Honey Bee Viruses: An Ongoing History of Discovery
Abstract
Numerous threats to honey bees‘ health exist, including climate change, pesticides, diseases, and pests, and their combined actions pose greater risks than a single factor. Viruses have been investigated for roughly 100 years since the first bee virus detection, Sacbrood virus from the United States in 1913. So far, seven viruses have been routinely included in honey bee health monitoring and surveillance systems in Korea, but new viruses have lately been found. However, understanding of viruses as a threat to honey bees remains insufficient. Although some of the threats to honey bee health posed by viruses and other factors have been identified, research is still in its initial step. In this paper, we have reviewed the diversity of honey bee viruses, virulence, mode of transmission, and potential molecular biological analysis approaches.
Keywords:
Honey bee viruses, Virome analysis, Apis mellifera, Varroa destructor, Nosema, High throughput sequencingINTRODUCTION
Honey bees (Apis mellifera) are indispensable to the global agricultural ecosystem since their pollination affects the yield of numerous crops (Gallai et al., 2009). Moreover, honey bees produce honey, pollen, royal jelly, wax, and other products that significantly contribute to the agricultural economy (Popovska Stojanov et al., 2021; Zacepins et al., 2021). Winter mortality, which is higher than summer mortality, is one of the key challenges (Steinhauer et al., 2014). In summer, honey bee activity is so high that the exchange of various pathogens among honey bees occurs outside, increasing the load of pathogens (Runckel et al., 2011), but the nutrients that honey bees can take are abundant, which does not appreciably affect the health of honey bees (Di Pasquale et al., 2013). In winter, nutrients are limited, and the Varroa mite, a vector of pathogens, increases (Traynor et al., 2016). As a result of the lengthy lifespan and the dense structure (winter cluster) inside the hive, and pathogens continuously infect the honey bees in winter that cause more harmful than in summer (Jung and Bae, 2022).
The physiological ecology of honey bees can be divided into summer honey bees, which have a short lifespan of 15 to 45 days, and winter honey bees, which have a lifespan of approximately 150 days or more (Doeke et al., 2015). Honey bees are known to prepare for the winter season around the autumnal equinox (Jung and Bae, 2022). The strength of a honey bee colony influences the ability of honey bee to survive during overwintering (Jung and Bae, 2022). In Korea, winter honey bees are born beginning mid-October, and if they fail to develop into adults during this period, they overwinter with weakened colonies or continue to make winter honey bees until December (Jung and Bae, 2022). Throughout the winter, honey bee colonies remain overcrowded to form winter clusters for temperature management; as a result, winter honey bees have more antibiotic activity than summer honey bees, but they are more vulnerable to infectious diseases (Jung and Bae, 2022). Winter clusters begin to form when the external temperature drops to 10 to 14℃ for more than three days (Stabentheiner et al., 2010). The inside of a winter cluster must be at least 13℃, and typically needs to be maintained between 20 to 25℃ for honey bees to be able to carry out colony maintenance activities (Southwick and Mugaas, 1971). According to Steinhauer et al. (2014), honey bee mortality in overwintering colonies in the US, was caused by weak forces, a lack of food, unproductive queen honey bees, honey bee mites, and pesticides. Between 2011 and 2013, the average overwintering success rate in Korea was 82.6% (Jeong et al., 2016). Honey bee mites had the greatest influence on the mortality of winter colonies (Genersch et al., 2010; Guzman-Novoa et al., 2016). Winter honey bees infected with honey bee mites are reported to have a 20% higher overwinter mortality than healthy hives (Jung and Bae, 2022). If mite infection becomes severe during the winter honey bee breeding season, the risk of overwinter mortality increases due to losses of fat and vitellogenin, which are substances that enhance the resistance to cold in honey bees (Fries et al., 1994; Amdam et al., 2004). Viruses such as Deformed wing virus (DWV) and Israeli acute paralysis virus (IAPV) are closely associated with colony mortality, and the damage caused by these viruses can be exacerbated by honey bee mite infestations, leading to higher mortality rates in honey bee colonies (Gisder et al., 2009; Chen et al., 2014).
Worker honey bees are reduced by 10 to 20% in the average overwintering colony. In Korea, a drop of more than 30% is considered abnormal mass loss phenomenon (Kim, 2022). Colony collapse disorder (CCD), which first occurred in Pennsylvania in the US in 2006 (Cox-Foster et al., 2007; vanEngelsdorp et al., 2007), is a phenomenon similar to abnormal winter mortality in Korea. CCD is a phenomenon in which worker honey bees disappear with little evidence of mass mortality near the hive, leaving only the queen and some young honey bees in the colony despite having sufficient food in the colony (Johnson, 2010). In this review, we describe the viruses that have been reported thus far, as well as synergy between viruses and other factors and virome analysis.
HONEY BEE VIRUSES
Viruses are classified as DNA or RNA viruses depending on the nucleic acid genome, and can be single and double-stranded depending on the structure of the genome (Villarreal, 2004). In addition, RNA viruses are divided into positive-sense and negative-sense (Payne, 2017). Positive-sense viral RNA genomes can be translated in the same manner as mRNA, so they can be expressed immediately after the viral genome enters the cell, but negative-sense RNA viruses can only be expressed in cells after a transcription process to positive-sense RNA virus occurs (Nguyen and Haenni, 2003).
Viruses are highly mutable, but RNA viruses have a higher mutation rate than DNA viruses (Sanjuán et al., 2010). Positive-sense single-stranded RNA viruses are the most common viruses identified in honey bees (Brutscher et al., 2016). Recently, many DNA viruses have been discovered in honey bees (Kraberger et al., 2019). However, little is known about the impacts of DNA viruses other than Apis mellifera filamentous virus (Gauthier et al., 2015).
Thus far, 128 viruses found in honey bees have been reported in the NCBI GenBank database (Table 1). Viruses are mainly found in worker honey bees but are sometimes found in drones, queen honey bees, bumblebees, wasps, and honey bee mites. The pathways, symptoms, and risks of most viruses have not yet been uncovered. Acute bee paralysis virus (ABPV), Black queen cell virus (BQCV), Chronic bee paralysis virus (CBPV), DWV, IAPV, Kashmir bee virus (KBV), Sacbrood virus (SBV), Apis rhabdovirus (ARV) group, and Lake Sinai virus (LSV) group have been the focus of global research.
MAJOR TAXA OF HONEY BEE VIRUSES
The viruses discovered in honey bees reported to NCBI are divided into nine orders, 11 families, and six genera, with several unclassified viruses (Table 1). The major honey bee viruses are mainly found in Nodamuvirales and Picornavirales.
Nodamuvirales was named after the Nodamura virus found in Noda-mura, Tokyo, Japan (Scherer and Hurlbut, 1967). It includes two families, and the virion form has a non-enveloped icosahedral symmetric structure with a size of 25-33 nm and a genome of 4.5-6 kb. In the case of the family Nodaviridae, it consists of two molecules of positive-sense single-stranded RNA1 and RNA2 (Thiéry et al., 2022). However, viruses in the family Sinhaliviridae have one positive-sense single-stranded RNA genome (Runckel et al., 2011). Among the two families, the viruses found in honey bees belong to the family Sinhaliviridae. Sinhaliviridae was named after two phylogenetically similar virus groups, the virus found in the sweat bee (Halictus scabiosae, Adlikon virus (Bigot et al., 2017)) and the LSV found in honey bees.
Picornavirus is a combination of the words “pico”, which means “small” in Spanish, and RNA virus. Several phylogenetically similar “picornavirus-like” viruses have been identified since the first identification of Picornavirus in 1963 and the identification of the entire genome sequence in 1980, and the family Picornaviridae are used to bind them (Le Gall et al., 2008). It has now been reported in ICTV as the order Picornavirales, which is a higher group (Sanfaçon et al., 2022). Picornaviruses are classified into five families, among which the bee viruses are found in family Dicistroviridae and Iflaviridae.
The Dicistroviridae family has a positive-sense single-stranded RNA genome of 8-10 kb, and the virion type has an icosahedral structure with a size of 30 nm without an envelope (Valles et al., 2017). It also has a characteristic dicistronic genome, from which it was named, which includes the genera Aparavirus, Triatovirus, and Cripavirus. Aparavirus is derived from the Acute bee paralysis virus, Triatovirus is derived from the Triatoma virus, and Cripavirus is derived from the Cricket paralysis virus (ICTV, 2022). Major honey bee viruses belonging to this order include ABPV, KBV, and IAPV belonging to the genus Aparavirus, and BQCV belonging to the Triatovirus, and several viruses belonging to the genus Cripavirus have also been found (Table 1).
Iflaviridae has an RNA genome of 9-11 kb, and the virion form is 22-30 nm in size, without an envelope, and has an icosahedral symmetrical structure. Derived from the Infectious flacherie virus, both family and genus were named, and one genus was included (Chen et al., 2022). The major honey bee viruses are DWV, SBV, and Varroa destructor virus (Table 1).
CHARACTERISTICS OF HONEY BEE VIRUSES
1. Acute bee paralysis virus (ABPV)
ABPV is a positive-sense single-stranded virus belonging to the family Dicistroviridae and genus Aparavirus. ABPV, along with CBPV, was found in A. mellifera adult insects (Bailey et al., 1963). Honey bees infected with ABPV show paralysis within 2 to 4 days and die within 1 day (Bailey et al., 1963). The complete viral genome sequence for ABPV was first detected in A. mellifera in the United Kingdom (Govan et al., 2000). ABPV was found in A. mellifera, A. cerana, honey bee mites (MZ821785, and KY451691), Bombus sp. (MW196265), Vespa sp. (MN565031), and other insects (ON304226, ON304239, and MW442703). In Korea, the first ABPV was detected in 2009 (Yoo and Yoon, 2009b). Ultra-rapid PCR and nested ultra-rapid PCR methods for ABPV detection have been reported (Kim et al., 2014b; Lee et al., 2016; Kim et al., 2017; Kim et al., 2018). However, ABPV genome sequence has not yet been reported in Korea.
