Differential Effects of the Detoxicant on the Honey Bee Intoxicated with Seven Pesticides and its Chemical Characterization
Abstract
Honey bees (Apis mellifera), particularly foragers, are continuously exposed to an array of potentially toxic insecticides. While foraging, they directly encounter insecticides and other toxic materials, and they often carry residual pesticides in their pollen loads into their hive. Although they are distinctively sensitive to toxic compounds, honey bees possess detoxifying mechanisms to thrive in the presence of toxic compounds. G-3KM detoxicant is a commercially detoxicant bee product marketed in South Korea. Analyzed by gas chromatography-mass spectrometry, 1,8-cineole was identified as the only volatile organic constituent of G-3KM, after it was subjected to a liquid-liquid partition between hexane and water. In this study, G-3KM detoxicant and its only volatile constituent, 1,8-cineole, were evaluated for their potential in reducing the mortality of worker bees intoxicated with seven different insecticides (5 neonicotinoids, 1 organophosphate, 1 acaricide). On response to worker bees intoxicated with insecticides, G-3KM treatments were found to be effective in reduction of worker bees mortality in all treatments, particularly acetamiprid (cyano-neonicotinoid) and amitraz (acaricide) on all tested concentrations. There were no significant reductions in worker bee mortality when treated with 1,8-cineole. Therefore, the active ingredient of G-3KM detoxicant could be polar substance(s) presented in polar aqueous fraction.
Keywords:
Honey bees (Apis mellifera), Insecticides, G-3KM detoxicant, 1,8-Cineole, MortalityINTRODUCTION
Honeybees pollinate around one third of the world’s crop species, but their population decline has put the global food production at risk (Klein et al., 2007; Cox-Foster and van Engelsdorp, 2009). Honey bees forage as far as 10 km from their hives (Couvillon et al., 2014) to search of nectar, pollen, water, and propolis needed to sustain a colony. While foraging, they are exposed to enormous variety of agrochemicals used in crop production, and on their return, they may bring significant amount of toxic contaminants into hive. Sánchez-Bayo and Goka (2014) showed that residues of 173 different compounds were found in apiaries. Toxic Residue of carbaryl and lime sulfur were also detected on the European honey bee, Apis mellifera and buff-tailed bumble bee, Bombus terrestris (Kim et al., 2014).
Pesticides are toxic chemicals designed to specifically control a target group of organisms. However, the toxicity of pesticides is not exclusively limited to the target organisms. Often, they may affect non-target species that have similar metabolism (Lushchak et al., 2018). Pesticides are classified into several groups based on their mode of action, including; acetylcholinesterase (AChE) inhibitors (organophosphate and carbamates), GABA-gated chloride channel blockers (cyclodiene organochlorines), sodium channel modulators (pyrethroids, DDT), nicotinic acetylcholine receptor (nAChR) competitive modulators (neonicotinoids), octopamine receptor agonists (amitraz) and many others (IRAC MoA, 2020).
Neonicotinoid insecticides are used to protect a wide variety of crops, and they have become a foundation of pest management since their discovery in 1990s (Nauen and Denholm, 2005). However, the European Union banned the field use of three neonicotinoids (imidacloprid, clothianidin and thiamethoxam) in 2018 (EFSA, 2018; Jactela et al., 2019) as there was compiling evidence implicating neonicotinoids in CCDS in bees and in other threats to the environment (Wood and Goulson, 2017), although they may still be used in greenhouses. Neonicotinoids do not affect cholinesterase, but rather bind to acetylcholine receptors resulting in prolonged stimulation leading to insect death (Lushchak et al., 2018). Several studies have also demonstrated that neonicotinoids were found to be toxic to honey bees (Lee et al., 2016; Ghosh and Jung, 2017; Kang and Jung, 2017; Ulziibayar and Jung, 2019). Carbamates and organophosphate are the most toxic substances because they inhibit acetylcholinesterase (AChE), an important nervous system enzyme that inactivates synaptic the neurotransmitter acetylcholine (Galloway and Handy, 2003). The organophosphate insecticides bind and inactivate AChE irreversibly, leading to overstimulation of insect nervous system. Such inhibition of acetylcholinesterase finally kills animals. However, carbamates are reversibly inhibitor of AChE.
