New Record of the Black-based Humming-bird Hawkmoth, Macroglossum passalus (Lepidoptera: Sphingidae) from Korea
Abstract
The black-based hummingbird hawkmoth, Macroglossum passalus Drury, 1773 is newly recorded from Korea. M. passalus can be characterized by the blackish transverse antemedial band on the forewing with the yellowish medial band on the hindwing. The female genitalia of M. passalus can be characterized by the tubular ostium burse, the long ductus bursae with multiple parallel stripes and posteriorly curved, and the ovate ductus bursae with a long triangular patch of minute signa. To date, seven species of the genus Macroglossum have been recorded in Korea.
Keywords:
Pollinator, Hawkmoth, Flower visit, KoreaINTRODUCTION
The hawkmoths including humming-bird hawkmoths are usually active during daytime, exhibit powerful flight maneuvers such as hovering during nectar feeding, and play an important role in the pollination of numerous plants. Hawkmoths are attracted to the wavelengths of 440 nm, which are preferred by other flower-visiting insects such as bufferflies (Scherer and Kolb, 1987a, 1987b; Kelber, 1997) and honeybees (Giurfa et al., 1995). The interaction between pollinators and plants is mediated by the concentration and traits of the common sugars found in nectars of flowers (Baker and Baker, 1983): some nectarivorous birds prefer sucrose and fructose to glucose (Lotz and Nicolson, 1996), bees and many hummingbirds prefer sucrose to glucose and glucose to fructose (Wykes, 1952; Stiles, 1976), butterflies and hummingbird hawkmoths prefer sucrose to fructose and fructose to glucose (Erhardt, 1991, 1992; Kelber, 2003).
The monophyly of the Sphingidae was defined based on morphology and five protein-coding nuclear genes (Kawahara et al., 2009). The genus Macroglossum, “humming-bird hawkmoth,” was erected by Scopoli (1777) with the type species Sphinx stellatarum Linnaeus. It comprises more than 120 species worldwide (Kitching and Cadiou, 2000). The members of the genus are diagnosed by the narrow, grayish to dark gray or black forewings with transverse, often sinuous fasciae, the blackish hindwings with a broad yellow or orange medial to subbasal band, the broad thorax, and the broad abdomen that has lateral and subventral yellow or white patches and the distal fan of flattened scales (Holloway, 1987).
To date, six species of Macroglossum have been known in Korea (NIBR, 2019; Choi et al., 2020): M. bombylans (Boisduval, 1875), M. corythus Walker, 1856, M. heliophila (Boisduval, 1875), M. pyrrhostictum (Butler, 1875), M. saga (Butler, 1878), and M. stellatarum (Linnaeus, 1758). A female specimen of M. passulus Drury, 1773 was recently collected from the southwestern island, Heuksan-do, Shinan-gun, Jeonnam. Therefore, it is reported that one of hummingbird hawkmoths, M. passalus was found for the first time in Korea.
MATERIALS AND METHODS
An adult humming-bird hawkmoth was collected during the night using a UV light and mounted for examination. For slide preparation of female genitalia, each specimen was prepared by boiling the abdomen in 10% KOH for approximately 20 min. Scales and tissues were removed, stained with Chlorazol black, and mounted on slides in Euparal solution. For wingspan measurements, the distance from the tip of the left forewing to the tip of the right forewing was used.
Genomic DNA was extracted from moth legs using the DNeasy Blood and Tissue Extraction Kit (Qiagen, UK) according to the manufacturer’s instructions. We targeted the mitochondrial protein-coding gene (cytochrome oxidase subunit I gene, COI). The procedure from amplification to DNA sequence comparison followed Choi et al. (2021).
Terminology of adults, including the female genitalia, refers to Holloway (1987). All material was deposited in the Collection of Insects of the Department of Environmental Education, Mokpo National University. Abbreviations are as follows: JN, Jeollanam-do, TL, type locality.
