The Mechanism of Anti-inflammation Effects of Propolis Components in Raw264.7 Macrophage Cell
Abstract
The study investigated the anti-inflammatory effects of propolis components on Raw264.7 macrophage cells. There were nine representative propolis components selected such as pinocembrin, quercetin, chrysin, naringin, gallic acid, p-coumaric acid, cinnamic acid, caffeic acid and CAPE. The cell cytotoxicity evaluation of diluted propolis components was determined by EZ-cytox cell viability assay. The anti-inflammation factors of propolis components were determined by confirming NO levels. All propolis components were able to decrease the generation of NO by LPS stimulation as inflammation inducer. However, quercetin, chrysin and CAPE components were significantly decreased the generation of NO and have synergistic effects for inflammation response. The expression of iNOS as inflammatory protein, IL-1β as representative cytokine and NF-κB as immune signaling molecule was evaluated using Western blotting. Quercetin, chrysin and CAPE involving inflammation response reduction play different role protein in expression in the cells. The results showed that propolis components have anti-inflammatory effects on Raw264.7 and each component may be related to immune-regulation. Thus, each propolis component may be used as a source of health-functional food materials for anti-inflammation response.
Keywords:
Propolis, Inflammation, Raw264.7, Quercetin, Chrysin, CAPEINTRODUCTION
The inflammatory response is one of the body̓s defense mechanisms against external stimuli as it is caused by bacteria and viruses penetrating into the body (Willoughby, 1975; Ismaki and Punnonen, 1997). As the core of the defense mechanism are various immune cells existing in the body. Proliferation and differentiation of immune cells by stimulation play a pivotal role in the defense system. Immune cells of human body are divided into three groups such as T lymphocytes, B lymphocytes and macrophages. Among these, macrophages are distributed in tissues and they are responsible for detecting and expelling aging cells outwards in normal conditions (Zhang et al., 2007; Hamsa and Kuttan, 2011). However, in response to the influx of inflammatory agents from the outside, macrophages allow the overexpression of inflammation mediators resulting to carcinogenesis of the cells to occur (McDaniel et al., 1996; Nishida et al., 2007; Cheon et al., 2009). When an inflammatory response occurs, the expression and secretion of various cytokine molecules are activated in macrophages, and promotes the release of the inflammatory mediators such as nitric oxide (NO) or prostaglandin E2 (PGE2) (Nathan, 1992; Lee et al., 2004; Kim et al., 2009). These molecules generate inflammatory reactions accompanied by pain, heat and immune cells that were activated for responses (Ialenti et al., 1992). Among the inflammatory mediators, NO is generated from L-arginine by an inducible NO synthase (iNOS) when a macrophage is activated as a highly reactive substance. The generated NO may act as a toxin to bacteria and cancer cells penetrating into the body, but overexpressed NO may cause inflammation, tissue damage, and genetic mutation (Geller et al., 1993; Sunyer et al., 1996; Bogdan, 2001). In the induction of inflammatory reactions, external toxins such as smoking causes cell stress (Ryu et al., 2003). The inflammatory response acts as a result of an increase oxidative stress and intake of antioxidant is known to reduce oxidative stress (Uttara et al., 2009).
Propolis is a resinous material that honey bee produce by mixing saliva and beeswax with exudate gathered from a variety of trees and plants to build and protect their hive (Burdock, 1998). Although propolis contains many components such as flavonoids, caffeic acid and beeswax, it has already been used by early civilization to cure wounds since around 300 B.C. Propolis contains various physiologically active substances that has been studied for its antimicrobials (Sforcin, 2000; Schnitzler et al., 2010), antioxidants (Jeong, 2004), anti-inflammatory (Khayyal et al., 1993; Ledon et al., 1997) and anti-tumor activities (Bazo et al., 2002; Orsolic et al., 2005; Kunimasa et al., 2010). Although the effects of propolis have been reported in various reactions, the associated molecular mechanism is still not sufficient. In particular, only the research of antimicrobial and antioxidant activities of propolis has been reported. These activities are functions of many flavonoids and phenyl compounds constituting propolis. However, the specific mechanism of each component of propolis in the immune-regulatory response has not been reported. In this study, nine representative components of propolis were selected, and then confirmed for cytotoxicity of mouse macrophage Raw264.7. Based on the cytotoxicity concentration of propolis components in Raw264.7 cells, inflammation response by lipopolysaccharide (LPS) was activated. To investigate the function of propolis components, the generation of NO inhibition was determined by the expression of protein related to iNOS and interleukin-1β (IL-1β) for inflammation regulation protein and representative cytokine, respectively. As a result, propolis may be suggested as the health-functional food for immunomodulation.
