Morphological Prevalence on Characterization of Varroa Mites from Myanmar
Abstract
Varroosis is a common disease affecting honeybees worldwide, caused by Varroa destructor mites. This external parasite infects Apis mellifera worker, drone, queen, larvae, and pupae, causing lower survival rates and colony density. The study measured the width, length, and distance of various aspects of 200 Varroa mite samples. This study examined female V. destructor mites from four groups of Varroa infestation areas such as Nay Pyi Taw (NPT), Magway Region (MGW), Shan State (SHN) and Saggaing Region (SGG). And study the variance in the 18 morphological characters of female Varroa mites. The results showed that SHN and SGG had significantly larger genitoventral shields (p<0.05) than NPT and MGW. They also had significantly larger plural shields (p<0.05) than MGW. The distance between anal setae, tarsus IV, macrochaeta IV, and hypostome setae was also significantly larger in SHN and SGG. The percentage of mite infestations is SSG 95.2%, SHN 3.2%, MGW 1.5%, and NPT 0.1%. Female Varroa mites from infected drone broods of colonies exhibit the most significant variance in morphological measurements.
Keywords:
Varroa mites, Morphological characters, Apis melliferaINTRODUCTION
Beekeeping of the native honeybee species Apis cerana F. and A. mellifera L. has contributed to the development of Eastern and particularly Asian nations as opposed to that of Western countries (Oldroyd and Wongsiri, 2009). Every year has seen a growth in the global beekeeping business, with the beekeeping industry established worldwide in Americas, Europe, Africa, Australasia, and Asia (Chantawannakul et al., 2018). Global honeybee trade and transport of bees have introduced the mite to Europe, Africa, America, and other places, thus successfully transferring them to the new host, the A. mellifera bee. A. cerana bees have a long history of coevolution with Varroa mites leading to a balanced host-parasite relationship. The A. mellifera bees are highly susceptible, and their hygienic traits are insufficient to control the mites.