RESULTS AND DISCUSSION
- Order Lepidoptera Linnaeus, 1758
- Family Sphingidae Latreille, 1802
- Genus Macroglossum Scopoli, 1777
Macroglossum passalus (Drury, 1773) (Figs. 1, 2) 흑산벌꼬리박각시 (신칭)
- Sphinx passalus Drury, 1773, Illust. Nat. Hist. Exot. Insects 2: 52, t. 29. TL: Ryukyu Island, Formosa, China.
- Sphinx pandora Fabricius, 1793, Ent. Syst. 3(1): 380.
- Rhamphoschisma rectifascia R. Felder, 1874, Reise Novara, Lep.: 75, f. 7. TL: South India, Ceylon.
- Macroglossum rhebus Moore, 1858, Cat. Lep. Ins. Mus. East India Coy 1: 263. TL: Java, N. India, S. India (Canara).
- Macroglossa sturnus Boisduval, 1875, Spec. Gen. Lep. Het. 1.: 349.
Material examined. 1 female, Korea: JN: Shinan, Heuksan-do, 15 Sep 2022, Oh HY.
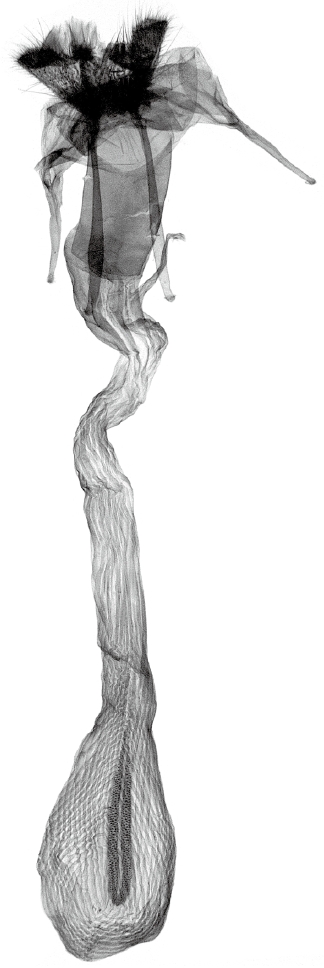
Diagnosis. Wingspan 54 mm. This large sphingid species can be distinguished by the dark brown basal part with a blackish transverse antemedial band, the light brown fascia that is followed by a medially projected blackish postmedial line of the forewing and a broad yellowish medial band of the hindwing (Fig. 1). The female genitalia of M. passalus can be distinguished by the simple tubular ostium bursae, long tubular ductus bursae, and ovate corpus bursae with a long triangular pouch-shaped patch of minute signa (Fig. 2).
DNA barcoding. We sequenced the COI (Genbank Accession number OG978941) and the sequence of the Korean specimen resulted in 100% similarity with the available BIN (BOLD:AAB1316) that was based on four specimens of M. passalus from Japan, Laos, and Vietnam (average distance 0.12% p-distance) (Ratnasingham and Hebert, 2007).
Distribution. Korea, Japan, China (southeast), Taiwan, Philippines, Indonesia, Thailand, India, and Sri Lanka.
Remarks. Macroglossum passalus is externally similar to M. pyrrhostictum but can be distinguished from the latter by the blackish basal part with a blackish transverse antemedial band on the forewing and the even-width of yellow medial band on the hindwing.
Macroglossum passalus feeds on Daphniphyllum calycinum Benth. (Daphniphyllaceae) (Hong Kong), Photinia glabra (Thunb.) Franch. and Sav. (Rosaceae) (Japan), and P. lindleyana Wight and Arn. (India) (Pittaway and Kitching, 2018).
Acknowledgments
The study was supported by a grant from the National Institute of Biological Resources (NIBR), funded by the Ministry of Environment (MOE) of the Republic of Korea (NIBR202304203, NIBR202333201).
CONTRIBUTION OF AUTHORS
Collection, HYO; investigation, resources, BS, YJC, KNG, SSK, SWC; writing, and editing, SWC; visualization, SSK; supervision, SWC; project administration, funding acquisition, SWC.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the correspondent author. The genetic data are publicly available.
References
- Baker, H. G. and I. Baker. 1983. Floral nectar sugar constituents in relation to pollinator type. pp. 117-141. in Handbook of experimental pollination biology, eds. by Jones, C.E. and R.L. Little. Scientific and Academic Editions, New York.
- Boisduval, J. B. 1875. Histoire Naturelle des Insectes. Spécies Général des Lépidoptères Hétérocères. Tome Premier. Sphingides, Sésiides, Castnides Hist. Nat. Ins., Spec. gén. Lépid. Hétérocères 1: 1-568, pl. 1-11.
- Choi, S. W., B. Shin, S. Ahn and S. S. Kim. 2021. Agonopterix issikii (Lepidoptera: Depressariidae), New to Korea. Anim. Syst. Evol. Divers. 37: 322-329.
- Choi, S. W., S. S. Kim and J.A. Jeon. 2020. First Record of Sphingid Moth, Macroglossum corythus (Lepidoptera: Sphingidae) from Korea. Anim. Syst. Evol. Divers. 36(1): 78-80.
- Drury, D. 1773. Illustrations of natural history; wherein are exhibited. Illust. Nat. Hist. Exot. Insects 2: 1-90, pl. 1-50.
-
Erhardt, A. 1991. Nectar sugar and amino acid preferences of Battus philenor (Lepidoptera, Papilioniae). Ecol. Entomol. 16: 425-434.
[https://doi.org/10.1111/j.1365-2311.1991.tb00235.x]