MATERIALS AND METHODS
1. Reagents
A flavonoid component and a phenyl compound are selected as nine propolis components were used in the experiment. The selected components are the most closely related components to the propolis functions. Nine selected components were as follows: Pinocembrin, quercetin, chrysin, naringin, gallic acid, p-coumaric acid, cinnamic acid, caffeic acid and caffeic acid penethyl ester (CAPE). The nine propolis components were purchased from Sigma Aldrich (U.S.A.).
2. Cell culture
The raw264.7 mouse macrophages were purchased from the Korea Cell Line Bank (KCLB) and maintained with high glucose Dulbecco’s modified eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) and 100 units/mL penicillin-streptomycin as antibiotics. DMEM, FBS and penicillin-streptomycin solution for subculture of Raw264.7 macrophages were purchased from Gibco (U.S.A.). FBS was used after inactivation in 56℃ for 50 min. Subculture of the cells are aided with the use of cell scraper without a trypsin cell detachment solution and maintained in an incubator set at 37℃ and 5% CO2.
3. Cytotoxicity of Raw264.7 for propolis components
To investigate how each propolis components affect the cell survival, cytotoxicity assay was carried out. Cytotoxicity assay used were EZ-Cytox MTT assay kit was purchased from Dainbio (Korea). Raw264.7 macrophage was inoculated into 96-well plate on 2×104 cells/well and incubated for 24 hr. Components of propolis were dissolved to absolute EtOH at 1 μg/μL. Each propolis component was filtered by 0.2 μm syringe filter for treatment. Raw264.7 macrophage was treated with each propolis component on dose-dependent (1, 2.5, 5, 10, 20, 25 and 50 μg/mL) exposure for 24 hr. As a control in this experiment, we compared only media treated cells. As a solvent control, only EtOH treatment was compared. After 24 hr exposure, EZ-Cytox solution was added to media at 1/10 volume and the cell was incubated for another 2 hr. The optical density of the media was measured at a wavelength of 450 nm.
4. Measurement of NO production
Raw264.7 macrophage inoculated into 24-well plate was 2×105 cells / well incubated for 24 hr. LPS used as positive reagent for inflammation reaction was purchased from Sigma Aldrich. The components of propolis were treated into the cells was 25 μg/mL for 24 hr except CAPE. Treated CAPE concentration is at 1 μg/mL. To compare the NO production, only the media treated cells and EtOH treated cells was considered as a negative control. LPS was treated into the cells at 1 μg/mL was made as the positive control. Cell supernatants were subjected to centrifugation at 3,000 rpm for 10 min after 24 hr. The supernatants without cell debris were collected to be used for NO assay. Generated NO was measured with NO assay reagents kit supplied by iNtRON (Korea). NO assay was carried out following the manufacture’s protocol. The optical density of produced NO was determined at a wavelength of 560 nm.
5. Measurement of produced NO by component of propolis in inflammation progression
To investigate the effect of propolis components in LPS-mediated inflammation progression three groups of experimental set up for NO generation was used. First, pre-incubation of LPS for 24 hr on Raw264.7 cells and then added with the components of propolis for 24 hr. Second, pre-incubation of the components of propolis for 24 hr on Raw264.7 cells and then added with LPS for 24 hr. Lastly, LPS and the components of propolis simultaneously treated on Raw264.7 cells for 48 hr. The experiment procedure was progressed in the same protocol as described above. However, three propolis components such as quercetin, chrysin and CAPE were selected based on the result of the NO assay. Treated LPS concentration was 1 μg/mL, and quercetin and chrysin were treated on three dose-dependent levels (1, 5 and 25 μg/mL). The CAPE concentration used was 0.01, 0.1 and 1 μg/mL.
6. Western blot