Many bee mites associated with honeybees were cosmopolitan species that only occasionally enter bee nests, and if they do, cause no significant harm (Sammataro et al., 2000). There are non-parasitic bee mites and parasitic bee mites. Non-parasitic bee mites are pollen feeder. Parasitic mites, which are tiny, blood-feeding parasites of bees and they consumed fat body from bees (Ramsey et al., 2019). There are two main species of parasitic mites for honeybees such as Varroa and Tropilaelaps species which are affected by honeybees (Nanork et al., 2006). Varroa mites are arachnids that belong to subclass Acari and suborder Mesostigmata. Varroa mites are natural parasites on their tropical host A. cerana. Up to four species of Varroa and two species EuVarroa mites have been identified Table 1. V. destructor Korean haplotype is the most common species found on A. mellifera bees (Anderson and Trueman, 2000).
The life cycle of the Varroa mite is very closely associated with its host, the honeybee. The mite has two distinct stages, the phoretic phase on the adult bee and the reproductive phase in the capped brood cells. The female mites phoretic life stage, attached to the adult bee and feeding the fat body. During this phase, the mites can shift to new host individuals in proximity within the hive or during foraging etc. (Rosenkranz et al., 2010). As the adult female mite approaches reproductive maturity, they transfer herself to nurse bees and quickly transported to the brood cells. The mites are attracted to the larvae by means of chemical signals (Le Conte et al., 1989). The mite enters a brood cell before the larvae are capped and move to the bottom of the cell, consuming the larval food and feeding on the larval fat body. The first egg is usually unfertilized and develops into a haploid male. The fertilized eggs develop into females. The males are smaller, pear-shaped and have yellowish colouration. An adult female mite produces 4-6 offspring in a single worker cell. The mite offspring pass through well-defined developmental stages, namely larvae, protonymph, deutonymph and finally the adult stage. The immobile stages referred to as protocrysalis and deutocrysalis. The females gradually change from an oblong shape to an elliptical shape in the final phase of change, which includes a changing colouration to reddish-brown. The developmental time of the offspring from egg to adult is between 5-6 days. The now matured offspring females mate with the single matured male to produce further offspring. The life of the male mite is short and does not have a phoretic stage. The female mites (usually only one) crawl out from the uncapped cells and find new host individuals (Ifantidis, 1983; Ifantidis and Rosenkranz, 1988).
