
Antiviral Effect of Korean Native Bee-honey against Influenza A Virus in A549 Cells
Abstract
Influenza viruses are responsible for respiratory infections in humans, which results in significant morbidity and mortality. Among the drugs commonly employed to treat influenza virus infections, neuraminidase inhibitors, such as oseltamivir and peramivir, feature prominently. However, the emergence of drug-resistant viruses underscores the need for the development of new anti-influenza medications. Korean Native bee-honey has been used for medicinal and food. Korean native bee-honey exhibits pharmacological effects, including antioxidant, anti-inflammatory, and antimicrobial activity; however, an anti-influenza effect of Korean native bee-honey has not been reported. In this study, we determined whether Korean native bee-honey from nine different regions (samples A-I) exhibits antiviral activity in pre-, co-, and posttreatment assays using the green fluorescent protein (GFP)-tagged influenza A/PR/8/34 (A/PR/8/34-GFP) virus. The results indicated that sample G (native bee-honey in Nonsan-si, Chungcheongnam-do) exhibited inhibitory effects in the pre-treatment assay against IAV. Also, samples C (native bee-honey in Boseong-gun, Jeollanam-do) and H (native bee-honey in Muju-gun, Jeollabuk-do) had anti-influenza effect under co-treatment conditions, and samples D (native bee-honey in Gapyeong-gun, Gyeonggi-do) and F (native bee-honey in Hamyang-gun, Gyeongsangnam-do) showed antiviral activity under post-treatment conditions. These results warrant further studies to identify the active ingredients and mechanisms underlying the anti-influenza effect of Korean native bee-honey.
Keywords:
Influenza A virus, Korean native bee-honey, Antiviral-effect, HoneyINTRODUCTION
Influenza A viruses (IAV), commonly called flu viruses, are highly contagious, constantly evolving pathogens that cause seasonal influenza outbreaks (Sooryanarain and Elankumaran, 2015). Influenza A virus infection can result in a range of symptoms, including fever, cough, sore throat, muscle aches, fatigue, and respiratory issues (Javanian et al., 2021). Severe cases may result in pneumonia, hospitalization, and even death, particularly in vulnerable populations (Attaway et al., 2021). Influenza A virus belongs to the Orthomyxoviridae family and is categorized into several subtypes based on the two major surface proteins: hemagglutinin (HA) and neuraminidase (NA). These two viral glycoproteins are essential for viral entry and exit from host cells (Abbas and Abidin, 2013). There are 18 different HA and 11 different known NA subtypes, which results in numerous possible combinations and many variants (Shao et al., 2017). Preventing and treating IAV involves a combination of vaccination and antiviral medications. The most common method to prevent influenza A virus infection is to administer an influenza vaccine, known as a flu vaccine or flu shot (Ramos and Fernandez-Sesma, 2015). Such vaccines are a preventative measure designed to provide immunity against the influenza virus. In addition, Tamiflu® (oseltamivir phosphate), Relenza® (Zanamivir), and Rapivab® (peramivir) are antiviral medications used to treat influenza A and B (Hussain et al., 2017). Although these drugs are generally considered safe and effective, they may have some side effects. Also, because of the emergence of drug-resistant viral strains, the development of new treatments is essential.
Bee-honey is a naturally sweet substance produced by bees from the nectar of flowers. It has been used for culinary and medicinal purposes for centuries (Chirsanova et al., 2021). Honey is a source of carbohydrates, primarily in the form of glucose and fructose, which provides a quick source of energy. It contains trace amounts of vitamins and minerals, such as vitamin C, calcium, and iron, as well as flavonoids and phenolic compounds (Kamal et al., 2019). Bee-honey may also contain antioxidants, which can protect cells from damage caused by free radicals. It also exhibits natural antibacterial and anti-inflammatory properties that promote healing (Liyanage and Mawatha, 2017).