The proteins for Western blotting were extracted from Raw264.7 cells. Cells were washed twice with 1× phosphate-buffered saline (PBS) and lysed with 200 μL of nonidet P (NP)-40 lysis buffer (Dainbio). The lysate supernatants were cleared by centrifugation for 15 min at 13,000 rpm. The concentrations of cleared lysates were determined by bicinchronic acid (BCA) assay. Cell lysated at 20 μg were used in Western blotting. The lysates were added with 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene fluoride (PVDF) membrane. Non-specific binding was blocked by incubation of membranes in 2% skim milk at 1X Tris-buffered Tween 20 (1× TBST) for 1 hr at room temperature. The membranes were probed with primary antibodies such as anti-iNOS, anti-IL-1β, anti-NF-κB and anti-GAPDH antibodies. iNOS and IL-1β antibody were purchased from Cell Signaling (U.S.A.) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and glyceraldehyde 3-phosphate dehydrogtenase (GAPDH) antibody were purchased from Santacruz biotechnology (U.S.A.). Except for anti-GAPDH, all antibodies were incubated for 16 hr. Anti-GAPDH were incubated for only 2 hr. Protein expression were detected by incubation with secondary horseradish peroxide (HRP)-conjugated anti-goat antibody and was visualized with an enhanced chemiluminescence (ECL) pico detection system.
7. Statistical analysis
The statistical significance of the results obtained from the experiment was used by the R (3.4.1 version, NewZealnd) statistics program, and the mean significance of the experiment was verified by the ANOVA test at p<0.05.
RESULTS AND DISCUSSION
1. Propolis components influenced Raw264.7 cell viability
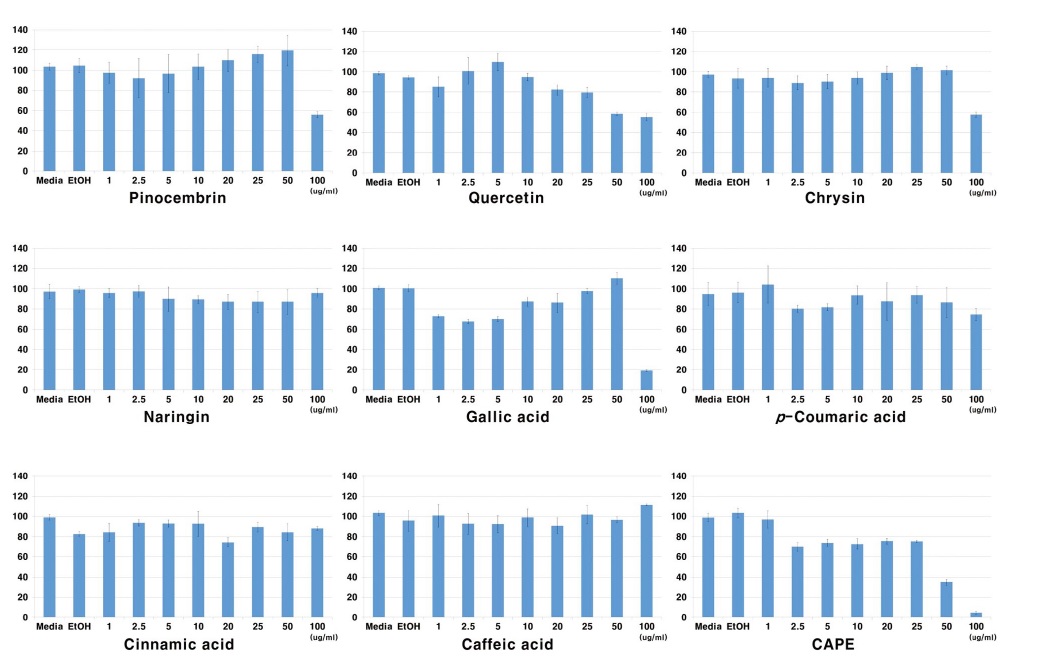
To investigate the cell viability of propolis components, nine propolis components were treated as described previously to Raw264.7 macrophage. The cells were incubated with each propolis component at different dose-dependent levels (0, 1, 2.5, 5, 10, 20, 50 and 100 μg/mL) for 24 hr. The components: naringin, p-coumaric acid, cinnamic acid and caffeic acid have not affected cell viability. In this result, four propolis components did not show cytotoxicity. However, pinocembrin, quercetin, chrysin and gallic acid decreased the cell viability in dosage of more than 50 μg/mL. As shown in Fig. 1, CAPE showed the most potent cytotoxicity effect of more than 2.5 μg/mL dosage. The component can be grouped into compounds that have cytotoxicity and no cytotoxicity effects. Based on the results, the concentration of propolis components for immune-regulation can be determined. The concentration of another component for immune-regulation was set up at 25 μg/mL maximum except CAPE which was determined at 1 μg/mL.
2. Quercetin, chrysin and CAPE play essential role in reduction of generated NO by inflammation reaction
NO molecule as representative marker in inflammation mediator is produced from L-arginine by nitric oxide synthase (NOS). L-arginine is converted to L-citrulline and NO, and iNOS are known as key molecule in this process. When cells are stimulated by external factor such as inflammation factor, bacteria and virus, it was expressed as iNOS. Generated NO molecule through this reaction were severely promoting inflammation responses such as cytotoxicity and tissue injury. To confirm the propolis components related to NO production, LPS was treated as inflammation inducing factor and each propolis component was prepared at 1 μg/mL for 24 hr. As a control, LPS treatment group was not only set up but also each propolis component treatment group. EtOH treatment group is a solvent control for propolis diluent.
As shown in Fig. 2A, inflammation response significantly increased in LPS-treated group, but not in propolis component-treated group. However, LPS and propolis components-treated group has different NO generation. Although all propolis components have decreased NO production, the component such as quercetin, chrysin and CAPE has significantly reduced NO production by more than half. The results indicate that the specific component of propolis has directly participated in NO reduction. It has been confirmed that inhibition of three components specially quercetin, chrysin and CAPE, whether the dose-dependent have been the most effective in NO reduction. Among the most effective concentrations for quercetin and chyrsin are 1, 5 and 25 μg/mL, and for CAPE is 0.01, 0.1 and 1 μg/mL. As shown in Fig. 2B, three components of propolis reduced the generated NO by LPS depending on dose-dependent levels. The results indicate that three components of propolis play essential role in reducing NO generation by inflammation response.
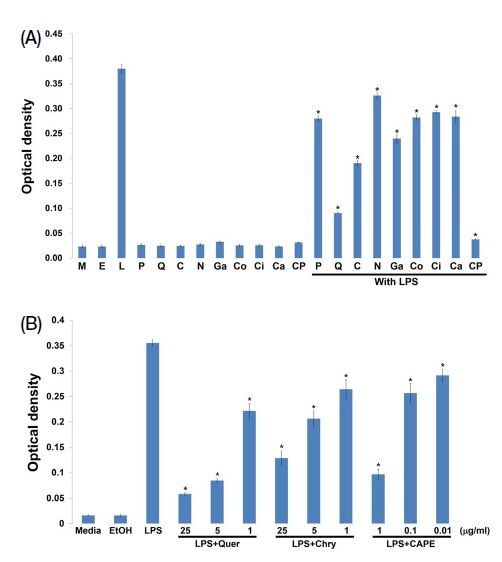
