Initial Results of Rearing Honey bee Apis ceranain vitro
An in vitro method of rearing worker European honey bee (Apis mellifera) was applied to Apis cerana larvae. Our study demonstrated successful Apis cerana production from larvae to adult in vitro. The onset of adult emergence started on day 17 from grafting. Worker bee emergence peak on day 18 and declined thereafter. The average survival rate from larvae to prepupae stage was 74.6%. The average survival rates from prepupae to adult stage and from larvae to adult stage were 40.7% and 30.4% respectively.
Keywords:
in vitro, Apis mellifera, Apis cerana, Emergence, Survival ratesINTRODUCTION
Honey bees play a vital role in pollination of crops and wild flora, and contribute to the conservation of biodiversity (Klein et al., 2007; Gallai et al., 2009; Corlett, 2011). Honey bees Apis cerana Farbricius 1793 is one of nine species of honey bees in the world (Sheppard and Smith, 2000). This honey bee species is also known by different name such as Asiatic honey bee or Oriental honey bee. A. cerana have well adapted to the climate and flowers in the local area of Asia, and has been domesticated for honey production for a long time in Asian countries (Chinh, 1996; Toan, 2012).
However, A. cerana is being declined and is threatened with extinction in its entire native habitat due to pest, disease, deforestation, pesticide positioning, and the replacement of exotic honey bee A. mellifera (Verma, 1993). Therefore the development of honey bee research tool to enhance our understanding of honey bee biology and to prevent honey bee population from declining is an urgent need. An important tool for this research is the rearing of honey bee larvae in vitro (i.e. in the laboratory and in the absence of nurse bees) because it allows more controllable conditions compared to in vivo (i.e. in the hive by nurse bees) (Crailsheime et al., 2013).
Initiation of in vitro culture of honey bee larvae began with A. mellifera. The first report of rearing honey bee larvae in the laboratory was published by Rhein (1933). He successfully used royal jelly to rear two to three-day-old worker larvae to adults. After that many attempts have been made to rear honey bee in vitro (Michael and Abromovitz, 1955; Weaver, 1955, 1974; Jay, 1963; Rembold et al., 1974; Rembold and Lackner, 1981; Shuel et al., 1978). Jay (1964) reared A. melliferain vitro by brood food obtaining from worker or queen cells in which 40~50% larvae reached adult stage. Woyke (1963, 1969) reared artificially diploid drone larvae in incubator for sex allel determination in honey bee A. mellifera and A. cerana. After successful larva rearing in the laboratory, studies on artificial infestation of honey bee disease was applied. Inoculate larvae in the laboratory with European foulbrood were conducted by Michael and Abramovitz (1955).
However, information of in vitro rearing of A. cerana is rare. In this paper, we report the partial results of the research conducted to rear A. cerana larvae using basic diets of 6% glucose, 6% fructose, 1% yeast and 50% royal jelly.
MATERIALS AND METHODS
The studies were carried out in the Honey bee Lab, Department of Agricultural Biology, NAAS. The standard methods for artificial rearing honey bee A. mellifera larvae (Huang, 2009; Crailsheim et al., 2013) were applied to rear A. cerana larvae.
Larval honey bee diet preparation
Royal jelly collected from A. mellifera colonies at the apiary of NAAS in 2013 stored at temperature about -20°C in a freezer was thawed by placing it at room temperature for 2 hours. 50 grams of royal jelly (50%) were weighed and put in the beaker (100ml). Distilled water was boiled for 5 minutes, then the water was cooled. A component including 6 grams of D-glucose (6%), 6 grams of Dfructose (6%), and 1 gram of yeast (1%) extract were weighed and put in the same beaker (50ml). When the water was cooled to about 45°C, 37ml of water was measured, and added to the beaker (50ml) containing sugars and yeast above. This mixture was dissolved by using a spatula to stir. The dissolved sugars and yeast extract was decanted into the beaker (100ml) containing royal jelly and mixing them thoroughly using a spatula. The larval diet is now ready for use. The beaker was labeled with date kept in refrigerator at 4°C to use only for 3 days.