Native honey refers to honey produced by bees from nectar collected in a specific region or ecosystem (Schaffer et al., 1983; Winfree et al., 2007). The characteristics of native honey can vary significantly depending on the geographical location, climate, and the type of flowers available to the bees in that area (Requier et al., 2019). Korean native bee-honey typically retains the nutritional qualities of the plants from which the nectar is collected. This results in variations in the content of antioxidants, vitamins, minerals, and other bioactive compounds (Namin et al., 2022). The pharmacological effects include: antibacterial, antimicrobial effect, respiratory health, and antioxidant activities (Nikhat and Fazil, 2022). Although various physiological activities associated with Korean native bee-honey have been reported, there are not many region-specific studies involving these honeys. Furthermore, there are no reports on the antiviral effects of Korean native bee-honey from different regions. Therefore, we evaluated the antiviral effects of Korean native bee-honey collected from various regions against influenza viruses.
MATERIALS AND METHODS
1. Cells and virus
The A549 human lung epithelial cell line was obtained from the American Type Culture Collection in Manassas, Virginia, USA and cultured in RPMI 1,640 medium containing 10% fetal bovine serum (Gibco, Grand Island, NY) and 1% penicillin-streptomycin (P/S). The cells were maintained at 37°C in a 5% CO2 atmosphere. Influenza virus strains of A/PR/8/34-green fluorescence protein (GFP, H1N1) were used (Kwon et al., 2021). These strains were obtained from the allantoic fluid of 10-day-old chicken embryos.
2. Korean native bee-honey
Korean native bee-honey from different regions of Korea in 2022 and 2023 was procured through the Korea Beekeeping Cooperative. In addition, some Korean native bee-honey was provided by the Rural Development Administration (Table 1).
3. MTT assay
A549 cells were seeded in 24-well plates at 1×105 cells/wells for 24 h in a CO2 incubator. The cells were treated with various concentrations (1-20 mg/mL) of Korean native bee-honey (A-I) from each region for 24 h. The cells were treated with 5 μg/mL 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) solution at 37℃ for 1 h. After reaction, the resulting purple formazan was dissolved in DMSO and the absorbance was read at 540 nm using an ELISA plate reader (Epoch, Bioteck, USA).
4. Antiviral assay
A549 cells were seeded in 24-well plates at 1×105 cells/wells for 24 h in a CO2 incubator. For the pretreatment assay, cells were pre-treated with various samples of Korean native bee-honey (A-I) for 24 h and then exposed to IAV (10 MOI) for 24 h. Human interferon-beta (IFN-β, 1000 unit) was used as a positive control. For the co-infection assay, various Korean native bee-honey (A-I) sample and IAV were mixed and incubated at 4℃ for 3 h. After incubation, the cells were exposed to the mixture at 4℃ for 3 h, washed, and complete medium was added. For the post-treatment assay, the cells were exposed to IAV-GFP for 3 h and treated with various Korean native bee-honey (A-I) samples for 24 h. After the reaction, viral GFP expression was measured by fluorescence microscopy (Nikon, Tokyo, Japan) and quantitative analysis was performed by flow cytometry (CytoFLEX; Beckman Coulter Inc., California, Pasa dena, USA).
5. Statistical analysis
Data are expressed as the mean±SEM. Statistically significant differences in the mean values between the treatment and control groups were determined using the chi-squared test with Bonferroni’s correction. An analysis of variance (ANOVA) was performed and Tukey’s post hoc test was used to analyze differences among three or more groups.
RESULTS AND DISCUSSION
1. Effect of Korean native bee-honey on cell viability
To determine whether Korean native bee-honey (A-I) exhibited cytotoxicity, we performed an MTT assay. A549 cells were treated with various concentrations (1, 2.5, 5, 10, and 20 mg/mL) of Korean native bee-honey from different regions. As shown in Fig. 1, none of the Korean native bee-honey (A-I) samples exhibited cytotoxicity in A549 cells. According to this data, a concentration of 5 mg/mL was chosen for all samples in the subsequent experiments, given that sample D exhibited cytotoxic effects starting at 10 mg/mL.