The mites growing stages process from egg to adult is 5-6 days for males and 7-8 days for a female. Once the adult bee emerges from the cell, adult female mites will enter new cells and begin the reproductive cycle again. Mite reproduction is more successful in drone cells than worker cells, the drone cells are larger than worker cells. The mites can stay a long period in the cells for their pre and post capping period. The drone body is bigger the worker, the mites can get higher protein content, and their requirements (Rosenkranz et al., 2010). The Varroa mite morphology and biology make it an excellent appropriate natural parasite of the honeybee life cycle. The size of the female body is 1.0-1.8 mm long by 1.5-1.9 mm wide and is reddish dark brown in colour, while the males are 0.7 mm by 7 mm, and yellowish grey in colour. Varroa mites have 8-legged ectoparasites that infest honeybees and bee colonies. They attach themselves to the bodies of adult bees using their tiny suction feet and feed on the bee fat body using their highly modified piercing mouth parts. The mites snuggle underneath the abdominal sternites to pierce the soft inter-segmental tissues and remain hidden from the hygienic surveillance of the bees (Spivak, 1996). The infestation of the Varroa destructor to the A. mellifera brood knew as varroosis. By feeding on the bee’s fat body, protein content and haemocytes up to 50% and 30%, respectively, which leads to weight loss and deformity in wings and limbs. In addition, the viruses and mites transmitted to the bees can reduce the lifespan of bees. Depending on the climate, season and level of infestation, the health of a honeybee colony can deteriorate within a few months’ time (de Guzman et al., 2008). At the individual level, this parasite infestation results in weight loss leading to reduced lifespan, immune suppression (Yang and Cox-Foster, 2007), decreased learning capabilities and other disorders depending on the level and time of infestation. At the colony level, the overall fitness is reduced due to lower numbers of swarms (Fries and Camazine, 2001), decreased flight performance in infected drones (Duay, 2002), scattered brood, crippled bees, and reduced bee population (Thompson et al., 2014). Extensive infestation of Varroa mites and the associated impact on the bee referred to as varroosis (Boecking and Genersch, 2008). Varroosis is the most devastating disease of honeybees causing widespread colony destruction and economically damaging the beekeeping industry.