Grafting larvae
Worker larvae were obtained from the honey bee A. cerana colonies collected in Cheon An province. These colonies were placed at the apiary of NAAS. A marked worker comb of empty cells was placed in a colony. On the fourth day, the bees were shaken off the marked comb and the comb was brought into the grafting room. Before grafting larvae, diet was pre-warmed (34°C, 15 minutes). Each well of 48-well cell culture plate was added 100μl of diet. First instar larvae (hatched within 24 hrs) were transferred gently into cells plates with one larva per well using the queen grafting tool. About every 10 larvae the grafting tool was sanitized with 70% ethyl alcohol to reduce cross contamination by pathogenic microorganisms. The larval rearing plates was transferred into a desiccator where the relative humidity was maintained at 95% (10% sulfuric acid solution in water, v/v). The desiccator was kept in a dark incubator maintained at 34°C. Each day, excessive diet was removed using a vacuum. Next, pre-warmed fresh diet was added to the larval rearing plate. The amount of food to add each day was showed in Table 1. Larvae will defecate when they are ready to pupate. Defecation is characterized by the presence of uric acid crystals and stringy material in the well. When a larva defecates, it is ready to be transferred to a pupation plate. Larval mortality is recorded each day.
Transfer of larvae to pupation plates
Pupating plates are 24-well plates matted with 2 layers of dust-free Kimwipes. The Kimwipes were previously rinsed with 70% ethyl alcohol and dried before being cut into pieces of 3×1.4cm. The mature larvae were dried on 2 layers of pre-cleaned Kimwipes and transferred to the pupation plates using a modified grafting tool (with the plunger removed). The pupation plates were kept in an incubator at 34°C and 75% relative humidity (maintained by a RH regulated incubator), until adult bees emerge. After 3 days the bees were inspected for survival to pupation plates as the pre-pupal stage is the most sensitive stage to movement and manipulations.
RESULTS AND DISCUSSION
The growth and developmental time from larvae to adult
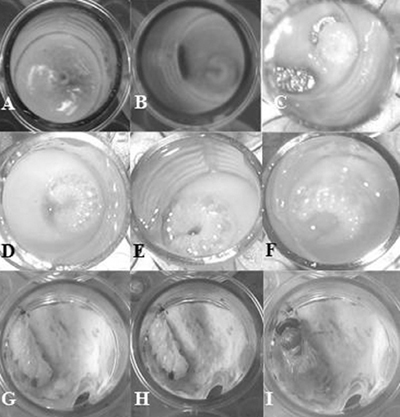
When larvae developed, their body gradually increased in size and often moved (Fig. 1). The surviving young larvae were characterized by the movement of larvae under a stereomicroscope for young larvae on day 3, and day 4 from grafting. From day 5, larval movement could be seen with the naked eye.
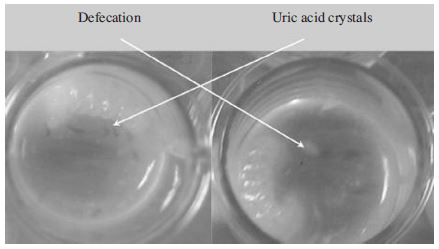
On the contrary dead larvae could be recognized by absence of movement, lack of turgidity and flattened body. Larvae defecated by day 6 or day 7 after grafting. In our experiment defecation were indicated by the presence of uric acid crystals and the appearance of light yellow feces (Fig. 2). When a larva defecates it is important to clean excessive diets and feces off the larvae and transferring to pupating plate.
A. mellifera larvae reared in vitro would defecate on day 8 or day 9 (Chan, 2012). Peng et al. (1992) showed that A. mellifera larval defecation began on the fifth day (9.5%), 30% did on sixth day, peaked on seventh day (55%) and declined thereafter. According to Peng et al. (1992) A. mellifera larvae that defecated on day 8 and day 9 probably did exhibit a delay growth and development.
The developmental time of larvae from grafting to emerging
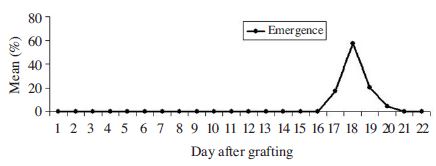
Fig. 3 demonstrates the emerge time of A. cerana worker bee reared in vitro. The observation on the emerge of 68 worker bees showed that 17.6% worker bees emerged on day 17 after grafting, worker bees emerged peak on day 18 (57.4%) and then declined.