Propolis component, quercetin, chrysin and CAPE inhibit NO generation. Raw264.7 macrophage cells placed into 24-well plates on a 2×105 cells/well. All propolis components treatments was at 25 μg/mL except CAPE (M: Media, E: EtOH, L: LPS, P: Pinocembrin, Q: Quercetin, C: Chrysin, N: Naringin, Ga: Gallic acid, Co: p-coumaric acid, Ci: Cinnamic acid, Ca: Caffeic acid, CP: CAPE) (A). Three components of propolis as quercetin, chrysin and CAPE was measured generated NO on dose-dependent (B). The supernatants were cleared with centrifugation at 3,000 rpm for 10 min. Generated NO was measured following manufacture’s protocol (Quer: Quercetin, Chry: Chrysin).
3. Synergistic effects of propolis components
The synergistic effects of quercetin, chrysin and CAPE in generated NO reduction by LPS-mediated inflammation response was investigated. The concentration of each propolis component was determined at 1 μg/mL and combined the components as follows. (1) Quercetin and chrysin; (2) Quercetin and CAPE; (3) Chrysin and CAPE; (4) Quercetin, chrysin and CAPE combination. Media and EtOH treatment is the control group for LPS and propolis components. LPS-treated group is a positive control for inflammation response.
As shown in Fig. 3, generated NO reduction is better in the combined components than single component treatment only. Especially, when quercetin and chrysin are combined reduced NO generation is about 50% compared with each quercetin and chrysin only. CAPE has shown the most reduction of generated NO. The results mean that CAPE plays essential role in reduction of NO production related to inflammation, while the combination of quercetin and chrysin provides synergistic effects on inflammation reduction.
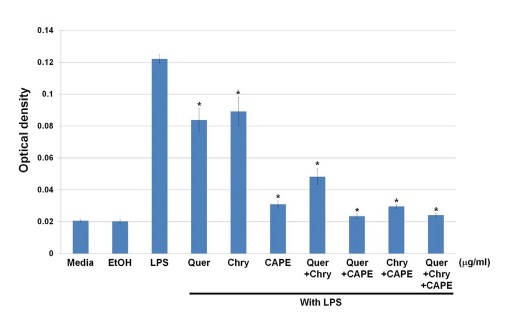
The synergistic effects on generated NO inhibition. Raw264.7 macrophage cells placed into 24-well plates on a 2×105 cells/well. The treatment of quercetin and chrysin were 25 μg/mL, and CAPE treatment was 1 μg/mL. LPS and each component combined mixture were incubated for 24 hr, and generated NO was measured with NO assay kit (Quer: Quercetin, Chry: Chrysin).
4. The effects of propolis components in prevention of inflammation
For inflammation prevention, three experimental groups are determined whether each propolis component exhibits therapeutic or prevention effects. The first group is induced inflammation response by LPS treatment for 24 hr, and was treated with the components of propolis. The second group has pre-treated propolis components for 24 hr, and was LPS-treated for 24 hr. The final group, propolis components and LPS were simultaneously used as treatment. The generated NO was measured on each experimental group. The media only was used as the negative control, EtOH treatment as the solvent control and LPS treatment group as the positive control. Propolis components that were selected are quercetin, chrysin and CAPE which had the most effect on the inhibition of NO production. All components concentration is set at 1 μg/mL.
As shown in Fig. 4A, when the component of propolis is pre-treated and LPS is treated, generated NO was decreased. However, when LPS is pre-treated then propolis component is treated, generated NO did not decrease. In simultaneously treatment group, CAPE has shown the most reduction of NO. Quercetin and chrysin have the same results as the previously held experiment. In other words, three components are related in terms of inflammation response reduction, with CAPE as the most efficacy on inflammation. Three components of propolis have the better effect when they are pre-treated to cells. Therefore, all three components are expected to have more prevention effects than therapeutic effects on inflammation.
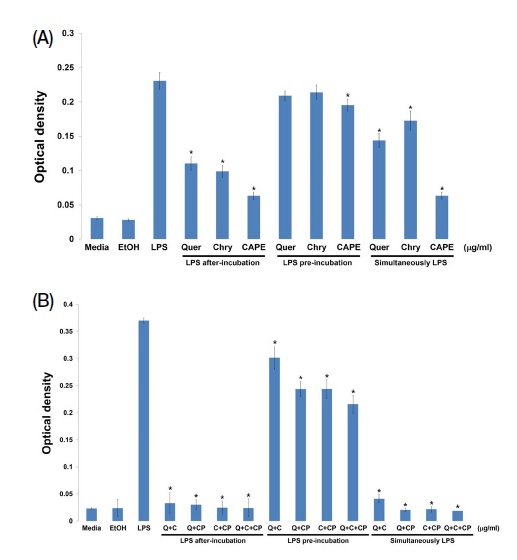
Propolis components prevention effects on inflammation response. Raw264.7 cells were placed into a 24-well plate on a 2×105 cells/well. The quercetin and chrysin treatments were set at 25 μg/mL while CAPE treatment was at 1 μg/mL for 24 hr. The treatment of LPS was 1 μg/mL for 24 hr (Quer: Quercetin, Chry: Chrysin) (A). Each propolis component mixture and LPS were treated for 24 hr. Each reagent concentration is same with previously conducted experiment (Q: Quercetin, C: Chrysin; CP: CAPE) (B).
Although three components of propolis showed NO reduction, confirmation of synergistic effects between three components were determined. As shown in Fig. 4B, the combination of the components showed more NO reduction effects than single component treatment. The results suggest that three components which showed NO reduction improved its effect by combination. Therefore, propolis is effective in inflammation prevention, especially quercetin, chrysin and CAPE in relation to NO reduction.