2. Black queen cell virus (BQCV)
BQCV is a positive-sense single-stranded RNA virus of the family Dicistroviridae and genus Triatovirus. It was first discovered in queen larvae and pupae. BQCV was named after the black cell wall color of the infected pupa (Bailey and Woods, 1977). Worker honey bees infected with severe BQCV exhibit symptoms of impaired orientation similar to those of DWV (Retschnig et al., 2019). In Korea, a complete viral sequence of BQCV was first reported in Anyang in 2012 (Reddy et al., 2013a). Infections have been documented in Vespa sp. (NCBI GenBank accession number MN902108), Bombus sp. (MN565034), A. mellifera, and A. cerana (MZ821802) (Dalmon et al., 2019). Because BQCV is a major diagnostic target among honey bee viruses in Korea, various diagnostic methods have been developed to detect BQCV, such as multi-point PCR, real-time PCR, ultra-rapid real-time PCR, and loop-mediated isothermal amplification (LAMP) (Cho et al., 2007; Yoo et al., 2008a; Lim et al., 2016; Kim et al., 2019c).
3. Chronic bee paralysis virus (CBPV)
CBPV is a positive-sense single-stranded virus with an unknown classification that belongs to the realm Riboviria (Chevin et al., 2012). Its official classification has yet to be established following its discovery with ABPV by Bailey et al. (1963). Infected honey bees exhibit the initial symptoms after approximately 6 days, after which they do not immediately die but continue to exhibit chronic paralysis symptoms (Bailey et al., 1963). The region estimated as the RNA-dependent RNA polymerase region of CBPV is similar to the families of Nodaviridae and Tombusviridae; however, at present, it is considered a new virus family with a positive RNA genome (Olivier et al., 2008). CBPV genome consists of two segments, and the complete viral genome sequence was first detected in A. mellifera in France (Olivier et al., 2008). The reported host species included A. mellifera, A. cerana, Bumbus sp. (ON448801), Vespa sp. (MZ151447), honey bee mites (MN114562, MN114563), and other insects (ON448799, ON448803, and ON448852). In Korea, a partial viral genome sequence has been reported in Cheongju (MH327931 and MH327932), whereas the complete viral genome sequence has not yet been reported. Diagnostic methods based on ultra-rapid PCR, RT-PCR, real-time PCR, and LAMP have been introduced to detect CBPV (Choi et al., 2008; No et al., 2010b; Yoo et al., 2010a, 2010b; Kim et al., 2019a).
4. Deformed wing virus (DWV)
DWV is a positive-sense single-stranded RNA virus belonging to the family Iflaviridae and genus Iflavirus. In honey bees, DWV infection causes immature development, wing deformity, and sometimes death (Bailey, 1968a). Bailey et al. (1979) named it the Egypt bee virus (EBV) after its first discovery during their research. In 1982, a virus similar to the EBV was discovered in malformed adults collected in Japan; the EBV was subsequently renamed DWV because of its similar symptoms (Ribière et al., 2008). Currently, there are three different types of DWV: DWV-A, DWV-B, and DWV-C. DWV-A corresponds to the known DWV. DWV-A is 97% identical to Kakugo virus (KV), which is only found in the brains of nurse honey bees and foragers, and its infection symptoms are more aggressive than those of DWV (Fujiyuki et al., 2004). It has also been demonstrated experimentally that KV can infect tissues other than the brains of honey bees (Lanzi et al., 2006). DWV-B, also known as the Varroa destructor virus-1 (VDV-1), was first detected in the Varroa mite, and it is 84% identical to DWV-A (Ongus et al., 2004). VDV-1 can be replicated in honey bees (Yue and Genersch, 2005). In temperate regions, DWV-B is a larger problem than other types of DWV (Natsopoulou et al., 2017). Recombination between type A and type B has also been reported (Moore et al., 2011). Overall, DWV-C shared 79% identity with type A and 78.9% identity with type B (Mordecai et al., 2016). In Korea, after a partial viral genome sequence (EU836051) was reported in 2008, a complete viral genome sequence was reported in 2012 (Reddy et al., 2013c). The documented hosts included A. mellifera, A. cerana (MH607198), Varroa destructor (MZ821838), Bumbus sp. (HQ655506 and MW222481), Vespa sp. (MN565036), and other insects (KF978606, KF314877, ON448657, KF314878, MF13482, and ON448672). DWV diagnostic manuals that include ultra-rapid PCR, ultra-rapid real time PCR, real time PCR, and LAMP methods have been developed (Lim, 2013; Lim and Yoon, 2013; Kim et al., 2014a; Kim et al., 2019b).
5. Israeli acute paralysis virus (IAPV)
IAPV was first reported in Israel as a positive-sense single-stranded RNA virus belonging to the family Dicistroviridae and genus Aparavirus (Maori et al., 2007). It is a significant virus that causes CCD and has symptoms similar to those of ABPV (Cox-Foster et al., 2007). Choi et al. (2007) discovered the first IAPV partial viral sequence (EU375538) in Korea. The complete viral genome sequence of IAPV in Korea was first reported by Andong, Guri, and Gangneung in 2013 (Reddy et al., 2013b). Its host species are A. mellifera, A. cerana, honey bee mites, Bumbus sp. (HQ655581), Vespa sp. (HQ655583), and other insects (LC581780, KT152165, and KT717338). Due to the significance of IAPV as a primary pathogen for honey bees in Korea, many diagnostic methods have been developed, including nested PCR, ultra-rapid PCR, RT-PCR, real-time PCR, and ultra-rapid real-time PCR techniques (Kang et al., 2008; Kim et al., 2009; Yoo et al., 2009; Yoo and Yoon, 2009a; No et al., 2010a; Lim et al., 2016).
6. Kashmir bee virus (KBV)
KBV is a positive-sense single-stranded virus belonging to the family Dicistroviridae and genus Aparavirus. A substance extracted from A. cerana collected in the Kashmir region was discovered through an injection experiment in A. mellifera (Bailey et al., 1976). Although no major symptoms appear after infection, external factors can result in death (Ward et al., 2007). Infection by the Varroa destructor, a vector that activates KBV, can result in sudden pupa and worker honey bee mortality (de Miranda et al., 2013). A complete viral genome sequence for KBV was first revealed in Pennsylvania, USA (de Miranda et al., 2004). The reported host was A. mellifera, A. cerana, Bumbus sp. (MF004374), Vespa sp. (MN565039), honey bee mites (AF200336 and MN114614), and several insects (MT068450, MT068447, and MT068448). In Korea, a complete viral genome sequence for KBV was first identified in A. mellifera sample from Anyang (Reddy et al., 2014) and its diagnostic method was based on real-time PCR (Yoo et al., 2008b).
7. Sacbrood virus (SBV)
SBV is a positive-sense single-stranded virus belonging to the family Iflaviridae and genus Iflavirus. It was the first virus identified in honey bees and described in 1913 (White, 1913). Infected larvae do not become pupae; instead, the molting fluid gradually accumulates in the integument, turns brown, and dies (Bailey, 1975). Hosts are A. mellifera, A. cerana, Varroa destructor (MN114616), Bumbus sp. (MH900073), Vespa sp. (MH133361), and other insects (MW435746, ON448730, ON448736, and MG737469). In Korea, a partial SBV viral genome sequence was first detected in A. mellifera (Kim et al., 2008), and its complete viral genome sequence was detected in A. cerana (HQ322114). SBV caused the large-scale extinction of A. cerana in Korea (Choi et al., 2010). Because of the substantial damage caused by SBV, the Animal and Plant Quarantine Agency in Korea strictly controls its occurrence. Various diagnostic methods based on nested PCR, Ultra-rapid PCR, RT-PCR, real-time PCR, Ultra-rapid real-time PCR, and LAMP techniques have been developed to detect SBV in Korea (Lee et al., 2011; Yoo et al., 2013; Lee et al., 2014; Wang et al., 2015; Truong et al., 2017).
8. Apis rhabdovirus (ARV) group
Apis rhabdoviruses are negative-sense single-stranded RNA viruses that have not been classified as a genus belonging to the family Rhabdoviridae (Remnant et al., 2017). Five ARV species have been reported. ARV1 (KY354230) and ARV2 (KY354233) were first reported in 2017 and ARV3 (MZ822104), ARV4 (MZ822105), and ARV5 (MZ822106) in 2021. ARV3 and 4 were only detected in A. cerana and have five open reading frames (N protein, P protein, M protein, G protein, and L protein) (Remnant et al., 2017). No symptoms have been identified yet. As of November 1st, 2022, viral genome sequences were reported to the NCBI GenBank database by New Zealand, South Africa, Tonga, Israel, USA, Sweden, China, Czech Republic, Austria, and Slovenia.
9. Lake Sinai virus (LSV) group
Lake Sinai viruses are positive-sense single-stranded viruses belonging to the family Sinhaliviridae and genus Sinaivirus (ICTV, 2019). They are classified into eight species. LSV1 (HQ871931) and LSV2 (HQ888865) were discovered for the first time in a sample from South Dakota, USA, where CCD occurred (Runckel et al., 2011). Thereafter, a partial LSV3 viral genome sequence was reported (Cornman et al., 2012), and its complete viral genome sequence was reported in 2018 (Thaduri et al., 2018). Ravoet et al. (2013) reported the partial viral genome sequences of LSV4 (JX878492) and LSV5 (KC880121), and a Chinese researcher reported the complete viral genome sequence for LSV4 (MZ821850). The complete viral genome sequence of LSV5 has not yet been confirmed. Daughenbaugh et al. (2015) reported partial viral genome sequences for LSV6 (KR021357) and LSV7 (KR021355). However, their complete viral genome sequences have not yet been identified. The complete viral genome sequence of LSV8 has been recently uncovered (PRJNA706851). However, in addition to the eight species, some viral genome sequences were reported under the names of unclassified LSV and LSV TO (KY354241), LSV NE (KY354242), LSV SA1 (KY354243), and LSV SA2 (KY354244). Several researchers have identified the full-length viral sequences of LSVs and proposed classification criteria among LSVs, but this classification has not been established yet (Roberts et al., 2017; Cornman, 2019). Because many recent studies have suggested that LSV may affect the occurrence of CCD, it has been reported as an important virus for honey bee health (Cornman et al., 2012; Daughenbaugh et al., 2015; Remnant et al., 2017; Thaduri et al., 2018; Faurot-Daniels et al., 2020). However, the symptoms of the LSV group have not yet been uncovered.
10. Other viruses
In addition to the main research subjects described above, many more viruses have been discovered, most of which lack data and pose unknown threats to honey bees. Recently, several new viruses have been reported in Australia, with the majority of the honey bee viruses belonging to the order Picornavirales. The symptoms of these novel viruses and whether they can be replicated in honey bees have not been determined yet; however, their nomenclature has been established, with novel viruses mainly being named “discovery area+honey bee virus”.
