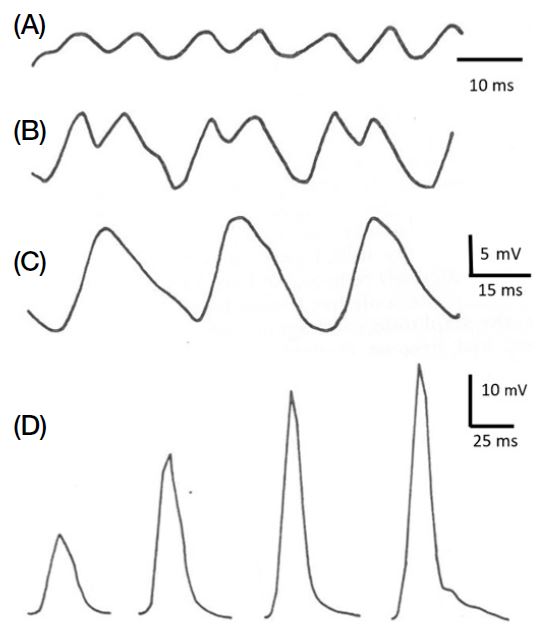
(A) Intracellularly recorded responses from a visual cell of the bumblebee eye to 10 μs flashes of white light (100 Hz). (B) Responses to twin flashes showing typical response characteristics to a frequency of 30 Hz. (C) Response to single flash of white light. (D) Intracellularly recorded responses from a visual cell of the bumblebee eye to 10 μs long stimulating flashes of increasingly bright white light from left to right.
The tasks that the small single lens eyes, the so-called ocelli on the bumblebee’s head, have are still not fully understood. In other insects the function of the ocelli has been compared to that of a light sensor that sets the sensitivity range of the large compound eyes on the sides of the head (Goodman, 1970), but there is also evidence that the ocelli are involved in navigational tasks and circadian control of locomotor rhythms (Honkanen et al., 2018). According to Wellington (1974) bumblebee ocelli are sensitive to polarized light and the detection of the plane of polarized light is often linked to an ability to perceive ultraviolet radiation. Meyer-Rochow (1981) showed that at least the median ocellus (the one flanked by the two smaller ones) in the bumblebee exhibits strong UV-sensitivity to light of 353 nm wavelength in addition to a somewhat weaker response to green light of 519 nm in Bombus hortorum.
To what extent covering one or all three of the ocelli of a bumblebee might affect the insect’s behaviour, when at the same time the compound eyes are also either painted over or are left uncovered, is unknown for this species. However, such experiments were done on the cockroach (Honkanen et al., 2018) and similar studies could possibly also shed some light on the role of the ocelli in the bumblebee. That the latter, however, with an acceptance angle of just 20° (Meyer-Rochow, 1981), would not be able to produce a useful image of objects in the bumblebee’s environment and thereby contribute to the sharpness of the world perceived by the insect, is obvious. At best a very crude under-focused image would result, which then would need significant processing by the insect’s visual centres of the brain as ocelli are unable to accommodate, i.e. move back and forward or change their shapes (Krapp, 2009). Garcia et al. (2017) have recently proposed on the basis of mathematical modelling that the ocelli could be involved in enabling the bees to make reliable colour decisions under changing levels of illumination.
SENSITIVITY AND ACUITY
This brings us to the problem of resolving power and visual sensitivity. Polymorphism in bumblebee species is common and that the position and size of the eye can affect binocular overlap, sensitivity, and visual resolution has recently been the subject of an investigation by Taylor et al. (2019). Resolving power is related to the finest detail that can be perceived by the eye. How the visual centres of the brain then process the information received from the eyes and whether the full capacity of the photoreceptors is made use of by the nervous system of the insect is another matter that needs to be assessed in behavioural tests. A constant struggle for any photoreceptor is how to balance resolving power (also known as ‘acuity’) with sensitivity, for given the structural constraints of an eye, the two functions are incompatible: high resolution in a compound eye requires small interommatidial angles and narrow photoreceptors with slim light-perceiving structures in the ommatidia (known as the rhabdoms mentioned earlier) that function as light guides when at least as wide as 1.5 μm in diameter and as wave guides when narrower. High absolute sensitivity to light on the other hand depends on wide apertures and in an apposition eye like that of a bumblebee this means large facets with large interommatidial angles and rhabdoms that aren’t thin but voluminous to transmit more light and to accommodate greater amounts of photopigment molecules in them. Problems that compound eyes generally face in this regard -and bumblebees are no exception-have been explained by Horridge (1977), who introduces the term “eye parameter” (p) in connection with the difficulty to reconcile sensitivity and resolution in a compound eye.