Myanmar beekeeping sector faced the heavy infestation of Varroa mites from 2019-2020 (Table 2), during the COVID-19 pandemic condition, the migration of beekeepers was restricted, and the bee colonies cannot move to other places. The most bee colonies are infected by Varroa and Tropilaelaps mites. All the beekeepers lost 50% of their bee colonies during the pandemic condition (DOA, 2022).
MATERIALS AND METHODS
The sample collection of Varroa mites from Apis mellifera colonies from four different regions from Myanmar were collected from 2022. A total of 200 Varroa samples were collected from 4 different places. All Varroa samples were store at 5 mL screw cap tube with 75% ethanol and keep in -4℃ to -20℃ refrigerator until morphology research. Photo taking by Leica Z16 APO disserting microscope attached by Scientific CMOS camera. Statistical analysis takes placed by ANOVA, SPSS V.25. All the 18 measurements take placed such as, Length of dorsal shield, Width of dorsal shield, no. of Lancet setae, width of pleural shield, Length of pleural shield, Width of genitoventral shield, Length of genitoventral shield, Width of anal shield, and width of gnathosome base. Distance between the first pair of setae of sternal shield, Distance between the first and second pairs of setae of sternal shield, Number of setae on sternal shield, Distance between anal setae, Length of tarsus IV, Length of macrochaeta IV, Distance between first and second of hypostome, Distance between second and third setae of hypostome, Distance between third pairs of setae of hypostome collapse (Fig. 2).
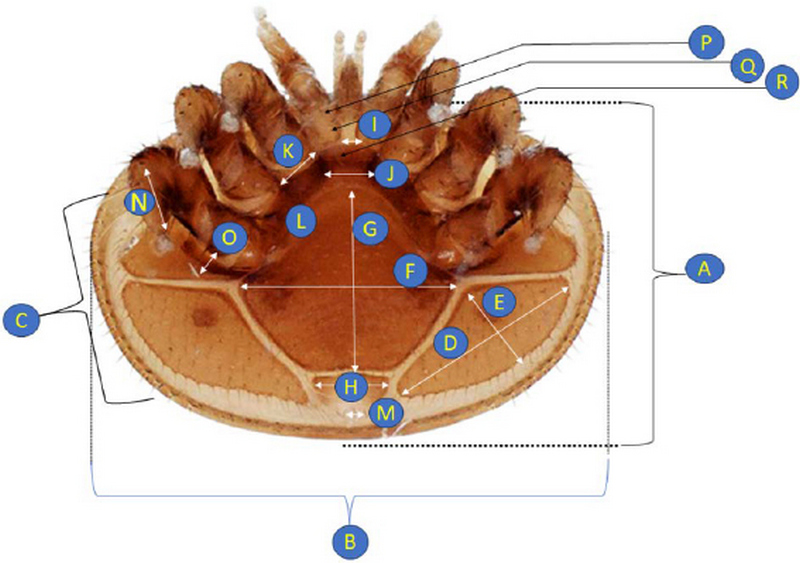
Length of dorsal shield (A), Width of dorsal shield (B), No. of Lancet setae (C), Width of pleural shield (D), Length of pleural shield (E), Width of Genitoventral shield (F), Length of genitoventral shield (G), Width of anal shield (H), Width of gnathosome base (I), Distance between the first pair of setae of sternal shield (J), Distance between the first and second pairs of setae of sternal shield (K), Number of setae on sternal shield (L), Distance between anal setae (M), Length of tarsus IV (N), Length of macrochaeta IV (O), Distance between first and second of hypostome (P), Distance between second and third setae of hypostome (Q), Distance between third pairs of setae of hypostome collapse (R).