NOSEMA
Nosema apis, a unicellular parasite belonging to Microsporidia, was first discovered in 1909 (Zander, 1909). Queen honey bees infected with N. apis have a reduced spawning potential owing to ovary degeneration, and queen honey bees located inside the bottom side of the hive during spring are predominantly infected with the N. apis (Fyg, 1945; Liu, 1992). In the case of worker bees, infections impair the hypopharyngeal glands, reducing the feeding capacity of spring nurse honey bees and making it impossible to properly raise larvae, thereby weakening the bee population (Lotmar, 1936). Moreover, RNA synthesis decreases in infected cells, and digestive enzymes release may cease, resulting in digestive disorders (Hartwig and Przelecka, 1971; Liu, 1984). When infected, worker honey bees develop into foraging honey bees faster than healthy bees, resulting in faster aging of infected honey bees (Hassanein, 1953; Wang and Mofller, 1970). The average life expectancy of infected honey bees is 17-31 days, 11-27 days shorter than honey bees that were not exposed to N. apis for seven years (Revell, 1960). As a result, it can reduce honey production (Moeller, 1962; L’Arrivee and Geiger, 1966; Cantwell and Shimanuki, 1969; Fries et al., 1984; Farrar, 2014). N. apis affects the wintering ability of the colony; winter loss is associated with the Nosema infection, and accumulation begins in the spring after surviving the winter (Farrar, 1942). In addition, when infected honey bees clean their honeycomb, the Nosema spores spread to the honeycomb itself and are then transmitted through the wax (Bailey and Ball, 2013). Honey bees infected with N. apis are suspected to have higher incidences of Malpighamoeba mellificae, however, more research is needed to confirm this (Bailey, 1968b). Recently, a TaqMan probe-based RT-qPCR diagnosis method for M. mellificae has been developed to study the effects of M. mellificae on honey bees (Schäfer et al., 2022). Several honey bee viruses are related to N. apis (Bailey, 1982). Considering that BQCV has a shorter lifespan in honey bees infected with both Nosema and BQCV than in single-infected honey bees, the simultaneous infection of BQCV and Nosema has been found to have a synergistic effect on the lifespan of honey bees (Gajda et al., 2021). In an experimental co-infection study, CBPV and Nosema had synergistic effects, as the co-infection resulted in faster honey bee mortality than individual infection (Toplak et al., 2013). Nosema infection did not significantly increase the DWV titer in the pollen supply process, however, compared to control, the DWV titer did increase significantly after the pollen supply was stopped, confirming that Nosema infection has a synergistic effect on the amplification of DWV when pollination is not properly performed (Zheng et al., 2015). In 1995, Nosema cerana was detected in A. cerana in China (Fries et al., 1996). N. cerana is known to have a greater impact on bumble bees than honey bees (Graystock et al., 2013). N. cerana was also found in pollen, which can cause infections, indicating that forager honey bees are more vulnerable to infection than queen honey bees and drones (Higes et al., 2008). N. cerana can be transmitted through N. apis, and can be infected by chewing contaminated wax when leaving the honeycomb (Malone and Gatehouse, 1998). Honey bees infected with N. cerana may have trouble returning to their hive due to stress (Wolf et al., 2016). This return ability has been demonstrated to reduce the level of trehalose involved in flight regardless of the degree of infection in infected honey bees (Kurze et al., 2016). Nosema-infected honey bees are also closely related to pesticides, with studies showing that honey bees exposed to high levels of pesticides are more susceptible to N. cerana infection than honey bees with low exposure levels (Pettis et al., 2013). After 8 days, honey bees infected with 1×105 N. cerana spores showed a 100% mortality rate (Higes et al., 2007). As a result, it may have a synergistic effect on the CCD phenomenon, which is the biggest threat to honey bees, by inflicting various damage (Bromenshenk et al., 2010).
VARROA MITE
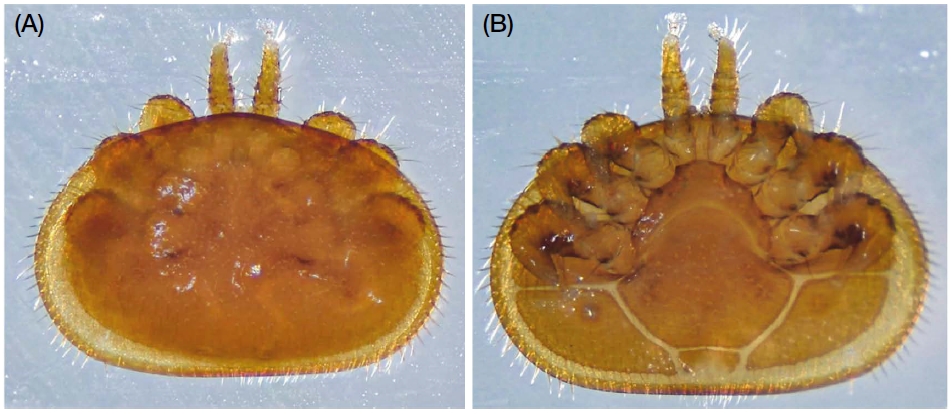
The Varroa mite is a honey bee pest known to cause considerable damage to honey bees (Fig. 2). To date, the Varroa mite comprises four species. Varroa jacobsoni Oudemans was discovered in 1904 in A. cerana, Java, Indonesia (Oudemans, 1904); V. underwoodi in A. cerana, Nepal (Delfinado-Baker and Aggarwal, 1987); and V. rindereri in A. koschevnikovi, Borneo, Malaysia (De Guzman and Delfinado-Baker, 1996). The fourth species, V. destructor, was recently separated as another species from being misclassified as V. jacobsoni (Anderson, 2000; Anderson and Trueman, 2000). Among these four Varroa mites, V. destructor is mostly distributed in mainland Asia, especially in the eastern part, and is the most destructive to honey bees (Rosenkranz et al., 2010). Several symptoms of Varroa infection in honey bees have been reported: first, some infected honey bees displayed shortened abdomens and a 6-25% weight loss compared to healthy honey bees, with the number of Varroa mites and the weight of the honey bees exhibiting a negative correlation (De Jong et al., 1982). Second, the life span of honey bees varied according to the number of Varroa mites. The average life span of honey bees was 18.1 days when infected by one Varroa mite and 8.9 days when infected by two or more mites, and the number of Varroa mites was correlated with the honey bee life-span (De Jong and De Jong, 1983). Third, infected worker honey bees were less efficient in major activities; when infected during the larval stage, the size of the hypopharyngeal glands decreased by up to 31% and the adult stage decreased by an average of 14% (Schneider and Drescher, 1987). This reduction in the hypopharyngeal gland size makes it difficult for the nurse honey bees to feed the larvae, which interferes with honey bee growth and weakens the colony. As a result, the Varroa mite directly injures bee larvae and adults, injures hemolymph and fat bodies, and harms them, affecting their life duration, weight, and endocrine organs (Ramsey et al., 2019). Fourth, the Varroa mite has a large effect on honey bee’s ability to fly. When infected by a Varroa mite in the larval stages, wing deformation can occur in some honey bees and the number of Varroa-infected honey bees that cannot return to the colony is twice that of healthy honey bees (Kralj and Fuchs, 2006). When a drone is infected by a Varroa mite, it has a great impact on the drone’s flight ability. There is no difference in flight abilities between a healthy drone and an infected drone through a single Varroa mite, but a drone infected by two or more Varroa mites showed a maximum flight time of up to 6 min compared to a healthy drone with an average flight time of 27 min (Duay et al., 2002). As such, Varroa mites, which cause substantial damage, also act as a mediator for transferring pathogens to honey bees (Fig. 3). Currently, there are 22 viruses registered in the GenBank that are associated with Varroa mites. Among them, ABPV (Ball and Allen, 1988), BQCV (Locke et al., 2012), CBPV (Celle et al., 2008), IAPV (Di Prisco et al., 2011), SBV (Shen et al., 2005), KBV (Chen et al., 2004), SBPV (Santillán-Galicia et al., 2010), DWV, and VDV1 (Ryabov et al., 2017) are known to infect honey bees. As a result, direct damage to bees can reduce their immune systems, resulting in synergistic effects in causing CCD, which is the primary cause of honey bee mortality in the world (Le Conte et al., 2010).
VIROME ANALYSIS USING HIGH-THROUGHPUT SEQUENCING (HTS)
Recent studies have focused not only on the diagnosis of individual viruses, but also on the analysis of all viral nucleic acids present in individuals, communities, and the environment, confirming the diversity within these groups. The virus or viral nucleic acid information of such a group is referred to as a “virome” (Zárate et al., 2017).
Virome analysis using HTS began in 2002 using seawater samples (Breitbart et al., 2002). HTS has demonstrated broad capabilities for the detection of known and novel viruses in a variety of different sample types, including environmental, clinical, and biological samples such as cell lines and biological products (Goodacre et al., 2018). HTS technologies have also provided new insights into intraspecific viral diversity, thereby facilitating the characterization of virus variants and improving the disentanglement of viral population genetics (Maclot et al., 2020).
HTS technology-based virome analysis has been widely used by several researchers to study honey bees (Remnant et al., 2017; Roberts et al., 2018; Kadlečková et al., 2022; Lester et al., 2022). Various novel viruses have been identified using virome analysis. As a result, the number of viruses detected in honey bees has increased from 18 in 2007 to 128 in 2022 (Chen and Siede, 2007; NCBI, 2022). Although Virome analysis has already been actively conducted by many researchers around the world, and a virome analysis was recently performed for the first time in honey bees in Korea (Kwon et al., 2023).
FUTURE DIRECTIONS
Since the CCD outbreak in 2006, many researchers around the world have been interested in honey bees’ health, with research on bees increasing annually. Several researchers in Korea have studied the effects of various factors on honey bee health, but research on viruses has not been conducted extensively. Countrywide, mass honey bee mortalities during the winter of 2021 were concluded to be caused by various factors (climate change, pesticides, and mites) without mentioning the influence of viruses (Lee and Choi, 2022). However, several studies have shown that viruses can have a significant impact on the health of bees, and further research is underway. Korea experienced severe damage from the SBV in Apis cerana during 2009-2010. Using past damage as a starting point, focusing on the risk of viruses in honey bees and accumulating data through various studies will lead to developments in Korean beekeeping.
Acknowledgments
This research was funded by the BSRP through the National Research Foundation of Korea (NRF), Ministry of Education (NRF-2018R1A6A1A03024862).
References
-
Amdam, G. V., K. Hartfelder, K. Norberg, A. Hagen and S. W. Omholt. 2004. Altered physiology in worker honey bees (Hymenoptera: Apidae) infested with the mite Varroa destructor (Acari: Varroidae): a factor in colony loss during overwintering? J. Econ. Entomol. 97: 741-747.
[https://doi.org/10.1093/jee/97.3.741]