Honey bees, like all other animals, are endowed with detoxification mechanisms that transform and eliminate most toxic chemicals. Several studies have demonstrated the involvement of the P450-, GST- or COE-enzyme families in pesticide and secondary metabolite detoxification in honey bees (Feyereisen 2012; Johnson et al., 2012). Honey bees activate detoxification mechanisms when they are exposed to toxic pesticides (Johnson et al., 2010). Enhancing the production level of acetylcholinesterase (AChE) is one of the main mechanisms when individuals exposed to pesticides such as carbamates and organophosphates (Walker et al., 2001), to pyrethroids (Badiou et al., 2008) and neonicotinoids (Boily et al., 2013). There are also a few phytoconstitutents known to detoxify honey bee from toxic pesticides (Balieira et al., 2018).
Honey bees are continuously exposed to various pesticides and other toxic materials. Hence, pesticides are one of the major factors that contribute to honey bee population decline. G-3KM is a bee detoxicant product marketed in South Korea. However, to the best of our knowledge, there were no prior studies on G-3KM detoxicant or 1,8-cineole as detoxicant. Therefore, the present study was designed to determine the effects of a detoxicant (G-3KM) and its volatile constituent (1,8-cineole) on reduction of mortality of worker bees intoxicated with five neonicotinoids (acetamiprid, imidacloprid, thiamethoxam, clothianidin, dinotefuran), one organophosphate (fenitrothion) and one acaricide (amitraz), separately.
MATERIALS AND METHODS
1. Materials
All insecticides information is listed below in Table 1. G-3KM detoxicant was purchased from Dogo Medical Company (Seoul, South Korea). 1,8-cineole (99% purity, CAS Number: 470-82-6, Sigma-Aldrich Korea) was purchased from Sigma Aldrich. Solvents (hexane, acetone) were also purchased from Sigma Aldrich. All pesticides were diluted in sugar syrup to prepare the test concentrations
We have collected young adult workers from healthy honey bee (Apis mellifera) colonies in the experimental apiary of Andong National University. The collected bees were released into the ventilated transparent plastic cups, and kept in the experimental room at 25±2℃ and 50~70% relative humidity.
2. Methods
G-3KM detoxicant (10 mL) was suspended in distilled water (100 mL) and was poured into a separatory funnel (capacity 500 mL; Schott/Duran, Germany). Hexane (100 mL) was added into it. The mixture was then manually shaken vigorously for 5 min and then allowed to stand for 30 min. Following this process, the organic and aqueous layers were separated. The aqueous layer was then further extracted again by shaking with hexane (100 mL). Both first and second hexane extracts were combined, and the residual water was removed from the extract by treating with an anhydrous sodium sulphate (5 g). The extract was filtered and transferred into a round-bottom flask, followed by evaporation. The extract was stored in a refrigerator until further use.
Hewlett-Packard HP 5977A Mass Spectrometer coupled with an HP-7890B GC was used to identify and quantify the VOC of G-3KM detoxicant. Separation was performed on a non-polar column (HP-5MS-fused silica column; 5% phenyl methylpolysiloxane; 30 m×0.25 mm id; 0.25 μm film thickness, Agilent Technologies). VOC of G-3KM detoxicant was diluted in acetone (1 : 10) prior to GC-MS analysis. VOC (2 μL) was injected in split mode with a 1 : 10 split ratio. Helium was used as a carrier gas at a flow rate of 1.0 mL/min. The oven was initially set at 40℃ for 3 min, then the temperature was programmed to increase by 6℃/min until it reached to 150℃, and then the temperature was further programmed to increase by 10℃/min until it reached a final temperature of 320℃, which was kept for 3 min. The MS readings were scanned in the range of 40~500 amu in full-scan mode with electron impact ionization energy of 70 eV. The temperatures of the ion source (EI) and injector port were set at 230 and 270℃, respectively. Compounds were identified by comparing the mass spectrum and Kovats retention index value of individual VOC with those listed in the MS Library Database and literature (Babushok et al., 2011), respectively.