Acceptance angles of individual photoreceptive cells determine the amount of detail an insect is able to obtain from an image the compound eye produces. Narrow acceptance angles lead to a greater density of pixels, but not brighter images. They are therefore useful for insects like honey and bumblebees that need to see not only the flowers they intend to visit, but details of the flower like the so-called bull’s eye of a flower and its pollen-bearing stamens. The smallest acceptance angles, determined through intracellular electrophysiological recordings from photoreceptive cells in the bumblebee Bombus hortorum, were 2.7° (Meyer-Rochow, 1981) and were therefore slightly broader than the 2.5° degrees reported for the worker honeybee photoreceptor cells by Laughlin and Horridge (1971).
The somewhat larger acceptance angle of the bumblebee’s visual cells and the wider rhabdoms in its ommatidia (greatest widths of 3 μm when compared with the 2.5 μm for the honeybee) in combination with the slightly bigger facets are almost certainly the reason why these insects continue to forage late into the evenings and at relatively low light levels. That the visual centre of the brains of these and other bee species can further improve the sharpness of the image it receives from the eye is to be expected, so that even finer details may be discerned. Behavioural tests by Macuda et al. (2001) actually suggested that the eyes of the bumblebee Bombus impatiens conferred resolutions that were 25% better than those obtained from the tested honeybees, but recently Rigosi et al. (2017) published electrophysiologically determined acceptance angles in worker honey bees of 1.9°, representing a value “30% smaller than that previously reported”.
The term ‘visual sensitivity’ requires further definition, because the absolute sensitivity of an eye to white light measured as an ERG (electro-retinogram) is less accurate than intracellular recordings from single photoreceptive cells in which an increase in cellular depolarization with increasing stimulus brightness accurately describes the cell’s sensitivity over a range of light intensities as a V/log I curve (Fig. 2D). Spectral sensitivity curves obtained by the ERG method may differ from the spectral sensitivities of individual receptors and the degree to which polarized light can be perceived. For honeybees it has long been known that their eyes are sensitive to linearly polarized light (cf., Kirschfeld, 1972; Wehner, 1976; Zeil et al., 2014) and through intracellular recordings it has been established that they contain three kinds of spectrally sensitive photoreceptive cells (UV, blue, and green sensitive cells: Menzel, 1979). Honeybees, furthermore, are alsoknown to remember colours very well (Menzel, 1968).
In the eyes of the bumblebee Bombus hortorum Meyer-Rochow (1981) determined through intracellular recordings from 75 retinula cells of 12 bumblebees a ratio of approximately 1 : 1: 6 of UV : blue : green sensitive cells, peaking at 353, 430 and 548 nm wavelengths, respectively (Fig. 3A, B). In one other study based on intracelllar recordings Skorupski et al. (2007) identified spectral peaks in B. terrestris dalmatinus (UV=348; blue=435; green=533) from the Italian mainland and B. terrestris sassaricus (UV=347; blue=436; green=536) from the island of Sardinia. The small differences with regard to the B. hortorum peaks are likely to be species-specific or due to the high northern latitude with a different photic environment to that of the Mediterranean region that B. hortorum vision had to adapt to. Overall agreement, however, confirming trichromatic vision with peak sensitivities in the ultraviolet, blue, and green regions of the visual spectrum, is good.