- Anderson, D. and J. Trueman. 2000. Varroa jacobsoni (Acari: Varroidae) is more than one species. Exp. Appl. Acarol. 24: 165-189.
-
Anderson, D. L. 2000. Variation in the parasitic bee mite Varroa jacobsoni Oud. Apidologie 31: 281-292.
[https://doi.org/10.1051/apido:2000122]

-
Bailey, L. 1968a. Honey bee pathology. Ann. Rev. Entomol. 13: 191-212.
[https://doi.org/10.1146/annurev.en.13.010168.001203]

-
Bailey, L. 1968b. The measurement and interrelationships of infections with Nosema apis and Malpighamoeba mellificae of honey-bee populations. J. Invertebr. Pathol. 12: 175-179.
[https://doi.org/10.1016/0022-2011(68)90174-2]

-
Bailey, L. 1975. Recent research on honeybee viruses. Bee World 56: 55-64.
[https://doi.org/10.1080/0005772X.1975.11097544]

-
Bailey, L. 1982. Viruses of honeybees. Bee World 63: 165-173.
[https://doi.org/10.1080/0005772X.1982.11097891]

-
Bailey, L., B. V. Ball and R. Woods. 1976. An iridovirus from bees. J. Gen. Virol. 31: 459-461.
[https://doi.org/10.1099/0022-1317-31-3-459]

-
Bailey, L., J. Carpenter and R. Woods. 1979. Egypt bee virus and Australian isolates of Kashmir bee virus. J. Gen. Virol. 43: 641-647.
[https://doi.org/10.1099/0022-1317-43-3-641]

-
Bailey, L., A. Gibbs and R. Woods. 1963. Two viruses from adult honey bees (Apis mellifera Linnaeus). Virology 21: 390-395.
[https://doi.org/10.1016/0042-6822(63)90200-9]

-
Bailey, L. and R. Woods. 1977. Two more small RNA viruses from honey bees and further observations on sacbrood and acute bee-paralysis viruses. J. Gen. Virol. 37: 175-182.
[https://doi.org/10.1099/0022-1317-37-1-175]

- Bailey, L. L. and B. V. Ball. 2013. Honey bee pathology. Elsevier.
-
Ball, B. and M. Allen. 1988. The prevalence of pathogens in honey bee (Apis mellifera) colonies infested with the parasitic mite Varroa jacobsoni. Ann. Appl. Biol. 113: 237-244.
[https://doi.org/10.1111/j.1744-7348.1988.tb03300.x]

-
Bigot, D., A. Dalmon, B. Roy, C. Hou, M. Germain, M. Romary, S. Deng, Q. Diao, L. Weinert, J. M. Cook, E. A. Herniou and P. Gayral. 2017. The discovery of Halictivirus resolves the Sinaivirus phylogeny. J. Gen. Virol. 98(11): 2864-2875.
[https://doi.org/10.1099/jgv.0.000957]

-
Breitbart, M., P. Salamon, B. Andresen, J. M. Mahaffy, A. M. Segall, D. Mead, F. Azam and F. Rohwer. 2002. Genomic analysis of uncultured marine viral communities. Proc. Natl. Acad. Sci. 99: 14250-14255.
[https://doi.org/10.1073/pnas.202488399]

-
Bromenshenk, J. J., C. B. Henderson, C. H. Wick, M. F. Stanford, A. W. Zulich, R. E. Jabbour, S. V. Deshpande, P. E. McCubbin, R. A. Seccomb, P. M. Welch, T. Williams, D. R. Firth, E. Skowronski, M. M. Lehmann, S. L. Bilimoria, J. Gress, K. W. Wanner and R. A. Cramer Jr. 2010. Iridovirus and microsporidian linked to honey bee colony decline. PLoS One 5: e13181.
[https://doi.org/10.1371/journal.pone.0013181]

-
Brutscher, L. M., A. J. McMenamin and M. L. Flenniken. 2016. The buzz about honey bee viruses. PLoS Pathog. 12: e1005757.
[https://doi.org/10.1371/journal.ppat.1005757]

- Cantwell, G. and H. Shimanuki. 1969. Heat treatment as a means of eliminating Nosema and increasing production. American Bee Journal 109: 52-54.
-
Celle, O., P. Blanchard, V. Olivier, F. Schurr, N. Cougoule, J.-P. Faucon and M. Ribière. 2008. Detection of Chronic bee paralysis virus(CBPV) genome and its replicative RNA form in various hosts and possible ways of spread. Virus Res. 133: 280-284.
[https://doi.org/10.1016/j.virusres.2007.12.011]

-
Chen, Y., J. S. Pettis, J. D. Evans, M. Kramer and M. F. Feldlaufer. 2004. Transmission of Kashmir bee virus by the ectoparasitic mite Varroa destructor. Apidologie 35: 441-448.
[https://doi.org/10.1051/apido:2004031]

- Chen, Y. P., N. Nakashima, P. D. Christian, T. Bakonyi, B. C. Bonning, S. M. Valles and D. Lightner. 2022. Family: Iflaviridae, Chapter Version: ICTV Ninth Report; 2009 Taxonomy Release [Online]. ICTV. Available: https://ictv.global/report_9th/RNApos/Picornavirales/Iflaviridae, [Accessed 2022.12.25].
-
Chen, Y. P., J. S. Pettis, M. Corona, W. P. Chen, C. J. Li, M. Spivak, P. K. Visscher, G. DeGrandi-Hoffman, H. Boncristiani, Y. Zhao, D. vanEngelsdorp, K. Delaplane, L. Solter, F. Drummond, M. Kramer, W. I. Lipkin, G. Palacios, M. C. Hamilton, B. Smith, S. K. Huang, H. Q. Zheng, J. L. Li, X. Zhang, A. F. Zhou, L. Y. Wu, J. Z. Zhou, M.-L. Lee, E. W. Teixeira, Z. G. Li and J. D. Evans. 2014. Israeli acute paralysis virus: epidemiology, pathogenesis and implications for honey bee health. PLoS Pathog. 10: e1004261.
[https://doi.org/10.1371/journal.ppat.1004261]

-
Chen, Y. P. and R. Siede. 2007. Honey bee viruses. Adv. Virus Res. 70: 33-80.
[https://doi.org/10.1016/S0065-3527(07)70002-7]

-
Chevin, A., F. Schurr, P. Blanchard, R. Thiéry and M. Ribière. 2012. Experimental infection of the honeybee (Apis mellifera L.) with the chronic bee paralysis virus (CBPV): infectivity of naked CBPV RNAs. Virus Res. 167: 173-178.
[https://doi.org/10.1016/j.virusres.2012.04.012]

- Cho, Y.-H., M.-S. Yoo, E.-H. Kim, D.-W. Lee, I.-W. Kim, M.-H. Kang, S.-H. Han and B.-S. Yoon. 2007. Development of Rapid Detection Method for Black Queen Cell Virus (BQCV) using the Loop-mediated Isothermal Amplification (LAMP) in Honeybees. J. Apic. 22: 139-146.
- Choi, Y.-S., M.-L. Lee and M.-Y. Lee. 2007. Detection of Israle Acute Paralysis Virus (IAPV) from the Honeybee in Korea. J. Apic. 22: 159-165.
- Choi, Y. S., H. K. Kim, M. L. Lee, M. Y. Lee and K. G. Lee. 2008. Detection of Chronic Bee Paralysis Virus (CBPV) by Minus-strand-specific RT-PCR. J. Apic. 23: 119-126.
- Choi, Y. S., M. Y. Lee, I. P. Hong, N. S. Kim, H. K. Kim, K. G. Lee and M. L. Lee. 2010. Occurrence of sacbrood virus in Korean apiaries from Apis cerana (Hymenoptera: Apidae). J. Apic. 25: 187-191.
-
Cornman, R. S. 2019. Relative abundance and molecular evolution of Lake Sinai Virus (Sinaivirus) clades. PeerJ 7: e6305.
[https://doi.org/10.7717/peerj.6305]

-
Cornman, R. S., D. R. Tarpy, Y. Chen, L. Jeffreys, D. Lopez, J. S. Pettis, D. vanEngelsdorp and J. D. Evans. 2012. Pathogen webs in collapsing honey bee colonies. PLoS One 7(8): e43562.
[https://doi.org/10.1371/journal.pone.0043562]

-
Cox-Foster, D. L., S. Conlan, E. C. Holmes, G. Palacios, J. D. Evans, N. A. Moran, P.-L. Quan, T. Briese, M. Hornig, D. M. Geiser, V. Martinson, D. vanEngelsdorp, A. L. Kalkstein, A. Drysdale, J. Hui, J. Zhai, L. Cui, S. K. Hutchison, J. F. Simons, M. Egholm, J. S. Pettis and W. I. Lipkin. 2007. A metagenomic survey of microbes in honey bee colony collapse disorder. Science 318: 283-287.
[https://doi.org/10.1126/science.1146498]

-
Dalmon, A., P. Gayral, D. Decante, C. Klopp, D. Bigot, M. Thomasson, E. A. Herniou, C. Alaux and Y. Le Conte. 2019. Viruses in the invasive hornet Vespa velutina. Viruses 11: 1041.
[https://doi.org/10.3390/v11111041]