Test concentrations of all insecticides (0.01~500 ppm) were prepared by diluting the stock solution in 50% sucrose solution. A plastic Kovax-Syringe (5 mL) with the open end narrowed to about 2 mm diameter was used as feeder unit (Fig. 1). An amount of 5 mL of prepared doses was taken into the feeder unit without any air bubbles. Collected worker bees (20 bees, each) were transferred into clear conical plastic cages with dimensions of 9 cm of bottom diameter×12 cm of top diameter×8 cm in height, with side ventilation slots, where they were kept starved for 2 hrs. Followed this, the worker bees were fed with different pesticides-contaminated diets (syrup adulterated with different pesticides at different concentrations) for 1 hr. Subsequently, the worker bees were fed with syrup supplemented with G-3KM (2.5%; v/v field recommendation) or 1,8-cineole (2.5%; v/v) for 48 hrs, separately, and this procedure was repeated three times for each treatment. The feeder unit containing syrup alone was considered as control. The number of dead bees were recorded 48 hrs after feeding with syrup supplemented with G-3KM detoxicant or 1,8-cineole. Worker bees were considered dead if they did not move after being touched with the fine-tipped brush.
3. Statistical analysis
All toxicity response assays were carried out in triplicate, and the results were presented as the mean mortality (%). A two-way analysis of variance (ANOVA) was conducted, followed by Tukey multiple-range tests at p<0.05 for mean separation, using Statistical Package for the Social Sciences (SPSS) version 16 (Chicago, SPSS Inc., 2007).
RESULTS
1. Characterization of volatile organic compounds extracted from G-3KM detoxicant
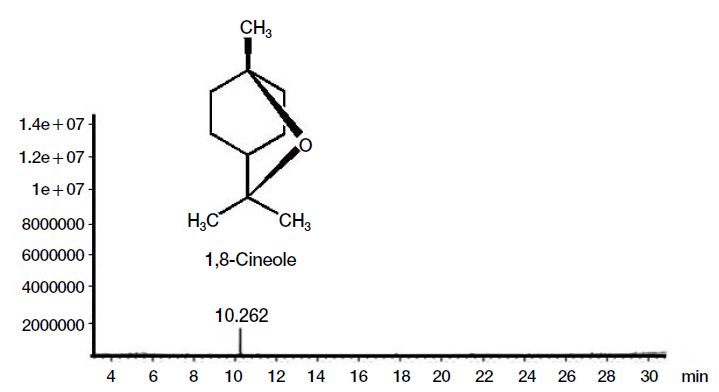
GC-MS analysis of the volatile constituents of G-3KM showed the presence of only one compound, accounting 100% of the total volatile constituents of G-3KM (Fig. 2). The compound was identified as 1,8-cineole. The identity was further confirmed by comparing the Kovats retention index values (RI of 1,8-cineole=1030 (experimental); 1032 (Babushok et al., 2011)). 1,8-cineole, also named eucalyptol, presents as a major constituents in many plant essential oils, such as eucalyptus oil (Dagne et al., 2000) and rosemary oil (Kovar et al., 1987).
2. Survival rate of worker bees intoxicated with seven pesticides after treatment with G-3KM
Honey bees activate detoxification mechanisms when they are exposed to toxic pesticides (Johnson et al., 2010). In this study, the mortality of worker bees supplemented with G-3KM detoxicant (2.5%; v/v) was reduced compared to control worker bees in all seven pesticides-intoxication (Figs. 3~5), although the effects of G-3KM detoxicant level on mortality did vary with concentrations and type of pesticides used.
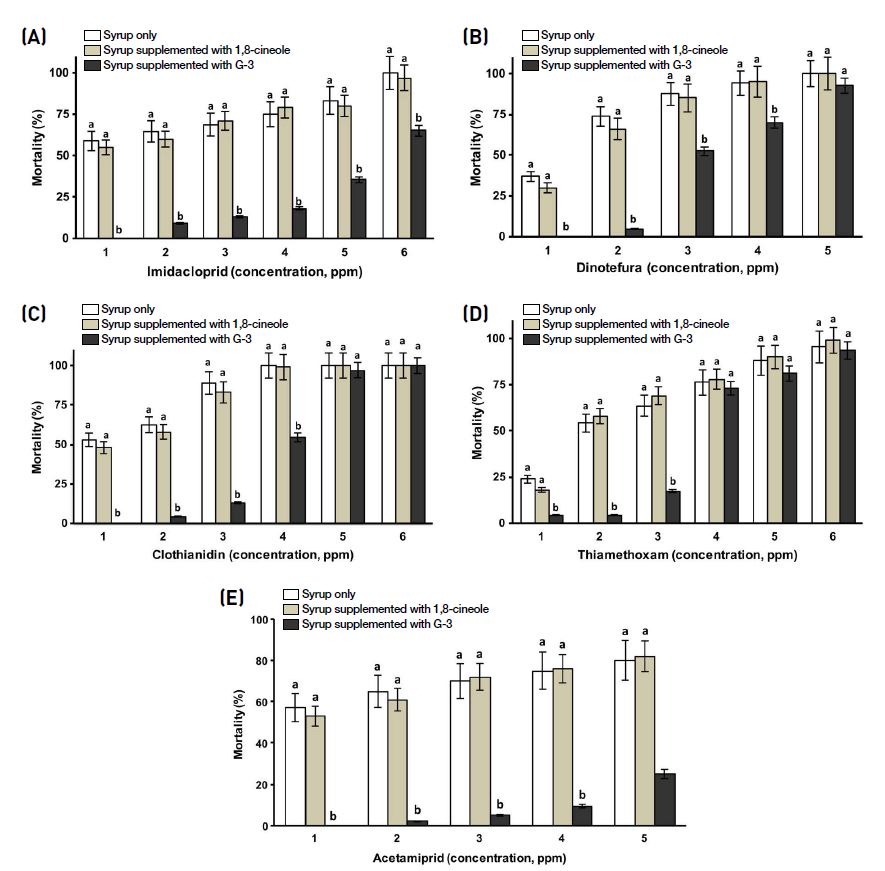
Effect of G-3KM detoxicant and 1,8-cineole on mortality of worker bees intoxicated with nitro-neonicotinoids, (A): Imidacloprid; (B): Dinotefuran; (C): Clothianidin; (D): Thiamethoxam and cyano-neonicotinoid, (E): Acetamiprid. Note: Same letters (a or b) refer to no significance difference (Two-way ANOVA; p>0.05) among the three tested samples (syrup only (control), syrup supplemented with 1,8-cineole and syrup supplemented with G-3KM detoxicant) for a single test concentration; Different letters (a or b) mean there is a significance difference among test samples (Two-way ANOVA; p<0.05) for a single test concentration.
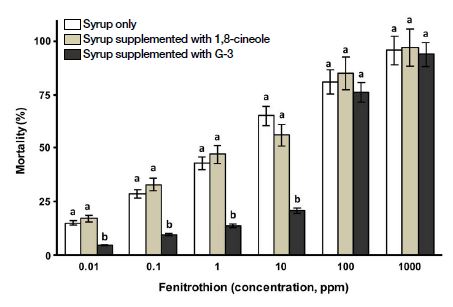
Effect of G-3KM detoxicant and 1,8-cineole on mortality of worker bees intoxicated with fenitrothion (organophosphate); Note: Same letters (a or b) refer to no significance difference (Two-way ANOVA; p>0.05) among the three tested samples (syrup only (control), syrup supplemented with 1,8-cineole and syrup supplemented with G-3KM detoxicant) for a single test concentration; Different letters (a or b) mean there is a significance difference among test samples (Two-way ANOVA; p<0.05) for a single test concentration.