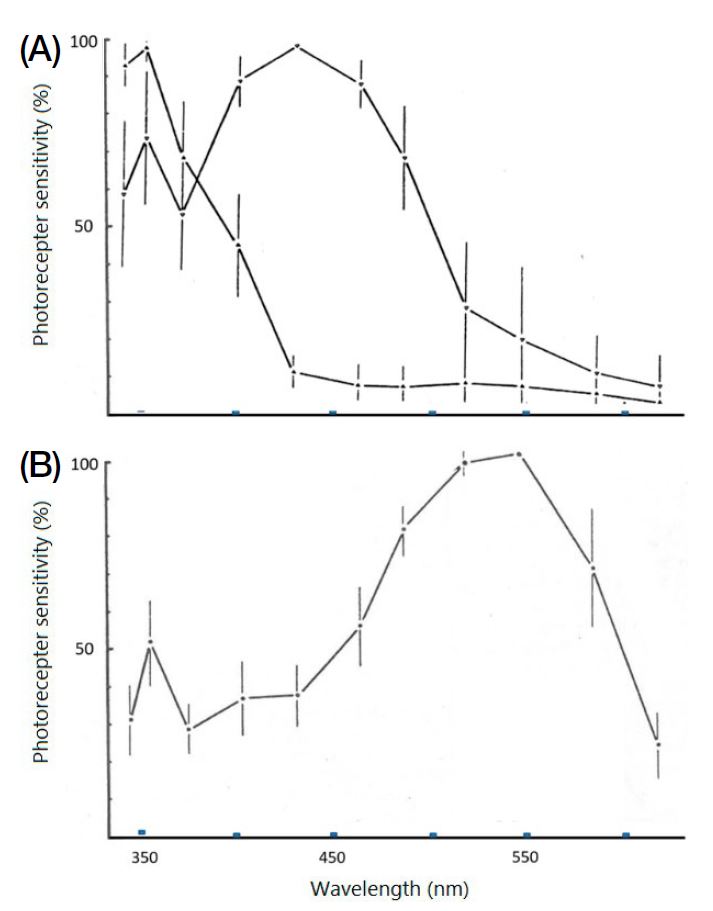
Results (with error bars) of the three spectrally different photoreceptive cells present in the eye of the bumblebee. Intracellular recordings revealed a ratio of 11 : 10 : 54 respective cell types. Sensitivity values were converted into percentages as in Meyer-Rochow (1981) and plotted on the ordinate. (A): Responses of 11 UV and 10 blue sensitive photoreceptive (=retinula) cells with respective peak sensitivities to lights of 353 nm and 430 nm wavelengths. (B). Responses of 54 green sensitive photoreceptive (=retinula) cells with peak sensitivity to light of 548 nm wavelength.
Given the fact that each ommatidium contains 8 photoreceptive cells and that the rhabdom and ommatidial organization are therefore not significantly different from what is known with regard to worker bee eyes, we can assume that there are 6 green sensitive cells per ommatidium plus one blue and one UV sensitive cell each. What, however, we should not assume without further investigation is that the three castes (workers, males, and queens) all share the same eye structure and visual capacity. There are many species of insects in which males and females possess different eye structures (Meyer-Rochow, 2008) including the honeybee with its different castes (Perrelet, 1970; Menzel et al., 1991; Streinzer et al., 2013). Whether structure and function of male and female bumblebee eyes differ and whether the ultrastructural organizations of the photoreceptive cells vary in the eyes of hibernating or summer-active individuals is not known.
FLOWER-VISITING BEHAVIOUR AND THE ROLE OF VISION
What unites the different castes of both bumble and honeybees is their need to visit flowers in order to sample nectar (mostly required as an efficient energy source for physical activities like flight, brood cell construction, etc.) and pollen to feed the larvae with. Unsurprisingly there has been considerable interest in floral colour patterns (Fig. 4A, B) and a review on the functional significance of the optical properties of flowers for visual signalling has recently been published by Van der Kooi et al. (2018) as a follow-up to a study by Dyer et al. (2008) on the psychophysics of bumblebee and honeybee colour discrimination and object detection. In both flower-seeking species innate preferences and learning play a role. In a series of experiments by Lunau et al. (2015), in which, for example, “bumblebees collected glass powder, which is visually inconspicuous and scentless, as often as Pinus pollen” indicating that no chemical stimulus is needed in collecting pollen. That pollen themselves can possess means to make it more difficult to be collected became obvious when the sticky pollenkitt of hollyhock pollen (Alcea rosea) was removed and the pollen spines were bent by vortexing: bumblebees readily collected these pollen, but without such pollen pre-treatment bumblebees would avoid them, suggesting that it was a mechanical defence and not a toxin that protected the pollen (Lunau et al., 2015). It is clear that visual stimuli initiate flower visitations and pollen collecting, but the full repertoire to complete the behavioural sequences of pollen collection occurs with multimodal stimulation that combines visual, olfactory, gustatory and tactile responses (Wilmsen et al., 2016).