5. Propolis components regulated inflammation-related protein expression in Raw264.7
In relation to NO inhibition caused by each component of propolis we carried out Western blotting whether each component regulate expression of protein in the cells. The regulation of expression of protein cell was determined using iNOS which involved NO production process, IL-1β which representative cytokine and NF-κB expression in inflammation signaling cascade.
As shown in Fig. 5A, the single treatment of quercetin, chrysin and CAPE did not have related protein expression in the cells. The protein expression level, however, was present when LPS and propolis components was treated into the cells as shown in Fig. 5B. LPS-mediated iNOS protein level was decreased by crude propolis treatment. Quercetin directly regulated iNOS expression. On the other hand, chrysin and CAPE did not affect to iNOS expression. In the combined components, iNOS expression decreased when quercetin was combined, while chrysin and CAPE mixing did not show inhibition of iNOS expression.
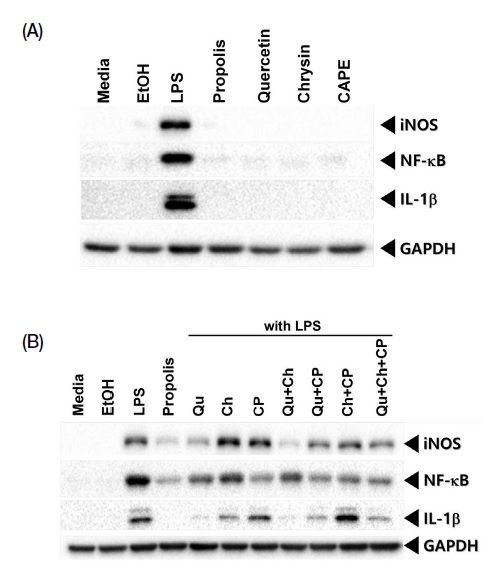
Propolis components influenced protein expression level in the cells. All lysates were used at 20 μg for Western blotting. Each antibody diluted at 1 : 1,000 in 2% skim milk incubated overnight except for GAPDH. GAPDH was made to be housekeeping control for Western blotting and its dilution is 1 : 5,000 (Qu: Quercetin, Ch: Chrysin, CP: CAPE).
In the NF-κB protein expression, which is important in cell immune signaling, CAPE treatment group has the lowest expression level. The component quercetin and chrysin have shown similar protein expression. For IL-1β which is known representative marker in inflammation response has shown the most reduction of protein expression in quercetin treatment group. The chrysin treatment group also showed reduced protein expression. CAPE, on the other hand, does not reduce the IL-1β protein expression level. The results of the combined components showed an inhibition in IL-1β expression only when quercetin was mixed, but chrysin and CAPE had no significant effect on IL-1β expression. GAPDH is a loading control in this protein expression experiment.
CONCLUSION
Propolis has been known to have an excellent effect as antioxidant and antibiotics. However, the study of propolis on immunomodulation is still not sufficient. The anti-inflammation effects of crude propolis and its protein expression regulation mechanism on LPS-mediated inflammation response was identified (Kim et al., 2018). Therefore, the molecular mechanisms for immune response by components of propolis are necessary to be studied.
Propolis has different activity and function following region and climate. Propolis functions depending on the composition of functional materials such as flavonoids and phenol compounds which has antioxidant, antibiotics and anticancer activity. Based on these facts, this study clarified the function of propolis compounds against inflammation response and its molecular mechanism. We selected nine representative propolis compounds and confirmed that quercetin, chrysin and CAPE are effective on LPS-mediated inflammation response. Among the three propolis components, CAPE played a direct role in NO reduction. In addition, mixing of propolis components has synergistic effect in relation to inflammation response. However, these three components have different roles in molecular regulation. All three components influence inhibition of NF-κB expression which serves as immune regulation factor. Although CAPE has the most inhibition effect of NO production, quercetin has directly inhibits iNOS expression. IL-1β expression was influenced by quercetin. These results mean that propolis was composed many components, and appears that it has a completely different function in immunomodulation. This study has shown not only the effect of inflammation inhibition but also provided important clues how each propolis component functions. Immune regulation as another component function must be clarified after this study. Based on this study, the developing health-functional foods containing propolis with specific effects can be suggested.
Acknowledgments
This study was carried out with the support of RDA grant (PJ016277).
References
-
Bazo, A. P., M. A. Rodrigues, S. M. Sforcin, J. L. de Camargo, L. R. Ribeiro and D. M. Salvadori. 2002. Protective action of propolis on the rat colon carcinogenesis. Teratog. Carcinog. Mutagen. 22: 183-194.
[https://doi.org/10.1002/tcm.10011]