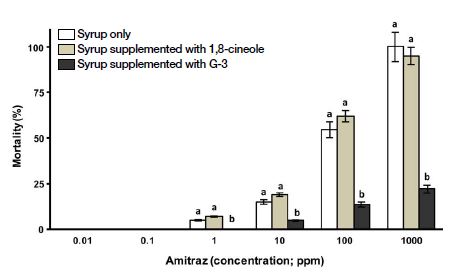
Effect of G-3KM detoxicant and 1,8-cineole on mortality of worker bees intoxicated with amitraz (acaricide). Note: Same letters (a or b) refer to no significance difference (Two-way ANOVA; p>0.05) among the three tested samples (syrup only (control), syrup supplemented with 1,8-cineole and syrup supplemented with G-3KM detoxicant) for a single test concentration; Different letters (a or b) mean there is a significance difference among test samples (Two-way ANOVA; p<0.05) for a single test concentration.
Pest insect control changed greatly with the discovery and development of the neonicotinoids. G-3KM detoxicant responses to worker bees intoxicated with acetamiprid (cyano-neonicotinoid) was found to be very effective. Compared to the control, there were significant reduction of mortality of worker bees intoxicated with acetamiprid across all tested concentrations when subjected to G-3KM detoxicant treatment (Fig. 3). Indeed, G-3KM reduced the percent mortality of worker bees by 69% when they were intoxicated with acetamiprid at the highest tested concentration (500 ppm). Likewise, on worker bees intoxicated with lower concentration of nitro-neonicotinoids (imidacloprid, dinotefuran, clothianidin, thiamethoxam), G-3KM detoxicant had a similar profile (Fig. 3). For instance, all worker bees were survived on G-3KM treatment on worker bees intoxicated with imidacloprid (0.01 ppm), although 58.8% worker bees were dead when subjected to feeding with only syrup (control). There was a statistically significant interaction on a two-way ANOVA analysis between the effects of treatment and imidacloprid concentration on percent mortality of worker bees intoxicated with imidacloprid (F(10,36)=3.658, p=0.002). While at higher concentration (500 ppm), there were no significant differences in worker bee mortality between the control and G-3KM treatment groups of dinotefuran, clothianidin and thiamethoxam (Fig. 3).
As shown in Fig. 4, worker bees fed with syrup intoxicated with fenitrothion (organophosphate) showed a similar profile as nitro-neonicotinoids. G-3KM treatments were found to be significantly effective (F(10,36)=4.764, p=0.001). When worker bees were exposed to lower concentration of fenitrothion. At higher concentrations of fenitrothion exposure (>100 ppm), there were no significant difference between G-3KM treatment and the control (p>0.05).
G-3KM treatments were most effective in reduction of mortality to worker bees intoxicated with amitraz across all concentrations (Fig. 5). Compared to the control, G-3KM treatments had significant mortality reduction in worker bees by 78% when worker bees were subjected to the highest test concentration of amitraz (1000 ppm).
3. Survival rate of worker bees intoxicated with seven pesticides on treatment with 1,8-cineole
There are a few phytoconstitutents known to detoxify honey bee from toxic pesticides (Balieira et al., 2018). In this study, 1,8-cineole was found as volatile constituent of G-3KM detoxicant. On treatment with 1,8-cineole, the survival effect of worker bees intoxicated with pesticides were evaluated at a single high oral dose (2.5%; v/v). In contrast to G-3KM, 1,8-cineole had no significant effect (p>0.05; for instance p=0.393; imidacloprid intoxicated worker bees) on reducing the mortality of worker bees intoxicated with diverse class of pesticides (Figs. 3~5).
DISCUSSION
Colony Collapse Disorder (CCD) is caused by multiple factors, but no particular factor has been singled out among the scientific community. Pesticides are one among the many causes. In this study, diverse groups of seven pesticides, including neonicotinoids, organophosphate, acaricide, were used to determine the effectiveness of G-3KM detoxicant. Neonicotinoids are economically important in crop protection as they are effective in the control of pests that have previously developed resistance to other classes of insecticides (Jeschke et al., 2011). In general, neonicotinoids comprise two sub groups, a nitro functional group (-NO2) and a cyano functional group (-C=N) as part of their structures. In the present findings, G-3KM treatments were found