(A): Example of a flower that exhibits strong ultraviolet absorbance in the centre, making it easy for flower-seeking insects with UV-vision (like bumble or honeybees) to locate the so-called bullseye with its pollen bearing stamens. (B): Human eyes are incapable of seeing UV and would see the same flower as in the photograph on the right.
The fact that ultraviolet patterns are a component of flower reflections and seen by flower-visiting hymenopterans like bumblebees and honeybees has been studied by Chittka et al. (1994) and photographs of flowers in ultraviolet of a large number of species have been made available by Rørslett (www.naturfotograf. com/UV_flowers_list.html#NYCTAGIX). Bees prefer the highest spectral purity and highest chromatic contrast against the background and respond sensitively to even small changes in flower coloration (Papiorek et al., 2013), something that can be expected to apply equally to bumblebees. Compared with human vision not only do bumblebees perceive ultraviolet as a colour, they also process colour vision much faster than humans (Skorupski and Chittka, 2010). The ultraviolet coloration or patterns that many flowers exhibit under UV-radiation are often very specifically distributed. Termed “floral or nectar guides” they usually affect stamens or patches on the petal bases that imitate stamen and are indicative of the flower’s “bulls eye” (Lunau et al., 1996). Most of the pollen due to their contents of flavonoids and carotenoids absorb ultraviolet radiation (Dyer et al., 2008).
Having collected pollen and ingested nectar, bumblebees need to find their way home. Navigation and determination of direction has been studied intensively and to great detail in hymenopterans like ants and honeybees (Dyer and Gould, 1983; Wehner and Srinivasan, 2003; Zeil et al., 2014), in which the perception of linearly polarized light, i.e. the E-vector, plays a critical role. Although the structural organization of the bumblebee rhabdom is virtually identical to that of the honeybee and polarization sensitivity in approximately 50% of the bumblebee’s UV and blue sensitive retinal cells were found to possess polarization sensitivities of 4 : 1, polarization-aided orientation in free living bumblebees has not yet been conclusively demonstrated.
What is known, is that bumblebees pay no or little attention to polarizing patterns during their flower visitations (Chittka et al., 1994), but are apparently able to learn to discriminate artificial flowers on the basis of their polarization characteristics (Orbán and Plowright, 2014) and may use celestial polarization patterns for navigation at dusk (Wellington, 1974). A clue that bumblebees could possibly employ magnetic compass orientation comes from observations by Chittka et al. (1999) who demonstrated that bumblebees were able to measure both distance and direction in total darkness even in the absence of odour trails. This suggests that bumblebees have available to them “backups” to supplement their sense of vision.
In order to locate a source of nectar and pollen, flower-visiting insects must be able to have spatial vision and see possible food sources over considerable distances (Wehner, 1981; Lehrer, 1994). Exactly from how far bumblebees would be able to determine whether it is worth the effort and energy to approach a possible food source is not known, but it is possible to argue like Brito et al. (2015) did that trees in the distance represent huge flowers and the flowers in them represent oversized floral guides. Flowers are of different shapes and sizes and bumblebees have been known to possess an excellent memory for flowers (Raine and Chittka, 2007). Because of the bumblebees’ and honeybees’ similar feeding habits, conclusions with regard to pattern discrimination studied extensively in the latter (see recent reviews by Horridge (2000) and Avargues-Weber et al. (2012) can be expected to apply to the former as well. Unsurprisingly then bumblebees can also be assumed to be capable of distinguishing many different patterns in the same way that honeybees and even mammals do (Srinivasan et al., 1994).
SOME OPEN QUESTIONS
Both honeybees and bumblebees often spend long times exposed to the bright light of sunshine. They cannot close their eyes, although adaptational changes and screening pigment migrations within the eye are known to occur and help protecting structures vulnerable to radiation damage (Kolb and Autrum, 1972). However, although UV-induced damage to either rhabdoms or visual cell ultrastructural features have been reported to impact the eyes of some insects (Meyer-Rochow et al., 2002), bumblebee eyes exposed to similar levels of UV-radiation seemed unaffected. Whether their resistance to UV-induced damage was due to their genetic make up and existed from the moment they eclose from the pupa or whether they build up some UV-resistance during the summer when they forage in bright sunlight could not be answered in the study by Meyer-Rochow et al. (2002). However, that age-related structural and functional changes can indeed occur and affect insect eyes has been shown (albeit not for bumblebees and other hymenopterans yet) by Butler et al. (1970) for adults of the black carpet beetle and by Bremer et al. (1993) for the compound eye of the termite Neotermes jouteli. To investigate this as well as some of the other questions raised in this brief review on bumblebee vision (like brain and eye differences in winter and summer bumblebees, the lack of interommatidial hairs, dissimilarities between male and female photoreceptor structure and function, distance vision, etc.) should be interesting tasks for the future.
Acknowledgments
The author wishes to thanks the staff of the Sophisticated Central Laboratory of Andong National University and Insect Industry R&D center for the instrumental support and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2018R1A6A1A03024862) and student Oh Jae Seok for their help with the scanning electron micrographs.
References
- Avargues-Weber, A., T. Mota, and M. Giurfa, (2012), New vistas on honey bee vision, Apidologie, 43, p244-268.
-
Bremer, S., H. Hertel, and E. Wachmann, (1993), Degeneration of the compound eye of the termite Neotermes jouteli (Isoptera) in darkness during the phase of reproduction, Zoomorphol, 113, p205-210.
[https://doi.org/10.1007/bf00394861]