-
Daughenbaugh, K. F., M. Martin, L. M. Brutscher, I. Cavigli, E. Garcia, M. Lavin and M. L. Flenniken. 2015. Honey bee infecting Lake Sinai viruses. Viruses 7: 3285-3309.
[https://doi.org/10.3390/v7062772]

-
De Guzman, L. and M. Delfinado-Baker. 1996. A new species of Varroa (Acari: Varroidae) associated with Apis koschevnikovi (Apidae: Hymenoptera) in Borneo. Int. J. Acarol. 22: 23-27.
[https://doi.org/10.1080/01647959608684077]

-
De Jong, D., P. De Jong and L. Goncalves. 1982. Weight loss and other damage to developing worker honeybees from infestation with Varroa jacobsoni. J. Apic. Res. 21: 165-167.
[https://doi.org/10.1080/00218839.1982.11100535]

-
De Jong, D. and P. H. De Jong. 1983. Longevity of africanized honey bees (Hymenoptera: Apidae) infested by Varroa jacobsoni (Parasitiformes: Varroidae). J. Econ. Entomol. 76: 766-768.
[https://doi.org/10.1093/jee/76.4.766]

- de Miranda, J., Y. Chen, M. Ribière and L. Gauthier. 2011. Varroa and viruses. Varroa - Still a problem in the 21st century, 11-31.
-
de Miranda, J. R., L. Bailey, B. V. Ball, P. Blanchard, G. E. Budge, N. Chejanovsky, Y.-P. Chen, L. Gauthier, E. Genersch, D. C. De Graaf, M. Ribière, E. Ryabov, L. De Smet and J. J. M. van der Steen. 2013. Standard methods for virus research in Apis mellifera. J. Apic. Res. 52: 1-56.
[https://doi.org/10.3896/IBRA.1.52.4.22]

-
de Miranda, J. R., M. Drebot, S. Tyler, M. Shen, C. Cameron, D. Stoltz and S. Camazine. 2004. Complete nucleotide sequence of Kashmir bee virus and comparison with acute bee paralysis virus. J. Gen. Virol. 85: 2263-2270.
[https://doi.org/10.1099/vir.0.79990-0]

-
Delfinado-Baker, M. and K. Aggarwal. 1987. A new Varroa (Acari: Varroidae) from the nest of Apis cerana (Apidae). Int. J. Acarol. 13: 233-237.
[https://doi.org/10.1080/01647958708683777]

-
Di Pasquale, G., M. Salignon, Y. Le Conte, L. P. Belzunces, A. Decourtye, A. Kretzschmar, S. Suchail, J.-L. Brunet and C. Alaux. 2013. Influence of pollen nutrition on honey bee health: do pollen quality and diversity matter? PLoS One 8: e72016.
[https://doi.org/10.1371/journal.pone.0072016]

-
Di Prisco, G., F. Pennacchio, E. Caprio, H. F. Boncristiani Jr, J. D. Evans and Y. Chen. 2011. Varroa destructor is an effective vector of Israeli acute paralysis virus in the honeybee, Apis mellifera. J. Gen. Virol. 92: 151-155.
[https://doi.org/10.1099/vir.0.023853-0]

-
Doeke, M. A., M. Frazier and C. M. Grozinger. 2015. Overwintering Honey bees: Biology and Management. Curr. Opin. Insect Sci. 10: 185-193.
[https://doi.org/10.1016/j.cois.2015.05.014]

- Duay, P., D. De Jong and W. Engels. 2002. Decreased flight performance and sperm production in drones of the honey bee (Apis mellifera) slightly infested by Varroa destructor mites during pupal development. Genet. Mol. Res. 1: 227-232.
- Farrar, C. 1942. Nosema disease contributes to winter losses and queen supersedure. Glean. Bee Cult. 70: 660-661.
-
Farrar, C. 2014. Nosema losses in package bees as related to queen supersedure and honey yields. J. Econ. Entomol. 40: 333-338.
[https://doi.org/10.1093/jee/40.3.333]

-
Faurot-Daniels, C., W. Glenny, K. F. Daughenbaugh, A. J. McMenamin, L. A. Burkle and M. L. Flenniken. 2020. Longitudinal monitoring of honey bee colonies reveals dynamic nature of virus abundance and indicates a negative impact of Lake Sinai virus 2 on colony health. PLoS One 15: e0237544.
[https://doi.org/10.1371/journal.pone.0237544]

-
Fries, I., S. Camazine and J. Sneyd. 1994. Population dynamics of Varroa jacobsoni: a model and a review. Bee World 75: 5-28.
[https://doi.org/10.1080/0005772X.1994.11099190]

-
Fries, I., G. Ekbohm and E. Villumstad. 1984. Nosema apis, sampling techniques and honey yield. J. Apic. Res. 23: 102-105.
[https://doi.org/10.1080/00218839.1984.11100617]

-
Fries, I., F. Feng, A. da Silva, S. B. Slemenda and N. J. Pieniazek. 1996. Nosema ceranae n. sp. (Microspora, Nosematidae), morphological and molecular characterization of a microsporidian parasite of the Asian honey bee Apis cerana (Hymenoptera, Apidae). Eur. J. Protistol. 32: 356-365.
[https://doi.org/10.1016/S0932-4739(96)80059-9]

-
Fujiyuki, T., H. Takeuchi, M. Ono, S. Ohka, T. Sasaki, A. Nomoto and T. Kubo. 2004. Novel insect picorna-like virus identified in the brains of aggressive worker honeybees. J. Virol. 78: 1093-1100.
[https://doi.org/10.1128/JVI.78.3.1093-1100.2004]

- Fyg, W. 1945. Der Einfluβ der Nosema - Infektion auf die Eierstöcke der Bienenkönigin. Schweizerische Bienenzeitung 68: 67-72.
-
Gajda, A. M., E. D. Mazur, A. M. Bober and M. Czopowicz. 2021. Nosema ceranae Interactions with Nosema apis and Black Queen Cell Virus. Agriculture 11: 963.
[https://doi.org/10.3390/agriculture11100963]

-
Gallai, N., J.-M. Salles, J. Settele and B. E. Vaissière. 2009. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 68: 810-821.
[https://doi.org/10.1016/j.ecolecon.2008.06.014]

-
Gauthier, L., S. Cornman, U. Hartmann, F. Cousserans, J. D. Evans, J. R. De Miranda and P. Neumann. 2015. The Apis mellifera filamentous virus genome. Viruses 7: 3798-3815.
[https://doi.org/10.3390/v7072798]

-
Genersch, E., W. Von Der Ohe, H. Kaatz, A. Schroeder, C. Otten, R. Büchler, S. Berg, W. Ritter, W. Mühlen, S. Gisder, M. Meixner, G. Liebig and P. Rosenkranz. 2010. The German bee monitoring project: a long term study to understand periodically high winter losses of honey bee colonies. Apidologie 41: 332-352.
[https://doi.org/10.1051/apido/2010014]

-
Gisder, S., P. Aumeier and E. Genersch. 2009. Deformed wing virus: replication and viral load in mites (Varroa destructor). J. Gen. Virol. 90: 463-467.
[https://doi.org/10.1099/vir.0.005579-0]

-
Goodacre, N., A. Aljanahi, S. Nandakumar, M. Mikailov and A. S. Khan. 2018. A reference viral database (RVDB) to enhance bioinformatics analysis of high-throughput sequencing for novel virus detection. mSphere 3: e00069-00018.
[https://doi.org/10.1128/mSphereDirect.00069-18]

-
Govan, V., N. Leat, M. Allsopp and S. Davison. 2000. Analysis of the complete genome sequence of acute bee paralysis virus shows that it belongs to the novel group of insect-infecting RNA viruses. Virology 277: 457-463.
[https://doi.org/10.1006/viro.2000.0616]

-
Graystock, P., K. Yates, B. Darvill, D. Goulson and W. O. Hughes. 2013. Emerging dangers: deadly effects of an emergent parasite in a new pollinator host. J. Invertebr. Pathol. 114: 114-119.
[https://doi.org/10.1016/j.jip.2013.06.005]

- Guzman-Novoa, E., S. Cork, D. Hall and K. Liljebjelke. 2016. Colony collapse disorder and other threats to honey bees. One health case studies: addressing complex problems in a changing world, 204-216.
-
Hartwig, A. and A. Przelecka. 1971. Nucleic acids in intestine of Apis mellifica infected with and treated with fumagillin DCH: cytochemical and autoradiographic studies. J. Invertebr. Pathol. 18: 331-336.
[https://doi.org/10.1016/0022-2011(71)90034-6]

-
Hassanein, M. 1953. The influence of infection with Nosema apis on the activities and longevity of the worker honeybee. Ann. Appl. Biol. 40: 418-423.
[https://doi.org/10.1111/j.1744-7348.1953.tb01093.x]

-
Higes, M., P. García-Palencia, R. Martín-Hernández and A. Meana. 2007. Experimental infection of Apis mellifera honeybees with Nosema ceranae (Microsporidia). J. Invertebr. Pathol. 94: 211-217.
[https://doi.org/10.1016/j.jip.2006.11.001]

-
Higes, M., R. Martín-Hernández, E. Garrido-Bailon, P. García-Palencia and A. Meana. 2008. Detection of infective Nosema ceranae (Microsporidia) spores in corbicular pollen of forager honeybees. J. Invertebr. Pathol. 97: 76-78.
[https://doi.org/10.1016/j.jip.2007.06.002]

- ICTV. 2019. Taxon Details (Species: Lake Sinai virus 1) [Online]. Available: https://ictv.global/taxonomy/taxondetails?taxnode_id=202105368, [Accessed].
- ICTV. 2022. Family: Dicistroviridae, Genus: Cripavirus [Online]. ICTV. Available: https://ictv.global/report/chapter/dicistroviridae/dicistroviridae/cripavirus, [Accessed 2022.12.25].
-
Jeong, S., C. Lee, D. Kim and C. Jung. 2016. Questionnaire study on the overwintering success and pest management of honeybee and damage assessment of Vespa hornets in Korea. J. Apic. 31(3): 201.
[https://doi.org/10.17519/apiculture.2016.09.31.3.201]