RESULTS
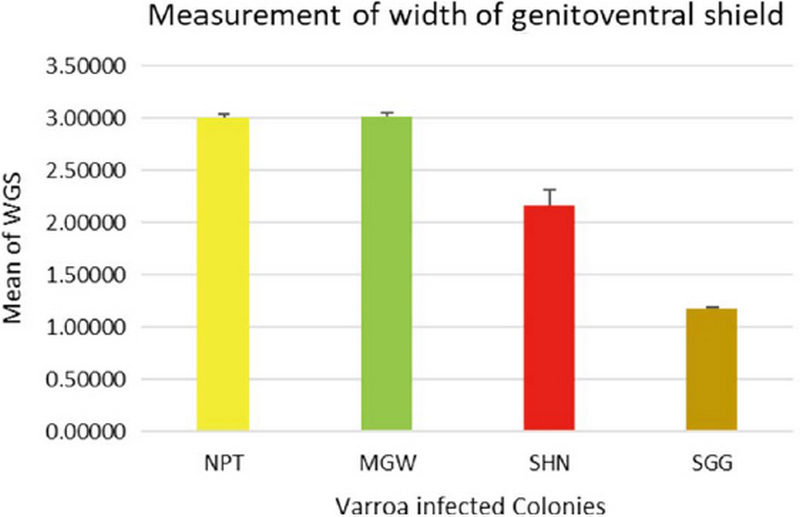
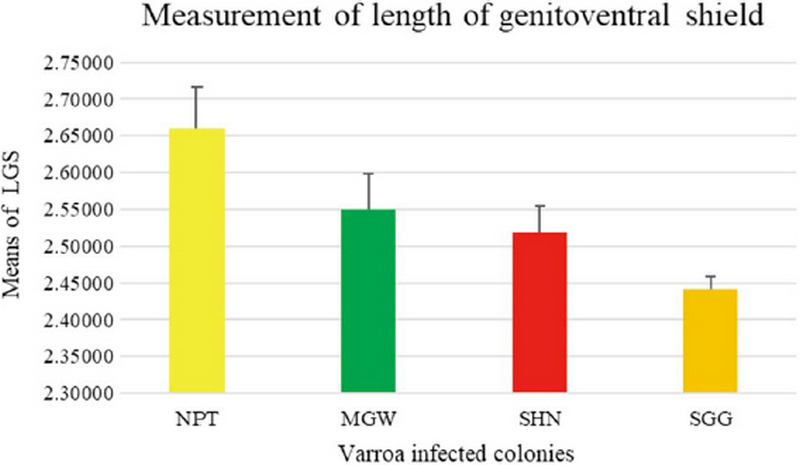
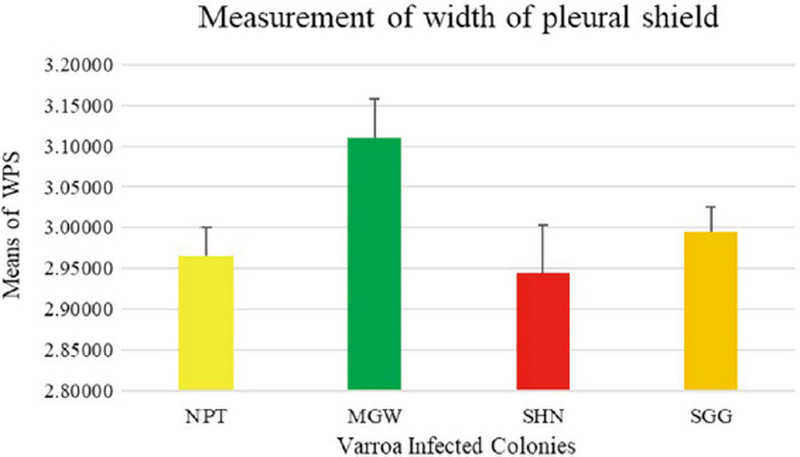
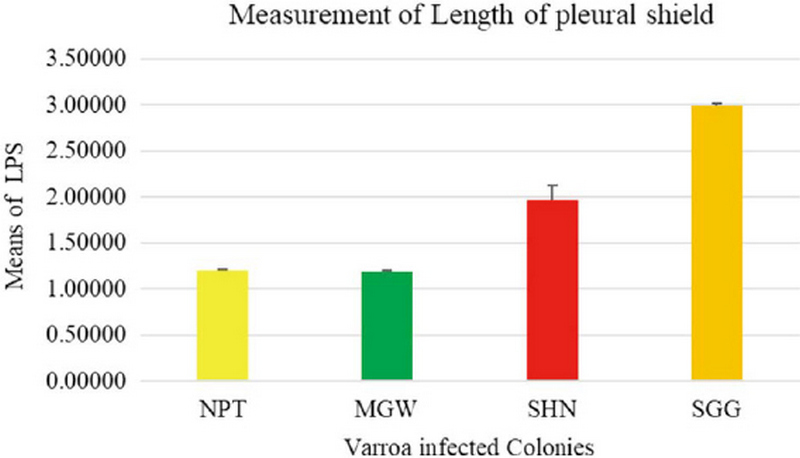
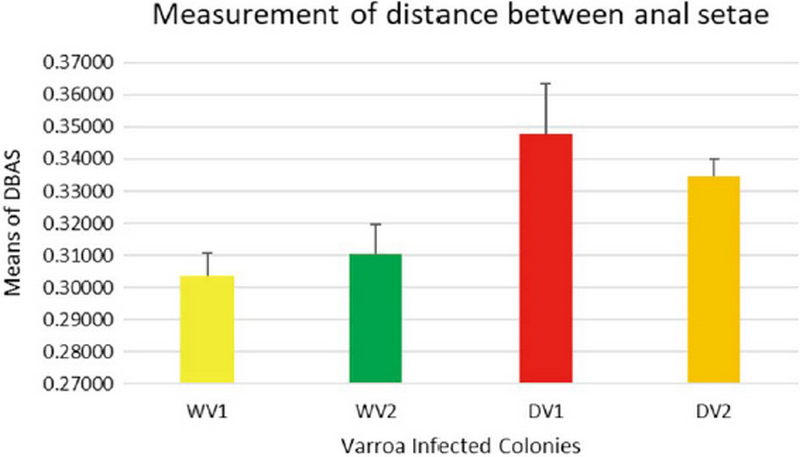
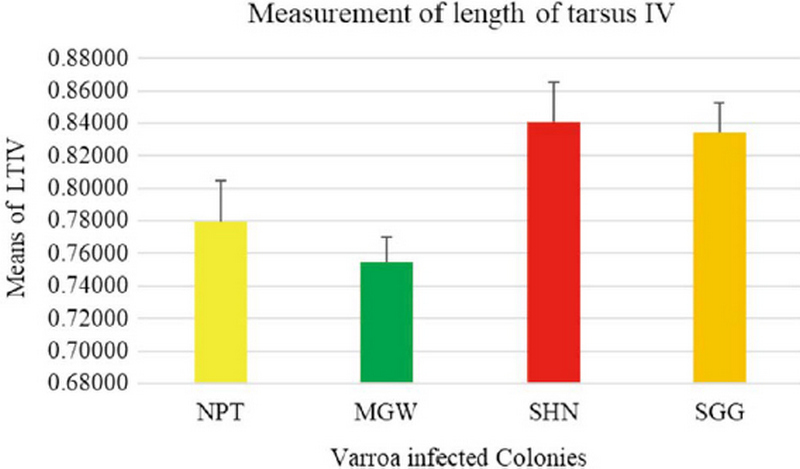
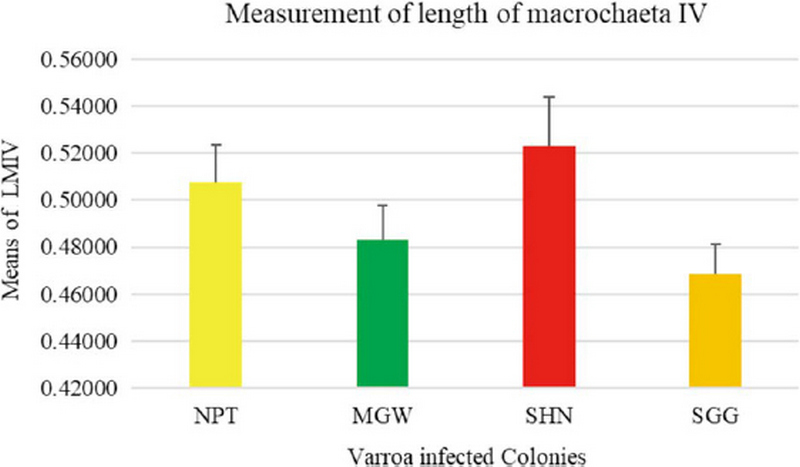
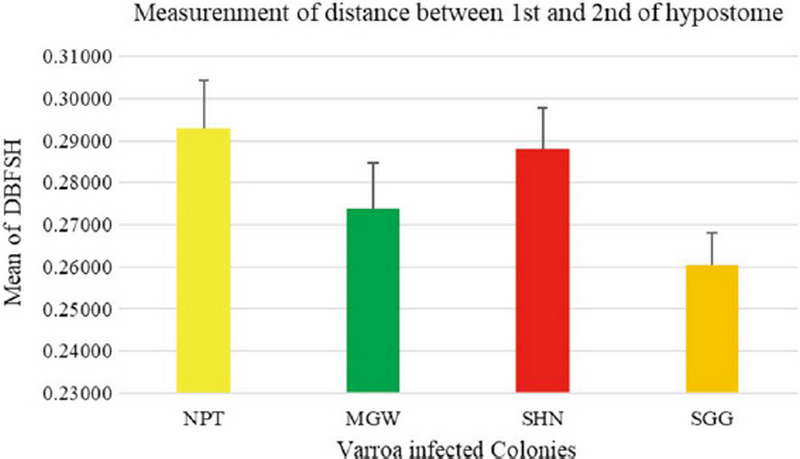
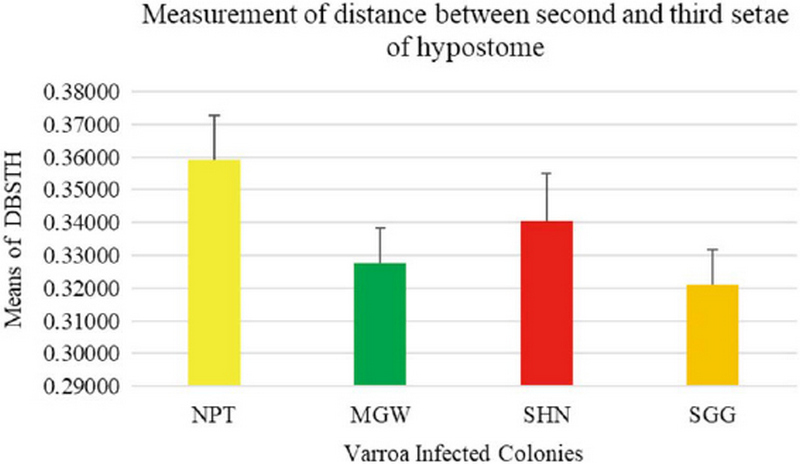
The measurement of 18 placed from Varroa mites were measured all the samples of 200 from four different groups. In this measurement of the Width of Genitoventral Shield of SHN and SGG are (p<0.05) Level significant than NPT and MGW (Fig. 3). And the measurement of the length of Genitoventral Shield of SHN and SGG is (p<0.05) Level significant than NPT (Fig. 4). In measurement of the width of plural Shield SHN is (p<0.05) Level significant than MGW. MGW is (p<0.05) Level significant than NPT and SHN (Fig. 5). In the measurement of Length of plural shield SHN and SGG are (p<0.05) Level significant than NPT and MGW. SGG is (p<0.05) Level significant than SHN (Fig. 6). In this measurement of the distance between anal setae of infected Varroa samples, SHN is (p<0.05) level significant than NPT and MGW. SGG is (p<0.05) level significant than NPT (Fig. 7). In the measurement of the length of tarsus IV of infected Varroa samples SHN is (p<0.05) level significant than NPT and MGW. SGG is (p<0.05) level significant than MGW (Fig. 8). In the measurement of the length of macrochaeta IV SGG is (p<0.05) level significant than SHN (Fig. 9) In the measurement of the distance between 1st and 2nd of hypostome of infected Varroa samples, SGG is (p<0.05) level significant than NPT (Fig. 10). In the measurement of the distance between second and Third setae of hypostome of infected Varroa samples SGG is (p<0.05) level significant than NPT (Fig. 11).
DISCUSSION
18 placed were measured all the samples of 200 Varroa mites. 9 characters were significant between groups and within groups. The development of reproductive area is relation of population. In the four group SGG is outbreak area of Varroa infestation, SHN is heavy infestation area, NPT and MGW are moderate infestation areas. When the surveillance of Varroa outbreak area and heavy infestation area, there have many drone brood frames with Varroa mites than moderate infected areas. When the compare of Varroa samples collected