-
Brito, V. L. G., K. Weynans, M. Sazima, and K. Lunau, (2015), Trees as huge flowers and flowers as oversized floral guides: the role of floral color change and retention of old flowers in Tibouchina pulchra, Front. Plant Sci.
[https://doi.org/10.3389/fpls.2015.00362]

- Butler, L., R. Roppel, and J. Zeigler, (1970), Post emergence maturation of the eye of the adult black carpet beetle, Attagenus megatoma (Fab.), J. Morphol., 130, p103-128.
- Campan, R., A. Gallo, and Y. Queinnes, (1965), Détermination électrorétinographique de la fréquence critique de fusionnement visual: étude comparative portant sur les yeux composés de dix-sept espèces d’insectes, C. R. Soc. Biol., 159, p2521-2526.
- Chittka, L., A. Shmida, N. Troje, and R. Menzel, (1994), UV arrangement of flower reflections and the colour perception of Hymenoptera, Vision Res., 34, p1489-1508.
-
Chittka, L., N. M. Williams, H. Rasmussen, and D. Thomas, (1999), Navigation without vision: bumblebee orientation in complete darkness, Proc. Biol. Sci. B, 266(1414), p45-50.
[https://doi.org/10.1098/rspb.1999.0602]

-
Dyer, A. G., J. Spaethe, and S. Prack, (2008), Comparative psychophysics of bumblebee and honeybee colour discrimination and object detection, J. Comp. Physiol. A, 194, p614-627.
[https://doi.org/10.1007/s00359-008-0335-1]

- Dyer, F. C., and J. L. Gould, (1983), Honey Bee Navigation: The honey bee̓s ability to find its way depends on a hierarchy of sophisticated orientation mechanisms, Am. Scient., 71(6), p587-597.
- Eguchi, E., (1999), Polarized light vision and rhabdom, p33-46, in Atlas of arthropod sensory receptors eds. byE. Eguchi, and Y. Tominaga, Springer, Tokyo, Berlin, New York.
-
Eisen, J. S., and N. N. Youssef, (1980), Fine structural aspects of the developing compound eye of the honey bee Apis mellifera L., J. Ultrastruct. Res., 71(1), p79-94.
[https://doi.org/10.1016/s0022-5320(80)90038-6]

-
Garcia, J. E., Y.-S. Hung, A. D. Greentree, M. G. P. Rosa, J. A. Endler, and A. G. Dyer, (2017), Improved color constancy in honey bees enabled by parallel visual projections from dorsal ocelli, PNAS, 114(29), p7713-7718.
[https://doi.org/10.1073/pnas.1703454114]

- Goodman, L. J., (1970), The Structure and function of the insect dorsal ocellus, Adv. Insect Physiol., 7, p97-195.
- Gullan, P. J., and P. S. Cranston, (2000), The Insects-An Outline of Entomology, Blackwell Publishing, Oxford.
-
Honkanen, A., P. Saari, J. Takalo, K. Heimonen, and M. Weckström, (2018), The role of ocelli in cockroach optomotor performance, J. Comp. Physiol. A, 204(2), p231-243.
[https://doi.org/10.1007/s00359-017-1235-z]