In an A. cerana hive total of developmental time for worker caste is 19 days including 3 days egg stage; 5 days larvae stage and 11 days prepupae and pupae stage (Oldroyd and Wongsiri, 2006). in vitro condition our data demonstrated that the onset of adult emergence started on day 17 from grafting. In the A. mellifera hive average emergence time for the worker caste is 18 days after egg hatching (Winston, 1987). A. mellifera reared in vitro adult worker emerged after 17-18 days from grafting (Jay, 1963; Peng et al., 1992). This was rather similar to those in the hive. However, Chan (2012) reported that in vitro condition A. mellifera adult worker emerged after 21 days from grafting. According to Peng et al. (1992) there is much of the variation in developmental time in the hive and in vitro condition can be attributed to temperature, nutrition, and genetic differences.
Survival rates
In Table 2 showed the survival rates of A. cerana larvae reared in vitro for two trials with a total of 224 larvae grafted. The average survival rate from larvae to prepupae was 74.6%. While survival rates from prepupae to adults was 40.7%. Consequently survival rate from grafting larvae to adults was 30.4%.
This is considerably lower than the survival rates of larvae to adults on A. mellifera reported 50% up to 80% (Peng et al., 1992; Aupinel et al., 2005; Huang, 2009; Kaftanoglu et al., 2011; Chan, 2012). Our data demonstrated that most of them died in prepupae to adult stage. There are probably two main factors affecting the survival rates of larvae reared in vitro. Firstly, the quantity and quality of diets that larvae ingested was not enough to support normal growth. In our experiment royal jelly in the diet to feed A. cerana larvae were from A. mellifera colonies. The amount of diets was modified from Huang’s protocol of rearing A. mellifera larvae in vitro; 600μl of diets was fed to an A. cerana larva instead of 900μl of diets recommended for A. mellifera larva. Secondly, that is defecation of larvae as well as cleaning feces and excessive diets from larvae before transferring them to pupation plates. In the natural hive, worker larvae were barely fed with amount of diets by adult worker bee before the cells to be sealed (Chinh, 2012). In the cells larvae turned, defecated, and deposited feces at the bottom of the cells. While in vitro larvae remained in contact with the feces all over the body (Chan, 2012). Therefore, the process of cleaning feces and excessive diets may increase mortality. in vitro incomplete defecation of larvae might contribute to higher mortality, as defecation was an important way to excrete wastes and feces remaining inside larvae might be harmful for development (Peng et al., 2009; Chan, 2012).
In summary, A. cerana larvae can be reared in vitro at 34°C and 95% RH by using artificial diets including royal jelly, glucose, fructose, yeast extract and distilled water. However, in the present experiment the survival rate of grafted larvae to adult stage was still low (below 50%). This was probably due to diet quantity, royal jelly used in the experiment, and transferring methods of larvae to pupation plate. Therefore next experiments are needed to improve the survival rate and complete a standard method for rearing A. cerana in vitro that will be useful tools for further research on honey bee biology, pathology and the effect of pesticide to honey bee.
Acknowledgments
This research was supported by a grant from the Next- Generation BioGreen 21 Program (No. PJ00903103). Rural Development Administration. Republic of Korea. We would like to thank technicians in the honey bee laboratory for their kind helps to our research.
References
- Aupinel, P., D. Fortini, H. Dufour, J-N. Tasei, B. Michaud, J-F. Odoux, M-H. Pham-Delègue, (2005), Improvement of artificial feeding in a standard in vitro method for rearing Apis mellifera larvae, Bulletin of Insectology, 58, p107-111.
- Chan, M.Y.M., (2012), Development and application of honey bee in vitro systems, M.Sc. Biochemistry and molecular biology thesis, Uni. of British Columbia, Vancouver.
- Chinh, P.H., (2012), Raising domestic bee Apis cerana in Viet Nam, Raising domestic bee Apis cerana in Viet Nam.
- Chinh, P.H., (1996), A number of research and technical solutions to improve productivity and qualities native honeybee Apis cerana in northern Viet Nam. Doctoral thesis Agricultural Sciences, Hanoi Agricultural University.
- Corlett, R.T., (2011), Honeybees in natural ecosystems, honeybee of Asia, Springer - Verlag Berlin Heiderberg, p215-25.
-
Crailsheim, K., R. Brodschneider, P. Aupinel, D. Behrens, E. Genersch, J. Vollmann, and U. Riessberger-Gallé, (2013), Standard methods for artificial rearing of Apis mellifera larvae, in Standard methods for Apis mellifera research, eds. by V. Dietemann, J.D. Ellis, P. Neumann), COLOSS BEEBOOK, I.