-
Bogdan, C. 2001. Nitric oxide and the immune response. Nat. Immunol. 2: 2907-2916.
[https://doi.org/10.1038/ni1001-907]

-
Burdock, G. A. 1998. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem. Toxicol. 36: 347-363.
[https://doi.org/10.1016/S0278-6915(97)00145-2]

-
Cheon, Y. P., L. M. Mohammad, C. H. Park, J. H. Hong, G. D. Lee, J. C. Song and K. S. Kim. 2009. Bulnesia sarmienti aqueous extract inhibits inflammation in LPS-stimulated Raw264.7 cells. J. Life Sci. 19: 479-485.
[https://doi.org/10.5352/JLS.2009.19.4.479]

-
Geller, D. A., A. K. Nussler, M. D. Silvio, C. J. Lowenstein and R. A. Shapiro. 1993. Cytokines, endotoxin, and glucocorticoids regulate the expression of inducible nitric oxide synthase in hepatocytes. Proc. Natl. Acad. Sci. USA 90: 522-526.
[https://doi.org/10.1073/pnas.90.2.522]

-
Hamsa, T. P. and G. Kuttan. 2011. Evaluation of the anti-inflammatory and anti-tumor effect of Ipomoea obscura (L) and its mode of action through the inhibition of proinflammatory cytokines, nitric oxide and COX-2. Inflammation 34: 171-183.
[https://doi.org/10.1007/s10753-010-9221-4]