- Horridge, G. A., (1971), Alternatives to superposition images in clear-zone compound eyes, Proc. Roy. Soc. Lond. B, 179, p97-124.
-
Horridge, G. A., (1977), Insects which turn and look, Endeavour, 1, p7-17.
[https://doi.org/10.1016/0160-9327(77)90004-7]

-
Horridge, G. A., (2000), Seven experiments on pattern vision of the honeybee, with a model, Vision Res., 40, p2589-2603.
[https://doi.org/10.1016/s0042-6989(00)00096-1]

- Kirschfeld, K., (1972), Die notwendige Anzahl zur Bestimmung der Richtung des elektrischen Vektors linear polarisierten Lichtes, Z. Naturforsch., 27b, p578-579.
-
Kolb, G., and H. Autrum, (1972), Die Feinstruktur im Auge der Biene bei Hell und Dunkeladaptation, J. Comp. Physiol., 77, p113-125.
[https://doi.org/10.1007/bf00693601]

-
Krapp, H. G., (2007), Polarization vision: how insects find their way by watching the sky, Curr. Biol., 17, pR557-R560.
[https://doi.org/10.1016/j.cub.2007.05.022]

-
Laughlin, S. B., and G. A. Horridge, (1971), Angular sensitivity of the retinula cells of dark adapted worker bee, Z. Vergl. Physiol., 74, p329-335.
[https://doi.org/10.1007/bf00297733]

-
Lehrer, M., (1994), Spatial vision in the honey bee: the use of different cues in different tasks, Vision Res., 34, p2363-2385.
[https://doi.org/10.1016/0042-6989(94)90282-8]

- Lunau, K., V. Piorek, O. Krohn, and E. Pacini, (2015), Just spines - mechanical defence of malvaceous pollen against collection by corbiculate bees, Apidologie, 46, p144-149.
-
Lunau, K., K. Wacht, and L. Chittka, (1996), Colour choices of naïve bumble bees and their implication for colour perception, J. Comp. Physiol. A, 178, p477-489.
[https://doi.org/10.1007/bf00190178]

- Macuda, T., R. J. Gegear, T. M. Laverty, and B. Timney, (2001), Behavioural assessment of visual acuity in bumblebees (Bombus impatiens), J. Exp. Biol., 204(3), p559-564.
-
Menzel, J. G., H. Wunderer, and D. G. Stavenga, (1991), Functional morphology of the divided compound eye of the honeybee drone (Apis mellifera), Tissue Cell, 23(4), p525-535.
[https://doi.org/10.1016/0040-8166(91)90010-q]

- Menzel, R., (1968), Das Gedächtnis der Honigbiene für Spektralfarben. I. Kurzzeitiges und langzeitiges Behalten, Z. Vergl. Physiol., 60, p82-102.
- Menzel, R., (1979), Spectral sensitivity and colour vision in invertebrates, p503-580, in Handbook of Sensory Physiology Vol VII/6A, ed. byH. Autrum, Springer, Verlag, Berlin.
-
Meyer-Rochow, V. B., (1981), Electrophysiology and histology of the eye of the bumblebee Bombus hortorum (L.) (Hymenoptera; Apidae), J. Roy. Soc. N. Zld., 11(s), p123-153.
[https://doi.org/10.1080/03036758.1981.10419447]

-
Meyer-Rochow, V. B., (2002), Honeybee heads weigh less in winter than in summer: a possible explanation, Ethol. Ecol. Evol., 14, p69-71.
[https://doi.org/10.1080/08927014.2002.9522762]

- Meyer-Rochow, V. B., (2008), Zur funktionellen Bedeutung unterschiedlicher Augenstrukturen bei sexualdimorphen Nachtfaltern und Leuchtkäfern: eine kurze Zusammenfassung neuerer Ergebnisse, Entomologie Heute, 20, p193-208.
-
Meyer-Rochow, V. B., and J. Gal, (2004), Dimensional limits for arthropod eyes with superposition optics, Vision Res., 44, p2213-2223.
[https://doi.org/10.1016/j.visres.2004.04.009]

-
Meyer-Rochow, V. B., T. Kashiwagi, and E. Eguchi, (2002), Selective photoreceptor damage in four species of insects induced by experimental exposures to UV-irradiation, Micron, 33, p23-31.
[https://doi.org/10.1016/s0968-4328(00)00073-1]