to be more effective on worker bees intoxicated with cyano-neonicotinoid (across all concentration) than nitro-neonicotinoids (only at lower concentrations). Although the nitro- and cyano-groups of neonicotinoids display similar binding affinity for honey bee nAChR (Nauen et al., 2001), their relative tolerance of bees toward the cyano-group is likely due to rapid cytochrome P450 detoxification (Iwasa et al., 2004). In addition, the nitro-neonicotinoids, including imidacloprid, clothianidin, thiamethoxam, and dinotefuran, are highly toxic to bees, with acute LD50s from 0.004 to 0.075 μg/bee (Iwasa et al., 2004; Cresswell, 2011). The cyano-neonicotinoids, thiacloprid and acetamiprid, are much less toxic, with contact LD50s in the range 7.1~14.6 μg/bee (Iwasa et al., 2004). Interestingly, G-3KM was less active in mortality reduction of honey bees intoxicated with higher concentration of nitro-neonicotinoids. This partly because of nitro-groups are more slowly metabolized than cyano-groups, for instance Imidacloprid (a nitro-group) has a half-life of 4 h (Suchail et al., 2004), possibly through P450 activity (Iwasa et al., 2004). However, the metabolites of cyano-groups, for instance acetamiprid, demonstrate greatly reduced toxicity to honey bees (Iwasa et al., 2004). G-3KM had no significance values in reduction of worker bees intoxicated with at higher concentration of thiamethoxam and clothianidin. This is relatively due to poor affinity of thiamethoxam for the insect nAChR (Tomizawa and Casida, 2005), but it is rapidly converted to clothianidin, which has great affinity (Tomizawa and Casida, 2005).
It is well noted that G-3KM treatments were effective when worker bees intoxicated at lower concentrations of fenitrothion (organophosphate insecticide). There were no G-3KM responses when worker bees were subjected to higher concentrations of fenitrothion. Organophosphates, including fenitrothion, are highly toxic as they irreversibly bind and inactivate AChE, causes permanent damage to the enzyme (Galloway and Handy, 2003). Indeed, honey bees are extremely sensitive to pesticides due to a deficiency in the number of genes encoding detoxification enzymes (Atkins, 1992; Claudianos et al., 2006). It is believed that bees have a relatively weak immune system as a result, and that this may be an evolutionary consequence of feeding on nectar and pollen, which are plant materials lower in toxin levels than foliage (CCD Steering Committee, 2010).
G-3KM has become less effective when worker bees intoxicated with at higher concentration of highly toxic pesticides, including organophosphates and nitro-neonicotinoids. Results of pairwise comparison across different concentrations showed there were dose-dependent responses as the effects of G-3KM weakened as the concentration of intoxication increases for each of the test experiments.
CONCLUSIONS
G-3KM detoxicant mixed in a syrup diet was found to significantly reduced mortality of worker bees intoxicated with diverse pesticides. However, the degree of G-3KM treatment did vary with concentrations and type of pesticides used. Overall, G-3KM treatments were generally more beneficial when it was administrated to worker bees intoxicated with at lower concentrations of all tested pesticides or across all level of concentrations of less toxic pesticides, like acetamiprid (cyano-neonicotinoids) and amitraz (acarides). Unfortunately, there were no significant reductions in worker bee mortality when intoxicated bees were treated with 1,8-cineole. Therefore, the active ingredient of G-3KM detoxicant could be polar substance(s) presented in polar aqueous fraction. Further work should be carried out on aqueous fraction to identify the active ingredient(s) of G-3KM detoxicant.
Acknowledgments
This research was funded by the BSRP through the National Research Foundation of Korea (NRF), Ministry of Education, grant number NRF-2018R1A6A1A03024862.
References
- Atkins, E. L. 1992. Injury to honey bee poisoning. In The Hive and the Honey Bee. (pp. 1153-1208). Illinois: Dadant and Sons Inc.
-
Babushok, V. I., P. J. Linstrom and I. G. Zenkevich. 2011. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data. 40: 1-47.
[https://doi.org/10.1063/1.3653552]