-
Ialenti, A., A. Ianaro, S. Moncada and M. Di. Rosa. 1992. Modulation of acute inflammation by endogenous nitric oxide. Eur. J. Pharmacol. 211: 177-182.
[https://doi.org/10.1016/0014-2999(92)90526-A]

-
Ismaki, P. and J. Punnonen. 1997. Pro- and anti-inflammatory cytokines in rheumatoid arthritis. Ann. Med. 29: 449-507.
[https://doi.org/10.3109/07853899709007474]

-
Jeong, I. Y. 2004. Antioxidant activity and radioprotection of two flavonoids from propolis. J. Med. Food. 34: 162-166.
[https://doi.org/10.3746/jkfn.2005.34.2.162]

- Khayyal, M. T., M. A. el-Ghazaly and A. S. el-Khatib. 1993. Mechanisms involved in the anti-inflammatory effect of propolis extract. Drugs Exp. Clin. Res. 19: 197-203.
- Kim, D. H., S. J. Park, J. Y. Jung, S. C. Kim and S. H. Byun. 2009. Anti-inflammatory effects of the aqueous extract of Hwangnyenhaedok-tang in LPS-activated macrophage cells. Kor. J. Herbol. 24: 39-47.
-
Kim, S. K., S. O. Woo, S. M. Han, S. G. Kim, K. W. Bang, H. R. Jang, H. J. Moon and H. J. Kim. 2018. Anti-inflammatory effects of Korean propolis extracts on Raw264.7 macrophage cells. J. Apic. 33: 187-194.
[https://doi.org/10.17519/apiculture.2018.09.33.3.187]

-
Kunimasa, K., M. R. Ahn, T. Kobayashi, R. Eguchi, S. Kumazawa, Y. Fujimori, T. Nakano, T. Nakayama, K. Kaji and T. Ohta. 2010. Brazilian Propolis suppresses angiogenesis by inducing apoptosis in tube-forming endothelial cCells through inactivation of survival signal ERK1/2. Evid. Based Complement Alternat. Med.
[https://doi.org/10.1093/ecam/nep024]