from the drone brood infected colonies have greater the genitoventral areas and pleural areas than the measurement of worker brood infected colonies infected Varroa samples. The normal composition of worker cells and drones cells are different size and different composition of nutrition, when the female Varroa from drone broods are healthier and population than worker broods female Varroa mites. The Varroa mites invade the drone brood cells have 12 times more than worker broods (Boot et al., 1991; Boot, 1995). The number of Varroa infestation is higher in drone brood cells than worker broods (Marcangeli and Damiani, 2017). The Varroa infestation was approximately eight times higher in drone brood cells as compared to worker brood (Fuchs, 1990; Santillán-Galicia et al., 2002; Odemer, 2020). Therefore, the increased number of drone brood combs lead to the population of Varroa mites population increased.
Acknowledgments
The authors would like to thank the Korea Rural Development Administration Alumni Association (KoRAA), the Rural Development Administration (RDA). Additionally, thanks to the assistance of the Division of Apiculture, the Laboratory of Honeybee Breeding, and the National Institute of Agriculture Sciences (NIAS) in the Republic of Korea. We also thank the Ministry of Agriculture, Livestock, and Irrigations, Republic of the Union of Myanmar, and the Myanmar-Korea Rural Development Administration Alumni Association (Myan KoRAA). And, thanks to the Rector and staff from the University of Veterinary Science, Yezin, Nay Pyi Taw. Additional appreciation goes out to the Director and Staff from the Division of Apiculture, Livestock Breeding, and Veterinary Department. For their gracious assistance in providing data and information for the Myanmar Beekeeping industry, we thank the President and members of the Myanmar Apicultural Association. This study is part of the “2023 KoRAA Long-Term Training Program” of Rural Development Administration, Republic of Korea. This study was carried out with the support of the Rural Development Administration RandD project (PJ01418006).
References
- Anderson, D. and J. Trueman. 2000. Varroa jacobsoni (Acari: Varroidae) is more than one species. Exp. Appl. Acarol. 24: 165-189.
-
Boecking, O. and E. Genersch. 2008. Varroosis - the ongoing crisis in bee keeping. J. für Verbraucherschutz Leb. 3: 221-228.
[https://doi.org/10.1007/s00003-008-0331-y]