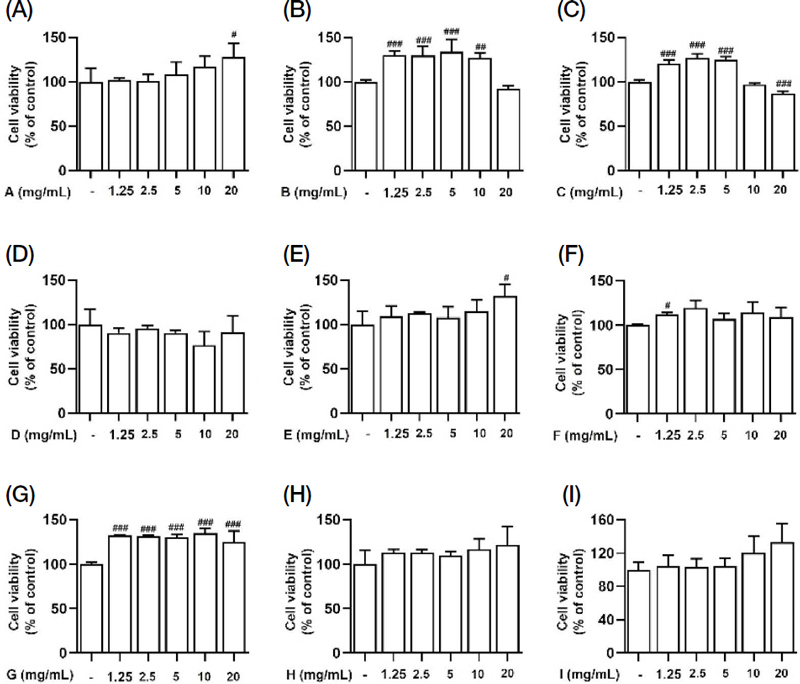
Cytotoxicity of Korean native bee-honey in A549 cells. The cells were treated with various concentrations of Korean native bee-honey (A-I) for 24 h and analyzed by an MTT assay. Bar graph (mean±SEM) statistics were determined using a one-way ANOVA with Tukey’s post hoc test. ###P<0.001, ##P<0.01; #P<0.05, compared with the untreated group.
2. Effect of Korean native bee-honey against IAV under pretreatment conditions
To evaluate the anti-IAV effects of Korean native bee-honey under pretreatment conditions, we used GFP-expressing viruses as previously described. A549 cells were infected with IAV-GFP for 3 h after a 24-h exposure to 5 mg/mL Korean native bee-honey. The results indicated that most Korean native bee-honey samples did not exhibit an antiviral effect under these pretreatment conditions (Fig. 2); however, sample G showed a 13.8% decrease compared with the virus infection group.
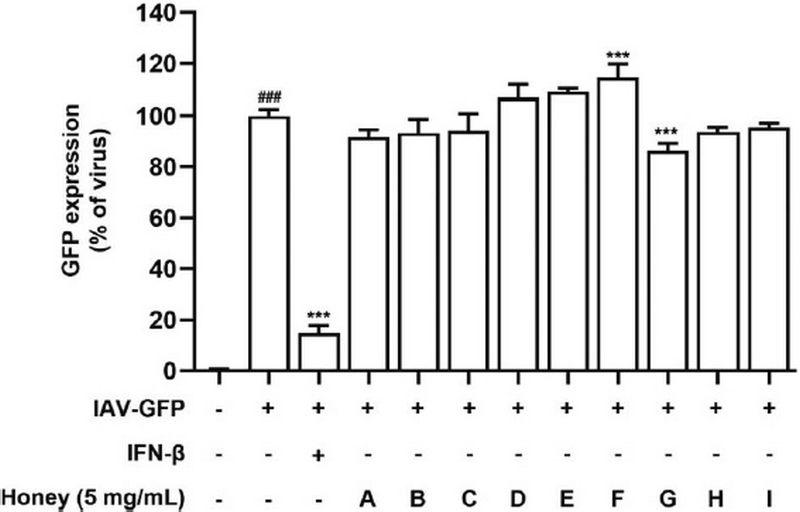
Antiviral effect of Korean native bee honey under pretreatment conditions. A549 cells were treated with Korean native bee honey (A-I) and IFN-β (1000 unit) for 24 h after infection with IAV-GFP for 2 h. IFN-β was used positive control. Bar graph (mean±SEM) statistics were determined using a one-way ANOVA with Tukey’s post hoc test. ###P<0.001, ##P<0.01; #P<0.05, compared with the untreated group. ***P<0.001, **P<0.01; *P<0.05, compared with the IAV-GFP infection group.