-
Badiou, A., M. Meled and L. P. Belzunces. 2008. Honeybee Apis mellifera acetylcholine esterase-A biomarker to detect deltamethrin exposure. Ecotoxicol. Environ. Safety. 69: 246-253.
[https://doi.org/10.1016/j.ecoenv.2006.11.020]

-
Balieira, K. V. B., M. Mazzo, P. F. V. Bizerra, A. R. de J. S Guimarães, D. Nicodemo and F. E. Mingatto. 2018. Imidacloprid-induced oxidative stress in honey bees and the antioxidant action of caffeine. Apidologie 49: 562-572.
[https://doi.org/10.1007/s13592-018-0583-1]

-
Boily, M., B. Sarrasin, C. DeBlois, P. Aras and M. Chagnon. 2013. Acetylcholinesterase in honey bees (Apis mellifera) exposed to neonicotinoids, atrazine and glyphosate: Laboratory and field experiments. Environ. Sci. Pollution Res. 20: 5603-5614.
[https://doi.org/10.1007/s11356-013-1568-2]

- CCD Steering Committee. 2010. Colony Collapse Disorder. Progress Report. The United States Department of Agriculture (USDA). pp. 1-43.
-
Claudianos, C., H. Ranson, R. M. Johnson, S. Biswas, M. A. Schuler, M. R. Barenbaum and J. G. Oakeshott. 2006. A deficit of detoxification enzymes: pesticide sensitivity and environmental response in the honeybee. Insect Mol. Biol. 15: 615- 636.
[https://doi.org/10.1111/j.1365-2583.2006.00672.x]