- Boot, W., J. Calis and J. Beetsma. 1991. Invasion of Varroa mites into honeybee brood cells; when do brood cells attract Varroa mites. Paper presented at the Proc. Exp. Appl. Entomol. NEV Amsterdam.
- Boot, W. J. 1995. Invasion of Varroa mites into honey bee brood cells: Wageningen University and Research.
-
Chantawannakul, P., S. Ramsey, K. Khongphinitbunjong and P. Phokasem. 2018. Tropilaelaps mite: an emerging threat to European honey bee. Curr. Opin. Insect Sci. 26: 69-75.
[https://doi.org/10.1016/j.cois.2018.01.012]

-
de Guzman, L. I. and M. Delfinado-Baker. 1996. A new species of Varroa (Acari: Varroidae) associated with Apis koschevnikovi (Apidae: Hymenoptera) in Borneo. Int. J. Acarol. 22(1): 23-27.
[https://doi.org/10.1080/01647959608684077]

-
de Guzman, L. I., T. E. Rinderer and A. M. Frake. 2008. Comparative reproduction of Varroa destructor in different types of Russian and Italian honey bee combs. Exp. Appl. Acarol. 44(3): 227-238.
[https://doi.org/10.1007/s10493-008-9142-1]

- DOA. 2022. Bees Diseases Record in Myanmar. Departmental Report.
-
Duay, P. 2002. Relation between the level of preimaginal infestation by the broodmite Varroa destructor and adult life expectancy in drone honeybees (Hymenoptera: Apidae: Apis mellifera). Entomol. Gen. 26(3): 213-218.
[https://doi.org/10.1127/entom.gen/26/2002/213]