-
Erhardt, A. 1992. Preferences and non-preferences for nectar constituents in Ornithoptera priamus poseidon (Lepidoptera, Papilionidae). Oecologia 90: 581-585.
[https://doi.org/10.1007/BF01875453]

- Fabricius, J. C. 1793. Entomologia systematica emendata et aucta. 3(1): 380.
- Felder, C., R. Felder and A. F. Rogenhofer. 1874. Lepidoptera. Heterocera. pp. 537-549, pls. 75-120. in Reise der österreichischen Fregatte Novara um die Erde in den Jahren 1857, 1858, 1859 unter den Behilfen des Commodore B. von Wüllerstorf-Urbair. Zoologischer Theil. Band 2. Abtheilung.
-
Giurfa, M., J. Nunez, L. Chittka and R. Menzel. 1995. Colour preferences of flower-naive honeybees. J. Comp. Physiol. A 177: 247-259.
[https://doi.org/10.1007/BF00192415]

- Holloway, J. D. 1987. The moths of Borneo, part 3. Superfamily Bombycoidea: families Lasiocampidae, Eupterotidae, Bombycidae, Brahmaeidae, Saturniidae, Sphingidae. Malayan Nature Society, Kuala Lumpur, pp. 1-199.
-
Kawahara, A. Y., A. A. Mignault, J. C. Regier, I. J. Kitching and C. Mitter. 2009. Phylogeny and biogeography of hawkmoths (Lepidoptera: Sphingidae): evidence from five nuclear genes. PLoS One 4(5): e5719.
[https://doi.org/10.1371/journal.pone.0005719]

-
Kelber, A. 1997. Innate preferences for flower features in the hawkmoth Macroglossum stellatarum. J. Exp. Biol. 200(4): 827-836.
[https://doi.org/10.1242/jeb.200.4.827]

-
Kelber, A. 2003. Sugar preferences and feeding strategies in the hawkmoth Macroglossum stellatarum. J. Comp. Physiol. A 189: 661-666.
[https://doi.org/10.1007/s00359-003-0440-0]

-
Kitching, I. J. and J. M. Cadiou. 2000. Hawkmoths of the world: an annotated and illustrated revisionary checklist (Lepidoptera: Sphingidae) (Vol. 93, No. 5, pp. 1195-1196). Oxford University Press.
[https://doi.org/10.1093/aesa/93.5.1195g]

-
Lotz, C. N. and S. W. Nicolson. 1996. Sugar preferences of a nectarivorous passerine bird, the lesser double-collared sunbird (Nectarinia chalybea). Funct. Ecol. 10: 360-365.
[https://doi.org/10.2307/2390284]

- Moore, F. 1858. A Catalogue of the Lepidopterous Insects in the Museum of the Hon. East-India Company in Horsfield and Moore. Cat. lep. Ins. Mus. East India Coy 1: 1-278, pl. 1-12, 1a, 2a, 3a, 4a, 5a, 6a.
- National Institute of Biological Resources (NIBR). 2019. National species list of Korea, III. Insects. National Institute of Biological Resources, Incheon, pp. 1-986.
- Pittaway, A. R. and I. J. Kitching. 2018. Macroglossum passalus (Drury, 1773). Sphingidae of the Eastern Palaearctic [Internet]. http://tpittaway.tripod.com/china/china.htm, . Accessed 31 Mar 2023.
-
Ratnasingham, S. and P. D. N. Hebert. 2007. BOLD: The Barcode of Life Data System (www.barcodinglife.org, ). Mol. Ecol. Notes 7: 355-364.
[https://doi.org/10.1111/j.1471-8286.2007.01678.x]

-
Scherer, C. and G. Kolb. 1987a. Behavioral experiments on the visual processing of color stimuli in Pieris brassicae L. (Lepidoptera). J. Comp. Physiol. A 160: 645-656.
[https://doi.org/10.1007/BF00611937]

-
Scherer, C. and G. Kolb. 1987b. The influence of color stimuli on visually controlled behavior in Aglais urticae L. and Pararge aegeria L. (Lepidoptera). J. Comp. Physiol. A 161: 891-898.
[https://doi.org/10.1007/BF00610230]

- Scopoli, G. A. 1777. Introductio ad historiam naturalem, sistens genera lepidum, Plantarum et animalium. Gerle, Prague, p. 414.
-
Stiles, F. G. 1976. Taste preferences, color preferences, and flower choice in hummingbirds. Condor 78: 10-26.
[https://doi.org/10.2307/1366912]

-
Wykes, G. R. 1952. The preferences of honeybees for solutions of various sugars which occur in nectar. J. Exp. Biol. 29: 511-519.
[https://doi.org/10.1242/jeb.29.4.511]