-
Johnson, R. 2010. Honey bee colony collapse disorder. Congressional Research Service Washington.
[https://doi.org/10.32473/edis-in720-2010]

-
Jung, C. and Y. H. Bae. 2022. Production and Characteristics of Witner Generation Honey Bees, Apis mellifera: Discussion with Overwintering Failure. J. Apic. 37: 265-274.
[https://doi.org/10.17519/apiculture.2022.09.37.3.265]

-
Kadlečková, D., R. Tachezy, T. Erban, W. Deboutte, J. Nunvář, M. Saláková and J. Matthijnssens. 2022. The Virome of Healthy Honey Bee Colonies: Ubiquitous Occurrence of Known and New Viruses in Bee Populations. mSystems 7(3): e0007222.
[https://doi.org/10.1128/msystems.00072-22]

- Kang, M.-H., I.-W. Kim, M.-S. Yoo, S.-H. Kwon and B.-S. Yoon. 2008. Development of PCR Method for Israeli Acute Paralysis Virus (IAPV) in Honeybees (Apis mellifera L.). J. Apic. 23: 29-36.
-
Kim, B., J. Kim, S. Kim, M. Kim, A. T. Truong, K. Cho and B. Yoon. 2019a. Detection of chronic bee paralysis virus using ultra-rapid PCR and nested ultra-rapid PCR. J. Apic. Res. 58: 133-140.
[https://doi.org/10.1080/00218839.2018.1517999]

-
Kim, H.-K. 2022. The Effect of Honey Bee Mites on the Winter Colony Losses. J. Apic. 37: 291-299.
[https://doi.org/10.17519/apiculture.2022.09.37.3.291]

- Kim, H. K., Y. S. Choi, M. L. Lee, M. Y. Lee, K. G. Lee and N. H. Ahn. 2008. Detection of Sacbrood Virus (SBV) fromthe Honeybee in Korea. J. Apic. 23: 103-109.
- Kim, I.-W., M.-S. Yoo, M.-H. Kang and B.-S. Yoon. 2009. Development of Nested PCR Method for the Detectionof Israel Acute Paralysis Virus (IAPV). J. Apic. 24: 93-99.
-
Kim, J.-M., S.-J. Lim, S. Kim, M. Kim, B. Kim, T. A. Tai, S. Kim and B. Yoon. 2019b. Rapid detection of deformed wing virus in honeybee using ultra-rapid qPCR and a DNA-chip. J. Vet. Sci. 21(1).
[https://doi.org/10.4142/jvs.2020.21.e4]

-
Kim, M., J. Kim, B. Kim, S. Kim, A. T. Truong and B. Yoon. 2018. Development of Nested Ultra-Rapid PCR Method for the Accurate Detection of Acute Bee Paralysis Virus (ABPV). J. Apic. 33: 165-180.
[https://doi.org/10.17519/apiculture.2018.09.33.3.165]

- Kim, N.-H., M.-s. Yoo, Y.-H. Kim, H.-N. Jung, K. E. Reddy, S.-C. Jung and S.-w. Kang. 2014a. 10 minutes PCR for detection of Deformed Wing Virus (DWV) in the honey bee. J. Apic., 60-60.
- Kim, N.-H., M.-s. Yoo, Y.-H. Kim, H.-N. Jung, K. E. Reddy, S.-C. Jung and S.-w. Kang. 2014b. 10 minutes PCR for detection of Acute Bee Paralysis Virus (ABPV) in the honey bee. J. Apic. 59-59.
- Kim, S.-m., B.-h. Kim, M.-j. Kim, J.-y. Park and B.-s. Yoon. 2017. How fast could we detect Acute bee paralysis virus (ABPV) from infected honeybee in apiary? J. Apic., 62-62.
-
Kim, S., B. Kim, M. Kim, J. Kim, A. T. Truong, S. Kim and B. Yoon. 2019c. Development of Diagnostic System to Black Queen Cell Virus (BQCV) Using Multi-point Detection. J. Apic. 34: 39-46.
[https://doi.org/10.17519/apiculture.2019.04.34.1.39]

-
Kraberger, S., C. N. Cook, K. Schmidlin, R. S. Fontenele, J. Bautista, B. Smith and A. Varsani. 2019. Diverse single-stranded DNA viruses associated with honey bees (Apis mellifera). Infect. Genet. Evol. 71: 179-188.
[https://doi.org/10.1016/j.meegid.2019.03.024]

-
Kralj, J. and S. Fuchs. 2006. Parasitic Varroa destructor mites influence flight duration and homing ability of infested Apis mellifera foragers. Apidologie 37: 577-587.
[https://doi.org/10.1051/apido:2006040]

-
Kurze, C., C. Mayack, F. Hirche, G. I. Stangl, Y. Le Conte, P. Kryger and R. F. Moritz. 2016. Nosema spp. infections cause no energetic stress in tolerant honeybees. Parasitol. Res. 115: 2381-2388.
[https://doi.org/10.1007/s00436-016-4988-3]

-
Kwon, M., C. Jung and E.-J. Kil. 2023. Metagenomic analysis of viromes in honey bee colonies (Apis mellifera; Hymenoptera: Apidae) after mass disappearance in Korea. Front. Cell. Infect. Microbiol. 13: 27.
[https://doi.org/10.3389/fcimb.2023.1124596]

- L’Arrivee, J. and J. Geiger. 1966. Size of package bees and honey yields. Am. Bee J. 106: 61.
-
Lanzi, G., J. R. De Miranda, M. B. Boniotti, C. E. Cameron, A. Lavazza, L. Capucci, S. M. Camazine and C. Rossi. 2006. Molecular and biological characterization of deformed wing virus of honeybees (Apis mellifera L.). J. Virol. 80: 4998-5009.
[https://doi.org/10.1128/JVI.80.10.4998-5009.2006]

-
Le Conte, Y., M. Ellis and W. Ritter. 2010. Varroa mites and honey bee health: can Varroa explain part of the colony losses? Apidologie 41: 353-363.
[https://doi.org/10.1051/apido/2010017]

-
Le Gall, O., P. Christian, C. M. Fauquet, A. M. King, N. J. Knowles, N. Nakashima, G. Stanway and A. E. Gorbalenya. 2008. Picornavirales, a proposed order of positive-sense single-stranded RNA viruses with a pseudo-T= 3 virion architecture. Arch. Virol. 153: 715-727.
[https://doi.org/10.1007/s00705-008-0041-x]

- Lee, B., J.-N. No, M.-S. Yoo and B. Yoon. 2011. Development of Method for the Detection of Sacbrood Virus by Loop-Mediated Isothermal Amplification J. Apic. 26: 267-274.
- Lee, J.-G., H.-Y. Lim and B. Yoon. 2014. Development of PCR method for distinguishment of Sacbrood virus (SBV) based on SBV nucleotide acid sequence. J. Apic., 37-37.
- Lee, J.-S., M.-S. Yoo, H.-J. Seo, W.-R. Bae, N.-R. Kim, S.-W. Kang, H.-S. Lee and Y.-S. Cho. 2016. High-performance PCR for Rapid Detection of Acute Bee Paralysis Virus (ABPV) in Honey Bee. J. Apic., 68-68.
- Lee, M.-Y. and Y.-S. Choi. 2022. In Korea, beekeeping farmhouse wintering honey bee damage public-private joint investigation result [Online]. Rural Development Administration. Available: https://www.rda.go.kr/board/board.do?boardId=farmprmninfo&prgId=day_farmprmninfoEntry&currPage=1&dataNo=100000777725&mode=updateCnt&searchSDate=&searchEDate=&searchOrgDeptKey=org&searchOrgDeptVal=&searchKey=subject&searchVal=%EA%BF%80%EB%B2%8C, [Accessed 2022.10.09 2022.03.14].
-
Lester, P. J., A. Felden, J. W. Baty, M. Bulgarella, J. Haywood, A. N. Mortensen, E. J. Remnant and Z. E. Smeele. 2022. Viral communities in the parasite Varroa destructor and in colonies of their honey bee host (Apis mellifera) in New Zealand. Sci. Rep. 12: 1-13.
[https://doi.org/10.1038/s41598-022-12888-w]

- Lim, H.-Y. 2013. Development of novel rapid detection method for deformed wing virus (DWV) using ultra-fast high-performance PCR (UF-HP PCR). J. Apic. 237-244.
- Lim, H.-Y. and B.-S. Yoon. 2013. Rapid and sensitive detection of Deformed Wing Virus (DWV) in honeybee using Ultra-rapid Real-time PCR. J. Apic. 28: 121-129.
- Lim, S.-J., S.-j. Yong, J. H. Wang, S. H. Min and B.-S. Yoon. 2016. Development of Point of Care Diagnostic for Israeli Acute Paralysis Virus (IAPV) in Honeybee using Ultra-fast High Performance PCR (UF-HP-PCR). J. Apic., 60-60.
-
Lim, S. J., G. T. H. Luong, S. H. Min, J. H. Wang and B.-S. Yoon. 2016. Rapid Detection of Black Queen Cell Virus from Honeybee using Reverse Transcription Real-Time Recombinase Polymerase Amplification (RT/RT RPA). J. Apic. 41-51.
[https://doi.org/10.17519/apiculture.2016.04.31.1.41]

-
Liu, T. 1984. Ultrastructure of the midgut of the worker honey bee Apis mellifera heavily infected with Nosema apis. J. Invertebr. Pathol. 44: 282-291.
[https://doi.org/10.1016/0022-2011(84)90026-0]

-
Liu, T. 1992. Oöcytes degeneration in the queen honey bee after infection by Nosema apis. Tissue Cell 24: 131-138.
[https://doi.org/10.1016/0040-8166(92)90087-N]

-
Locke, B., E. Forsgren, I. Fries and J. R. de Miranda. 2012. Acaricide treatment affects viral dynamics in Varroa destructor-infested honey bee colonies via both host physiology and mite control. Appl. Environ. Microbiol. 78: 227-235.
[https://doi.org/10.1128/AEM.06094-11]