-
Fries, I. and S. Camazine. 2001. Implications of horizontal and vertical pathogen transmission for honey bee epidemiology. Apidologie 32(3): 199-214.
[https://doi.org/10.1051/apido:2001122]

-
Fuchs, S. 1990. Preference for drone brood cells by Varroa jacobsoni Oud in colonies of Apis mellifera carnica. Apidologie 21(3): 193-199.
[https://doi.org/10.1051/apido:19900304]

-
Ifantidis, M. 1983. Ontogenesis of the mite Varroa jacobsoni in worker and drone honeybee brood cells. J. Apic.l Res. 22(3): 200-206.
[https://doi.org/10.1080/00218839.1983.11100588]

-
Ifantidis, M. D. and P. Rosenkranz. 1988. Reproduktion der Bienenmilbe Varroa jacobsoni (Acarina: Varroidae). Entomol. Gen. 14(1): 111-122.
[https://doi.org/10.1127/entom.gen/14/1988/111]

-
Koeniger, N., G. Koeniger, L. L. de Guzman and C. Lekprayoon. 1993. Survival of EuVarroa sinhai Delfinado and Baker (Acari: Varroidae) on workers of Apis cerana Fabr., Apis florea Fabr. and Apis mellifera L. in cages. Apidologie 24(4): 403-410.
[https://doi.org/10.1051/apido:19930407]

-
Le Conte, Y., G. Arnold, J. Trouiller, C. Masson, B. Chappe and G. Ourisson. 1989. Attraction of the parasitic mite Varroa to the drone larvae of honey bees by simple aliphatic esters. Science 245(4918): 638-639.
[https://doi.org/10.1126/science.245.4918.638]

-
Lekprayoon, C. and P. Tangkanasing. 1991. EuVarroa wongsirii, a new species of bee mite from Thailand. Internat. J. Acarol. 17(4): 255-258.
[https://doi.org/10.1080/01647959108683915]

- Marcangeli, J. and N. Damiani. 2017. Infestation levels of the mite Varroa destructor (Acari, Varroidae) in new and old honeybee brood combs of Apis mellifera (Hymenoptera, Apidae). Rev. Soc. Entomol. Argent 66: 1-2.
-
Nanork, P., S. Wongsiri and B. P. Oldroyd. 2006. The reproductive dilemmas of queenless red dwarf honeybee (Apis florea) workers. Behav. Ecol. Sociobiol. 61: 91-97.
[https://doi.org/10.1007/s00265-006-0239-4]

-
Odemer, R. 2020. Reproductive capacity of Varroa destructor in four different honey bee subspecies. Saudi J. Biol. Sci. 27(1): 247-250.
[https://doi.org/10.1016/j.sjbs.2019.09.002]

-
Oldroyd, B. P. and S. Wongsiri. 2009. Asian honey bees: biology, conservation, and human interactions: Harvard University Press.
[https://doi.org/10.2307/j.ctv2drhcfb]

-
Ramsey, S. D., R. Ochoa, G. Bauchan, C. Gulbronson, J. D. Mowery, A. Cohen and D. vanEngelsdorp. 2019. Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci. 116(5): 1792-1801.
[https://doi.org/10.1073/pnas.1818371116]

-
Rosenkranz, P., P. Aumeier and B. Ziegelmann. 2010. Biology and control of Varroa destructor. J. Invertebr. Pathol. 103: S96-S119.
[https://doi.org/10.1016/j.jip.2009.07.016]

-
Sammataro, D., U. Gerson and G. Needham. 2000. Parasitic mites of honey bees: life history, implications, and impact. Annu. Rev. Entomol. 45(1): 519-548.
[https://doi.org/10.1146/annurev.ento.45.1.519]

-
Santillán-Galicia, M., G. Otero-Colina, C. Romero-Vera and J. Cibrián-Tovar. 2002. Varroa destructor (Acari: Varroidae) infestation in queen, worker, and drone brood of Apis mellifera (Hymenoptera: Apidae). Can. Entomol. 134(3): 381-390.
[https://doi.org/10.4039/Ent134381-3]

-
Spivak, M. 1996. Honey bee hygienic behavior and defense against Varroa jacobsoni. Apidologie 27(4): 245-260.
[https://doi.org/10.1051/apido:19960407]

-
Thompson, C. E., J. C. Biesmeijer, T. R. Allnutt, S. Pietravalle and G. E. Budge. 2014. Parasite pressures on feral honey bees (Apis mellifera sp.). PLoS One 9(8): e105164.
[https://doi.org/10.1371/journal.pone.0105164]

-
Yang, X. and D. Cox-Foster. 2007. Effects of parasitization by Varroa destructor on survivorship and physiological traits of Apis mellifera in correlation with viral incidence and microbial challenge. Parasitology 134(3): 405-412.
[https://doi.org/10.1017/S0031182006000710]