-
Couvillon, M. J., R. Schurch and F. L. W. Ratnieks. 2014. Dancing bees communicate a foraging preference for rural lands in high-level agri-environment schemes. Curr. Biol. 24: 1212-1215.
[https://doi.org/10.1016/j.cub.2014.03.072]

-
Cox-Foster, D. and D. van Engelsdorp. 2009. Saving the honeybee. Sci. Am. 300: 40-47.
[https://doi.org/10.1038/scientificamerican0409-40]

-
Cresswell, J. 2011. A meta-analysis of experiments testing the effects of a neonicotinoid insecticide (imidacloprid) on honey bees. Ecotoxicol. 20:149-157.
[https://doi.org/10.1007/s10646-010-0566-0]

-
Dagne, E., D. Bisrat, M. Alemayehu and T. Worku. 2000. Essential Oils of Twelve Eucalyptus species from Ethiopia, J. Essen. Oil Res. 12: 467-470.
[https://doi.org/10.1080/10412905.2000.9699567]

- European Food Safety Authority (EFSA), 2018. Neonicotinoids: risks to bees confirmed. February 28. https://www.efsa.europa.eu/en/press/news/180228, (2018).
-
Feyereisen, R. 2012. Insect CYP genes and P450 enzymes. Insect Molecular Biology and Biochemistry, ed Gilbert LI (Elsevier/Academic Press, Amsterdam). pp. 236-316.
[https://doi.org/10.1016/B978-0-12-384747-8.10008-X]

-
Galloway, T. and R. Handy. 2003. Immunotoxicity of organophosphorous pesticides. Ecotoxicol. 12: 345-363.
[https://doi.org/10.1023/A:1022579416322]

-
Ghosh, S. and C. Jung. 2017. A short review on neonicotinoids; Use in crop protection and issue on honey bee and hive. J. Apic. 32: 333-344.
[https://doi.org/10.17519/apiculture.2017.11.32.4.333]

- IRAC. 2020. IRAC mode of action classification scheme. Version 9.4, CropLife International. pp. 1-30.
-
Iwasa, T., N. Motoyama, J. Ambrose and R. M. Roe. 2004. Mechanism for the differential toxicity of neonicotinoid insecticides in the honey bee, Apis mellifera. Crop Prot. 23: 371-378.
[https://doi.org/10.1016/j.cropro.2003.08.018]

-
Jactela, H., F. Verheggenb, D. Thiéryc, A. J. Escobar-Gutiérrezd, E. Gachete, N. Desneuxf, and the Neonicotinoids Working Group. 2019. Alternatives to neonicotinoids. Environ. Int. 2019: 423-429.
[https://doi.org/10.1016/j.envint.2019.04.045]

-
Jeschke, P., R. Nauen, M. Schindler and A. Elbert. 2011. Overview of the status and global strategy for neonicotinoids. J. Agric. Food Chem. 59:2897-2908
[https://doi.org/10.1021/jf101303g]

-
Johnson, R. M., M. D. Ellis, C. A. Mullin and M. Frazier. 2010. Pesticides and honey bee toxicity - USA. Apidologie 41: 312-331.
[https://doi.org/10.1051/apido/2010018]

-
Johnson, R. M., W. Mao, H. S. Pollock, G. Niu, M. A. Schuler and M. R. Berenbaum. 2012. Ecologically appropriate xenobiotics induce cytochrome P450s in Apis mellifera. PLoS ONE 7: e3105.
[https://doi.org/10.1371/journal.pone.0031051]