- Lotmar, R. 1936. Nosema-Infektion und ihr Einfluss auf die Entwicklung der Futtersaftdrüse.
-
Maclot, F., T. Candresse, D. Filloux, C. M. Malmstrom, P. Roumagnac, R. Van der Vlugt and S. Massart. 2020. Illuminating an ecological blackbox: using high throughput sequencing to characterize the plant virome across scales. Front. Microbiol. 11: 578064.
[https://doi.org/10.3389/fmicb.2020.578064]

-
Malone, L. A. and H. S. Gatehouse. 1998. Effects of Nosema apis Infection on Honey Bee (Apis mellifera) Digestive Proteolytic Enzyme Activity. J. Invertebr. Pathol. 71: 169-174.
[https://doi.org/10.1006/jipa.1997.4715]

-
Maori, E., S. Lavi, R. Mozes-Koch, Y. Gantman, Y. Peretz, O. Edelbaum, E. Tanne and I. Sela. 2007. Isolation and characterization of Israeli acute paralysis virus, a dicistrovirus affecting honeybees in Israel: evidence for diversity due to intra-and inter-species recombination. J. Gen. Virol. 88: 3428-3438.
[https://doi.org/10.1099/vir.0.83284-0]

- Moeller, F. 1962. Nosema disease control in package bees. Am. Bee J. 102: 390-392.
-
Moore, J., A. Jironkin, D. Chandler, N. Burroughs, D. J. Evans and E. V. Ryabov. 2011. Recombinants between Deformed wing virus and Varroa destructor virus-1 may prevail in Varroa destructor-infested honeybee colonies. J. Gen. Virol. 92: 156-161.
[https://doi.org/10.1099/vir.0.025965-0]

-
Mordecai, G. J., L. Wilfert, S. J. Martin, I. M. Jones and D. C. Schroeder. 2016. Diversity in a honey bee pathogen: first report of a third master variant of the Deformed Wing Virus quasispecies. ISME J 10: 1264-1273.
[https://doi.org/10.1038/ismej.2015.178]

-
Natsopoulou, M. E., D. P. McMahon, V. Doublet, E. Frey, P. Rosenkranz and R. J. Paxton. 2017. The virulent, emerging genotype B of Deformed wing virus is closely linked to overwinter honeybee worker loss. Sci. Rep. 7: 1-9.
[https://doi.org/10.1038/s41598-017-05596-3]

- NCBI. 2022. NCBI Virus: Sequences for discovery [Online]. Available: https://www.ncbi.nlm.nih.gov/labs/virus/vssi/#/virus?SeqType_s=Nucleotide&VirusLineage_ss=Viruses,%20taxid:10239&HostLineage_ss=Apis%20mellifera%20(honey%20bee),%20taxid:7460, [Accessed 12.20 2022].
-
Nguyen, M. and A.-L. Haenni. 2003. Expression strategies of ambisense viruses. Virus Res. 93: 141-150.
[https://doi.org/10.1016/S0168-1702(03)00094-7]

- No, J.-N., N. V. Phu, M.-S. Yoo and B.-S. Yoon. 2010a. Development of Semi-nested PCR Method for the Detection of Israeli Acute Paralysis Virus (IAPV). J. Apic. 25: 97-103.
- No, J.-N., M.-S. Yoo, V. P. Nugyen and B. S. Yoon. 2010b. Development of a Loop-mediated IsothermalAmplification (LAMP) for Easy Detectionof Chronic Bee Paralysis Virus (CBPV). J. Apic. 25: 253-258.
-
Olivier, V., P. Blanchard, S. Chaouch, P. Lallemand, F. Schurr, O. Celle, E. Dubois, N. Tordo, R. Thiéry, R. Houlgatte and M. Ribière. 2008. Molecular characterisation and phylogenetic analysis of Chronic bee paralysis virus, a honey bee virus. Virus Res. 132: 59-68.
[https://doi.org/10.1016/j.virusres.2007.10.014]

-
Ongus, J. R., D. Peters, J.-M. Bonmatin, E. Bengsch, J. M. Vlak and M. M. van Oers. 2004. Complete sequence of a picorna-like virus of the genus Iflavirus replicating in the mite Varroa destructor. J. Gen. Virol. 85: 3747-3755.
[https://doi.org/10.1099/vir.0.80470-0]

- Oudemans, A. C. 1904. On a new genus and species of parasitic acari. Notes from the Leyden Museum 24: 216-222.
-
Payne, S. 2017. Introduction to RNA viruses. Viruses, 97-105.
[https://doi.org/10.1016/B978-0-12-803109-4.00010-6]

-
Pettis, J. S., E. M. Lichtenberg, M. Andree, J. Stitzinger and R. Rose. 2013. Crop pollination exposes honey bees to pesticides which alters their susceptibility to the gut pathogen Nosema ceranae. PLoS One 8: e70182.
[https://doi.org/10.1371/journal.pone.0070182]

-
Popovska Stojanov, D., L. Dimitrov, J. Danihlík, A. Uzunov, M. Golubovski, S. Andonov and R. Brodschneider. 2021. Direct economic impact assessment of winter honeybee colony losses in Three European countries. Agriculture 11: 398.
[https://doi.org/10.3390/agriculture11050398]

-
Ramsey, S. D., R. Ochoa, G. Bauchan, C. Gulbronson, J. D. Mowery, A. Cohen, D. Lim, J. Joklik, J. M. Cicero, J. D. Ellis, D. Hawthorne and D. vanEngelsdorp. 2019. Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci. 116: 1792-1801.
[https://doi.org/10.1073/pnas.1818371116]

-
Ravoet, J., J. Maharramov, I. Meeus, L. De Smet, T. Wenseleers, G. Smagghe and D. C. De Graaf. 2013. Comprehensive bee pathogen screening in Belgium reveals Crithidia mellificae as a new contributory factor to winter mortality. PLoS One 8: e72443.
[https://doi.org/10.1371/journal.pone.0072443]

-
Reddy, K. E., J. H. Noh, S. E. Choe, C. H. Kweon, M. S. Yoo, H. T. T. Doan, M. Ramya, B.-S. Yoon, L. T. K. Nguyen and T. T. D. Nguyen. 2013a. Analysis of the complete genome sequence and capsid region of black queen cell viruses from infected honeybees (Apis mellifera) in Korea. Virus Genes 47: 126-132.
[https://doi.org/10.1007/s11262-013-0902-6]

-
Reddy, K. E., J. H. Noh, Y.-H. Kim, M. S. Yoo, H. T. T. Doan, M. Ramya, S.-C. Jung, D. Van Quyen and S.-W. Kang. 2013b. Analysis of the nonstructural and structural polyprotein regions, and complete genome sequences of Israel acute paralysis viruses identified from honeybees (Apis mellifera) in Korea. Virology 444: 211-217.
[https://doi.org/10.1016/j.virol.2013.06.012]

-
Reddy, K. E., J. H. Noh, M.-S. Yoo, Y.-H. Kim, N.-H. Kim, H. T. T. Doan, M. Ramya, S.-C. Jung, D. Van Quyen and S.-W. Kang. 2013c. Molecular characterization and phylogenetic analysis of deformed wing viruses isolated from South Korea. Vet. Microbiol. 167: 272-279.
[https://doi.org/10.1016/j.vetmic.2013.08.018]

-
Reddy, K. E., M.-S. Yoo, Y.-H. Kim, N.-H. Kim, H.-N. Jung, L. T. B. Thao, M. Ramya, H. T. T. Doan, L. T. K. Nguyen and S.-C. Jung. 2014. Analysis of the RdRp, intergenic and structural polyprotein regions, and the complete genome sequence of Kashmir bee virus from infected honeybees (Apis mellifera) in Korea. Virus Genes 49: 137-144.
[https://doi.org/10.1007/s11262-014-1074-8]

-
Remnant, E. J., M. Shi, G. Buchmann, T. Blacquière, E. C. Holmes, M. Beekman and A. Ashe. 2017. A diverse range of novel RNA viruses in geographically distinct honey bee populations. J. Virol. 91: e00158-00117.
[https://doi.org/10.1128/JVI.00158-17]

-
Retschnig, G., L. A. Kellermann, M. M. Mehmann, O. Yañez, P. Winiger, G. R. Williams and P. Neumann. 2019. Black queen cell virus and drifting of honey bee workers (Apis mellifera). J. Apic. Res. 58: 754-755.
[https://doi.org/10.1080/00218839.2019.1655133]

-
Revell, I. L. 1960. Longevity of refrigerated Nosema spores - Nosema apis, a parasite of honey bees. J. Econ. Entomol. 53: 1132-1133.
[https://doi.org/10.1093/jee/53.6.1132]

- Ribière, M., B. Ball and M. Aubert. 2008. Natural history and geographical distribution of honey bee viruses. Virology and the Honey Bee, European Communities, Luxembourg, 2008, pp. 15-84.
-
Roberts, J. M., D. L. Anderson and P. A. Durr. 2017. Absence of deformed wing virus and Varroa destructor in Australia provides unique perspectives on honeybee viral landscapes and colony losses. Sci. Rep. 7: 1-11.
[https://doi.org/10.1038/s41598-017-07290-w]

-
Roberts, J. M., D. L. Anderson and P. A. Durr. 2018. Metagenomic analysis of Varroa-free Australian honey bees (Apis mellifera) shows a diverse Picornavirales virome. J. Gen. Virol. 99: 818-826.
[https://doi.org/10.1099/jgv.0.001073]

-
Rosenkranz, P., P. Aumeier and B. Ziegelmann. 2010. Biology and control of Varroa destructor. J. Invertebr. Pathol. 103: S96-S119.
[https://doi.org/10.1016/j.jip.2009.07.016]

-
Runckel, C., M. L. Flenniken, J. C. Engel, J. G. Ruby, D. Ganem, R. Andino and J. L. DeRisi. 2011. Temporal analysis of the honey bee microbiome reveals four novel viruses and seasonal prevalence of known viruses, Nosema, and Crithidia. PLoS One 6: e20656.
[https://doi.org/10.1371/journal.pone.0020656]

-
Ryabov, E. V., A. K. Childers, Y. Chen, S. Madella, A. Nessa, D. vanEngelsdorp and J. D. Evans. 2017. Recent spread of Varroa destructor virus-1, a honey bee pathogen, in the United States. Sci. Rep. 7: 1-10.
[https://doi.org/10.1038/s41598-017-17802-3]