[https://doi.org/10.3896/IBRA.1.52.1.05]

-
Gallai, N., J-M. Salles, J. Settele, and B.E. Vaissiere, (2009), Economic valuation of the vulnerability of world agriculture with pollinator decline, Ecological Economics, 68, p810-821.
[https://doi.org/10.1016/j.ecolecon.2008.06.014]

- Huang, Z., (2009), A standardized procedure for the in vitro rearing of honey bee larvae ABSTC report, (https://www.google.com search), [2014/06/12].
- Jay, S.C., (1964), Rearing honeybee brood outside the hive, J. Apic. Res, 3, p51-60.
- Jay, S.C., (1963), The development of honeybee in their cells, J. Apic. Res, 2, p117-134.
- Kaftanoglu, O., T.A. Linksvayer, R.E. Page, (2011), Rearing honey bees, Apis mellifera, in vitro 1: Effects of sugar concentrations on survival and development, Journal of Insect Science, 11, p1-10.
-
Klein, A.M., B.E. Vaissière, J.H. Cane, I. Steffan-Dewenter, S.A. Cunningham, C. Kremen, T. Tscharntke, (2007), Importance of pollinators in changing landscapes for world crops, Proc. R. Soc. Lond. B Biol, 274, p303-313.
[https://doi.org/10.1098/rspb.2006.3721]

- Michael, A.S., M. Abramovitz, (1955), A new method of rearing honey bee larvae in vitro, Journal of Economic Entomology, 48, p43-44.
- Oldroyd, B.P., and S. Wongsiri, (2006), Asian honey bees. Biology, conservation and human interactions, Harvard University Press, Cambridge, MA, USA, p340.
- Peng, Y.S.C., E. Mussen, A. Fong, M.A. Montague, and T. Tyler, (1992), Effects of chlortetracycline of honey bee worker larvae reared in vitro, Journal of Invertebrate Pathology, 60, p127-133.
- Rhein, W.V., (1933), Uber die Entstehung des weiblichen Dimorphismus im Bienenstaate, Wilhelm Roux Archiv für Entwicklungsmechanik der Organismen, 129, p601-665.
-
Rembold, H., B. Lackner, I. Geistbeck, (1974), The chemical basis of honeybee, Apis mellifera, caste formation, Partial purification of queen bee determinator from royal jelly. Journal of Insect Physiology, 20, p307-314.
[https://doi.org/10.1016/0022-1910(74)90063-8]

- Rembold, H., and B. Lackner, (1981), Rearing of honeybee larvae in vitro: Effect of yeast extract on queen differentiation, J. Apic. Res, 20, p165-171.
-
Sheppard, W.S., and D.R. Smith, (2000), Identification of African - derived bees in the Americas: A survey of methods, Annals of Entomological Society of America, 93, p159-176.
[https://doi.org/10.1603/0013-8746(2000)093[0159:IOADBI]2.0.CO;2]

- Shuel, R.W., S.E. Dixon, G.B. Kinoshita, (1978), Growth and development of honey bees in the laboratory on altered queen and worker diets, J. Apic. Res, 17, p57-68.
- Toan, T.V., (2012), Study on some biological and ecological characteristics of the hybrid between Dong Van indigenous honeybee breed (Apis cerana cerana Fabricius) with the local indigenous honeybee breeds (Apis cerana indica Fabricius) in some provinces in the north of Viet Nam. Ph.D. thesis Plant protection, Hanoi University of Agriculture.
- Verma, L.R., (1993), Current state Himalayan honey bee (Apis cerana) biodiversity and strategies for its conservation, 428. in Himalayan biodiversity: conservation strategies ed. U. Dhar. Gyanodaya Prakashan, Nainital, India, p415-428.
- Weaver, N., (1995), Rearing of honey bee larvae on royal jelly in the laboratory, Science, 121, p509-510.
- Weaver, N., (1974), Control of dimorphism in the female honey bee. 2, Methods of rearing honeybee in the laboratory and of preserving royal jelly. J. Apic. Res, 13, p3-14.
- Winston, M.L., (1987), The biology of the honey bee, Harvard Uni. Press, Cambridge, MA.
- Woyke, J., (1963), Rearing and viability of diploid drone larvae, J. Apic. Res, 2, p77-84.
- Woyke, J., (1969), A method of rearing diploid drone in a honeybee colony, J. Apic. Res, 8, p65-74.