-
Kang, M. and C. Jung. 2017. Avoidance behavior of honey bee, Apis mellifera from commonly used fungicides, acaricides and insecticides in apple orchards. J. Apic. 32: 295-302.
[https://doi.org/10.17519/apiculture.2017.11.32.4.295]

-
Kim, D. W., W. K. Yun and C. Jung. 2014. Residual toxicity of carbaryl and lime sulfur on the European honey bee, Apis mellifera (Hymenoptera: Apidae) and buff-tailed bumble bee, bombus terrestris (Hymenoptera: Apidae). J. Apic. 29: 341-348.
[https://doi.org/10.17519/apiculture.2014.11.29.4.341]

-
Klein, A. M., B. E. Vassiere, J. H. Cane, I. Stean-Dewenter, S. A. Cunningham, C. Kremen and T. Tscharntke. 2007. Importance of crop pollinators in changing landscapes for world crops. Proc. R. Soc. Lond. Ser. B Biol. Sci. 274: 303-313.
[https://doi.org/10.1098/rspb.2006.3721]

-
Kovar, K. A., B. Gropper, D. Friess and H. P. Ammon. 1987. Blood levels of 1,8-cineole and locomotor activity of mice after inhalation and oral administration of rosemary oil. Planta Med. 53: 315-318.
[https://doi.org/10.1055/s-2006-962725]

-
Lee, C. Y., S. M. Jeong, C. Jung and M. Burgett. 2016. Acute oral toxicity of neonicotinoid insecticides to four species of honey bee, Apis florea, A. cerena, and A. dorsata. J. Apic. 31: 51-58.
[https://doi.org/10.17519/apiculture.2016.04.31.1.51]

- Lushchak, V. I., T. M. Matviishyn, V. V. Husak, J. M. Storey and K. B. Storey. 2018. Pesticide toxicity: a mechanistic approach. EXCLI J. 17: 1101-1136.
-
Nauen, R., U. Ebbinghaus-Kintscher and R. Schmuck. 2001. Toxicity and nicotinic acetylcholine receptor interaction of imidacloprid and its metabolites in Apis mellifera (Hymenoptera: Apidae). Pest Manag. Sci. 57: 577-586.
[https://doi.org/10.1002/ps.331]

-
Nauen, R. and I. Denholm. 2005. Resistance of insect pests to neonicotinoid insecticides: current status and future prospects. Arch. Insect Biochem. Physiol. 58: 200-215.
[https://doi.org/10.1002/arch.20043]

-
Sánchez-Bayo, F. and K. Goka. 2014. Pesticide residues and bees - A risk assessment. PLoS ONE 9: e94482.
[https://doi.org/10.1371/journal.pone.0094482]

-
Suchail, S., G. De Sousa, R. Rahmani and L. P. Belzunces. 2004. In vivo distribution and metabolisation of 14C-imidacloprid in different compartments of Apis mellifera L. Pest Manag. Sci. 60: 1056-1062.
[https://doi.org/10.1002/ps.895]

-
Tomizawa, M. and J. E. Casida. 2005. Neonicotinoid insecticide toxicology: mechanisms of selective action. Annu. Rev. Pharmacol. Toxicol. 45: 247-268.
[https://doi.org/10.1146/annurev.pharmtox.45.120403.095930]

-
Ulziibayar, D. and C. Jung. 2019. Comparison of Acute Toxicity of Different Groups of Pesticides to Honey Bee Workers (Apis Mellifera L.). J. Apic. 34: 305-313.
[https://doi.org/10.17519/apiculture.2019.11.34.4.305]

- Walker, A. H., S. P. Hopkin, R. M. Sibly and D. B. Peakall, Principles of Ecotoxicology, Taylor and Francis, London, UK, 2nd edition, 2001.
-
Wood, T. J. and D. Goulson. 2017. The environmental risks of neonicotinoid pesticideinsecticides: a review of the evidence post 2013. Environ. Sci. Pollut. Res. 24: 17285-17325.
[https://doi.org/10.1007/s11356-017-9240-x]