- Sanfaçon, H., A. E. Gorbalenya, N. J. Knowles and Y. P. Chen. 2022. Order: Picornavirales, Chapter Version: ICTV Ninth Report; 2009 Taxonomy Release [Online]. ICTV. Available: https://ictv.global/report_9th/RNApos/Picornavirales, [Accessed 2022.12.25].
-
Sanjuán, R., M. R. Nebot, N. Chirico, L. M. Mansky and R. Belshaw. 2010. Viral mutation rates. J. Virol. 84: 9733-9748.
[https://doi.org/10.1128/JVI.00694-10]

-
Santillán-Galicia, M. T., B. V. Ball, S. J. Clark and P. G. Alderson. 2010. Transmission of deformed wing virus and slow paralysis virus to adult bees (Apis mellifera L.) by Varroa destructor. J. Apic. Res. 49: 141-148.
[https://doi.org/10.3896/IBRA.1.49.2.01]

-
Schäfer, M. O., J. Horenk and C. Wylezich. 2022. Molecular Detection of Malpighamoeba mellificae in Honey Bees. Vet. Sci. 9: 148.
[https://doi.org/10.3390/vetsci9030148]

-
Scherer, W. and H. Hurlbut. 1967. Nodamura virus from Japan: a new and unusual arbovirus resistant to diethyl ether and chloroform. Am. J. Epidemiol. 86: 271-285.
[https://doi.org/10.1093/oxfordjournals.aje.a120737]

-
Schneider, P. and W. Drescher. 1987. Einfluss der Parasitierung durch die Milbe Varroa jacobsoni Oud. auf das Schlupfgewicht, die Gewichtsentwicklung, die Entwicklung der Hypopharynxdrüsen und die Lebensdauer von Apis mellifera L. Apidologie 18: 101-110.
[https://doi.org/10.1051/apido:19870108]

-
Shen, M., L. Cui, N. Ostiguy and D. Cox-Foster. 2005. Intricate transmission routes and interactions between picornalike viruses (Kashmir bee virus and sacbrood virus) with the honeybee host and the parasitic Varroa mite. J. Gen. Virol. 86: 2281-2289.
[https://doi.org/10.1099/vir.0.80824-0]

-
Southwick, E. E. and J. N. Mugaas. 1971. A hypothetical homeotherm: the honeybee hive. Comp. Biochem. Physiol. 40: 935-944.
[https://doi.org/10.1016/0300-9629(71)90282-9]

-
Stabentheiner, A., H. Kovac and R. Brodschneider. 2010. Honeybee colony thermoregulation-regulatory mechanisms and contribution of individuals in dependence on age, location and thermal stress. PLoS One 5: e8967.
[https://doi.org/10.1371/journal.pone.0008967]

-
Steinhauer, N. A., K. Rennich, M. E. Wilson, D. M. Caron, E. J. Lengerich, J. S. Pettis, R. Rose, J. A. Skinner, D. R. Tarpy, J. T. Wilkes and D. vanEngelsdorp. 2014. A national survey of managed honey bee 2012-2013 annual colony losses in the USA: results from the Bee Informed Partnership. J. Apic. Res. 53: 1-18.
[https://doi.org/10.3896/IBRA.1.53.1.01]

-
Thaduri, S., B. Locke, F. Granberg and J. R. de Miranda. 2018. Temporal changes in the viromes of Swedish Varroa-resistant and Varroa-susceptible honeybee populations. PLoS One 13: e0206938.
[https://doi.org/10.1371/journal.pone.0206938]

- Thiéry, R., K. L. Johnson, T. Nakai, A. Schneemann, J. R. Bonami and D. V. Lightner. 2022. Family: Nodaviridae, Chapter Version: ICTV Ninth Report; 2009 Taxonomy Release [Online]. ICTV. Available: https://ictv.global/report_9th/RNApos/Nodaviridae, [Accessed 2022.12.25].
-
Toplak, I., U. Jamnikar Ciglenečki, K. Aronstein and A. Gregorc. 2013. Chronic bee paralysis virus and Nosema ceranae experimental co-infection of winter honey bee workers (Apis mellifera L.). Viruses 5: 2282-2297.
[https://doi.org/10.3390/v5092282]

-
Traynor, K. S., K. Rennich, E. Forsgren, R. Rose, J. Pettis, G. Kunkel, S. Madella, J. Evans, D. Lopez and D. vanEngelsdorp. 2016. Multiyear survey targeting disease incidence in US honey bees. Apidologie 47: 325-347.
[https://doi.org/10.1007/s13592-016-0431-0]

- Truong, A.-T., J.-M. Kim, S.-J. Lim, M.-S. Yoo and B. Yoon. 2017. Sacbrood virus (SBV) genotypes detection by Ultra-Rapid Real-time PCR. J. Apic., 48-48.
-
Valles, S., Y. Chen, A. Firth, D. A. Guérin, Y. Hashimoto, S. Herrero, J. De Miranda, E. Ryabov and I. R. Consortium. 2017. ICTV virus taxonomy profile: Dicistroviridae. J. Gen. Virol. 98: 355.
[https://doi.org/10.1099/jgv.0.000756]

- vanEngelsdorp, D., D. C. Foster, M. Frazier, N. Ostiguy and J. Hayes. 2007. “Fall-dwindle Disease”: Investigations Into the Causes of Sudden and Alarming Colony Losses Experience by Beekeepers in the Fall of 2006. Florida Department of Agriculture.
-
Villarreal, L. P. 2004. Are viruses alive? Sci. Am. 291: 100-105.
[https://doi.org/10.1038/scientificamerican1204-100]

-
Wang, D.-I. and F. Mofller. 1970. The division of labor and queen attendance behavior of Nosema-infected worker honey bees. J. Econ. Entomol. 63: 1539-1541.
[https://doi.org/10.1093/jee/63.5.1539]

- Wang, J. H., J. G. Lee and B. S. Yoon. 2015. Convenient and Rapid Detection Method for Classification of Sacbrood Virus (SBV) Based on Mutant Nucleotide Sequence using Ultra-Fast High-Performance PCR (UF-HP PCR). J. Apic., 31-31.
-
Ward, L., R. Waite, N. Boonham, T. Fisher, K. Pescod, H. Thompson, P. Chantawannakul and M. Brown. 2007. First detection of Kashmir bee virus in the UK using real-time PCR. Apidologie 38: 181-190.
[https://doi.org/10.1051/apido:2006072]

-
White, G. F. 1913. Sacbrood, a disease of bees. US: Government Printing Office.
[https://doi.org/10.5962/bhl.title.56965]

-
Wolf, S., E. Nicholls, A. M. Reynolds, P. Wells, K. S. Lim, R. J. Paxton and J. L. Osborne. 2016. Optimal search patterns in honeybee orientation flights are robust against emerging infectious diseases. Sci. Rep. 6: 1-10.
[https://doi.org/10.1038/srep32612]

- Yoo, M.-S., Y. S. Choi, Y.-H. Park and B.-S. Yoon. 2010a. Development of Real-Time PCR Method for the Detection of Chronic Bee Paralysis Virus. J. Apic. 25: 31-37.
- Yoo, M.-S., I.-W. Kim, M.-H. Kang, S.-H. Han and B.-S. Yoon. 2008a. Development of Real-Time PCR Method for Balck Quen Cell Virus. J. Apic. 23: 37-42.
- Yoo, M.-S., I.-W. Kim, M.-H. Kang, S.-H. Han and B.-S. Yoon. 2008b. Development of Real-Time PCR Methodfor the Detection of Kashmir Bee Virus and Israel Acute Paralysis Virus. J. Apic. 23: 97-102.
- Yoo, M.-S., J.-N. No, Y. S. Choi, Y.-H. Park and B.-S. Yoon. 2010b. Development of ultra-rapid real-time PCR method for the detection of chronic bee paralysis virus. J. Apic. 25: 193-199.
- Yoo, M.-S., K. C. N. Thi, D.-S. Kim, I.-U. Kim, S.-H. Kwon and B.-S. Yoon. 2009. Development of Real-Time PCR Method for the Detection of Israel Acute Paralysis Virus. J. Apic. 24: 31-36.
- Yoo, M.-S. and B.-S. Yoon. 2009a. Development of Ultra-rapid Reverse Transcriptase Real-time PCR for the Detection of Israel acute paralysis virus (IAPV). J. Apic., 12-12.
- Yoo, M.-S. and B.-S. Yoon. 2009b. Incidence of Honeybee Disease in Korea 2009. J. Apic. 24: 273-278.
- Yoo, M. S., J. H. Noh, Y. H. Kim, H. T. T. Doan, S. E. Choe, K. E. Reddy and S. H. Kang. 2013. Development of Ultra-Rapid Real-Time PCR method for the Detection of Korean Sacbrood Virus (KSBV). Korean J. Vet. Res. 53.
-
Yue, C. and E. Genersch. 2005. RT-PCR analysis of Deformed wing virus in honeybees (Apis mellifera) and mites (Varroa destructor). J. Gen. Virol. 86: 3419-3424.
[https://doi.org/10.1099/vir.0.81401-0]

-
Zacepins, A., A. Kviesis, V. Komasilovs and R. Brodschneider. 2021. When It Pays to Catch a Swarm - Evaluation of the Economic Importance of Remote Honey Bee (Apis mellifera) Colony Swarming Detection. Agriculture 11: 967.
[https://doi.org/10.3390/agriculture11100967]

- Zander, E. 1909. Tierische parasiten als krankenheitserreger bei der biene. Münchener Bienenzeitung 31: 196-204.
-
Zárate, S., B. Taboada, M. Yocupicio-Monroy and C. F. Arias. 2017. Human virome. Arch. Med. Res. 48: 701-716.
[https://doi.org/10.1016/j.arcmed.2018.01.005]

- Zheng, H.-Q., H.-R. Gong, S.-K. Huang, A. Sohr, F.-L. Hu and Y. P. Chen. 2015. Evidence of the synergistic interaction of honey bee pathogens Nosema ceranae and deformed wing virus. Vet. Microbiol. 177: 1-6.