- Ledon, N., A. Casaco, R. Gonzalez, N. Merino, A. Gonzalez and Z. Tolon. 1997. Antipsoriatic, anti-inflammatory, and analgesic effects of an extract of red propolis. Acta Pharmacol. Sin. 18: 274-276.
-
Lee, E. S., H. K. Ju, T. C. Moon, E. K. Lee, Y. D. Jahng, S. H. Lee, K. S. Son, S. H. Baek and H. W. Chang. 2004. Inhibition of nitric oxide and tumor necrosis factor-alpha (TNF-alpha) production by propenone compound through blockade of nuclear factor (NF)-kappa B activation in cultured murine macrophages. Biol. Pharm. Bull. 27: 617-620.
[https://doi.org/10.1248/bpb.27.617]

-
McDaniel, M. L., G. Kwon, J. R. Hill, C. A. Marshall and J. A. Corbett. 1996. Cytokines and nitric oxide in islet inflammation and diabetes. Proc. Soc. Exp. Biol. Med. 211: 24-32.
[https://doi.org/10.3181/00379727-211-43950D]

-
Nathan, C. 1992. Nitric oxide as a secretary product of mammalian cells. FASEB J. 6: 3051-3064.
[https://doi.org/10.1096/fasebj.6.12.1381691]

-
Nishida, T., Y. Yabe, H. Y. Fu, Y. Hayashi, K. Asahi, H. Eguchi, S. Tsuji, M. Tsujii, N. Hayashi and S. Kawano. 2007. Geranylgeranylacetone induces cyclooxygenase-2 expression in cultured rat gastric epithelial cells through NF-kappaB. Dig. Dis. Sci. 52: 1890-1896.
[https://doi.org/10.1007/s10620-006-9661-8]

-
Orsolic, N., I. Kosalec and I. Basic. 2005. Synergistic antitumor effect of polyphenolic components of water soluble derivative of propolis against Ehrlich ascites tumour. Biol. Pharm. Bull. 28: 694-700.
[https://doi.org/10.1248/bpb.28.694]

-
Ryu, J. H., H. Ahn, J. Y. Kim and Y. K. Kim. 2003. Inhibitory activity of plant extracts on nitric oxide synthesis in LPS-activated macrophages. Phytother. Res. 17: 485-489.
[https://doi.org/10.1002/ptr.1180]

-
Schnitzler, P., A. Neuner, S. Nolkemper, C. Zundel, H. N. Karl, H. Sensch and J. Reichling. 2010. Antiviral activity and mode of action of propolis extracts and selected compounds. Phytother. Res. 24: S20-S28.
[https://doi.org/10.1002/ptr.2868]

-
Sforcin, J. M. 2000. Seasonal effect on Brazilian propolis anti-bacterial activity. J. Ethnopharmacol. 73: 243-249.
[https://doi.org/10.1016/S0378-8741(00)00320-2]

-
Sunyer, T., L. Rothe, X. Jiang, P. Osdoby and P. Collin-Osdoby. 1996. Proinflammatory agents, IL-8 and IL-10, upregulate inducible nitric oxide synthase expression and nitric oxide production in avian osteoclast-like cells. J. Cell. Biochem. 60: 469-483.
[https://doi.org/10.1002/(SICI)1097-4644(19960315)60:4<469::AID-JCB4>3.0.CO;2-Q]

-
Uttara, B., A. V. Singh, P. Zamboni and R. T. Mahajan. 2009. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 7: 65-74.
[https://doi.org/10.2174/157015909787602823]

-
Willoughby, D. A. 1975. Human arthritis applied to animal models. Towards a better therapy. Ann. Rheum. Dis. 34: 471-478.
[https://doi.org/10.1136/ard.34.6.471]

- Zhang, L., H. Zhu, Y. Lun, D. Yan, L. Yu, B. Du and X. Zhu. 2007. Proteomic analysis of macrophages: a potential way to identify novel proteins associated with activation of macrophages for tumor cell killing. Cell Mol. Immuno. 4: 359-367.