-
Orbán, L. L., and C. M. S. Plowright, (2014), Getting to the start line: how bumblebees and honeybees are visually guided towards their first floral contact, Insect Soc., 61, p325-336.
[https://doi.org/10.1007/s00040-014-0366-2]

-
Papiorek, S., K. Rohde, and K. Lunau, (2013), Bee’s subtle colour preferences: how bees respond to small changes in pigment concentration, Naturwissenschaften, 100, p633-643.
[https://doi.org/10.1007/s00114-013-1060-3]

-
Perrelet, A., (1970), The fine structure of the retina of the honey bee drone, Z. Zellforsch. Mikrosk. Anat., 108, p530-562.
[https://doi.org/10.1007/bf00339658]

- Raine, N., and L. Chittka, (2007), Flower constancy and memory dynamics in bumblebees (Hymenoptera: Apidae: Bombus), Entomol. Gener., 29(2-4), p179-199.
-
Rigosi, E., S. D. Wiederman, and D. O’Carroll, (2017), Visual acuity of the honey bee retina and the limits for feature detection, Sci. Rep., 7, 45972.
[https://doi.org/10.1038/srep45972]

-
Skorupski, P., and L. Chittka, (2010), Differences in photoreceptor processing speed for chromatic and achromatic vision in the bumblebee Bombus terrestris, J. Neurosci., 30(11), p3896-3903.
[https://doi.org/10.1523/jneurosci.5700-09.2010]

-
Skorupski, P., T. F. Döring, and L. Chittka, (2007), Photoreceptor spectral sensitivity in island and mainland populations of the bumblebee, Bombus terrestris, J. Comp. Physiol. A, 193, p485-494.
[https://doi.org/10.1007/s00359-006-0206-6]

-
Skrzipek, K. H., and H. Skrzipek, (1974), The ninth retinula cell in the ommatidium of the worker honey bee (Apis mellifica L.), Z. Zellforsch. Mikrosk. Anat., 147(4), p589-593.
[https://doi.org/10.1007/bf00307257]

- Srinivasan, M. V., S. W. Zhang, and K. Whitney, (1994), Visual discrimination of pattern orientation by honeybees, Phil. Trans. Roy. Soc. Lond., 343, p199-210.
-
Streinzer, M., A. Brockmann, N. Nagaraja, and J. Spaethe, (2013), Sex and caste-specific variation in compound eye morphology of five honeybee species, PloS One, 8(2), pe57702.
[https://doi.org/10.1371/journal.pone.0057702]

-
Taylor, G. J., P. Tichit, M. D. Schmidt, A. J. Bodey, C. Rau, and E. Baird, (2019), Bumblebee visual allometry results in locally improved resolution and globally improved sensitivity, eLife., 2019;8:e40613.
[https://doi.org/10.7554/eLife.40613]

- Van der Kooi, C. J., A. G. Dyer, P. G. Kevan, and K. Lunau, (2018), Functional significance of the optical properties of flowers for visual signalling, Annals of Botany, in press.
-
Wehner, R., (1976), Polarized light navigation by insects, Scient. Am., 235, p106-115.
[https://doi.org/10.1038/scientificamerican0776-106]

- Wehner, R., (1981), Spatial vision in arthropods, p287-616, in Handbook of Sensory Physiology Vol. VII/6c, ed. byH. Autrum, Springer, Berlin, Heidelberg, New York.
- Wehner, R., and M. V. Srinivasan, (2003), Path integration in insects, p9-30, in The Neurobiology of Spatial Behaviour, ed. byK. J. Jefferey, Oxford University Press, Oxford.
-
Wellington, W. G., (1974), Bumblebee ocelli and navigation at dusk, Science, 183(4124), p550-551.
[https://doi.org/10.1126/science.183.4124.550]

-
Wilmsen, S., R. Gottlieb, R. R. Junker, and K. Lunau, (2016), Bumblebees require visual pollen stimuli to initiate and multimodal stimuli to complete a full behavioral sequence in close-range flower orientation, Ecol. Evol., 7(5), p1384-1393.
[https://doi.org/10.1002/ece3.2768]

-
Zeil, J., W. A. Ribi, and A. Narendra, (2014), Polarisation vision in ants, bees and wasps, G. Horváth, (ed.)Polarized Light and Polarization Vision in Animal Sciences, Springer Series in Vision Research 2, Berlin, Heidelberg, New York.
[https://doi.org/10.1007/978-3-642-54718-8_3]