3. Effect of Korean native bee honey against IAV during cotreatment conditions
To determine the anti-IAV effects of Korean native bee honey under cotreatment conditions, we used GFP-expressing viruses as previously described. A mixture of 5 mg/mL of Korean native bee honey and IAV-GFP were incubated at 4℃ for 3 h. A549 cells were inoculated with the mixture for 3 h. After 24 hpi, samples C and H exhibited a 22.3% and 22.4% decrease compared with the virus-infection group, respectively. The results indicate that samples C and H have inhibitory activity against IAV-GFP (Fig. 3).
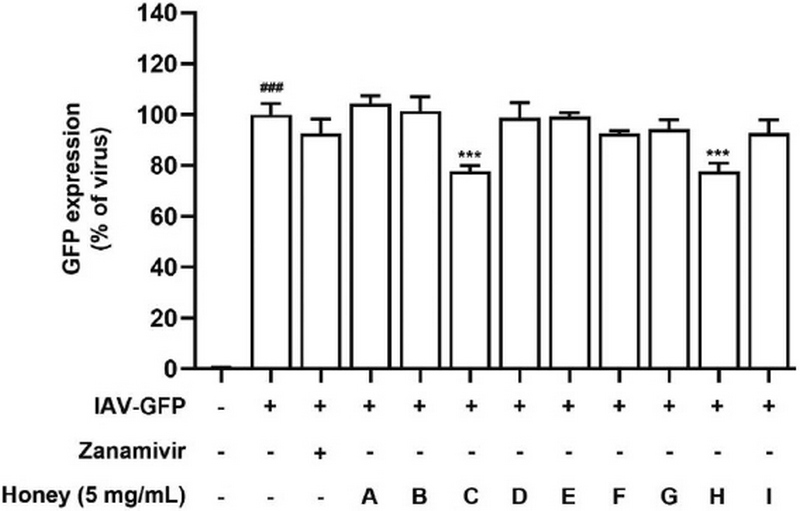
Antiviral effect of Korean native bee-honey under cotreatment conditions. A mixture of 5 mg/mL Korean native bee-honey or 30 μmol/L zanamivir with IAV-GFP was incubated at 4℃ for 3 h. Zanamivir was used as positive control. A549 cells were infected with the virus mixture at 37℃ for 3 h. Virus-expressing GFP was detected by flow cytometry. Bar graph (mean±SEM) statistics were determined using a one-way ANOVA with Tukey’s post hoc test. ###P<0.001, ##P<0.01; #P<0.05, compared with the untreated group. ***P<0.001, **P<0.01; *P<0.05, compared with the IAV-GFP infection group.
4. Effect of Korean native bee honey against IAV under posttreatment conditions
To evaluate the anti-IAV effects of Korean native bee honey under posttreatment conditions, we used GFP-expressing viruses as previously described. A549 cells were infected with IAV-GFP before treating with Korean native bee honey (5 mg/mL). The results indicated that samples D and F suppressed infection by 21.6% and 14.2% compared with the virus-infection group, respectively (Fig. 4). Ribavirin decreased infection by 55.1% compared with the virus-infection group. The results suggest that samples D and F have anti-influenza A virus activity under posttreatment assay conditions.
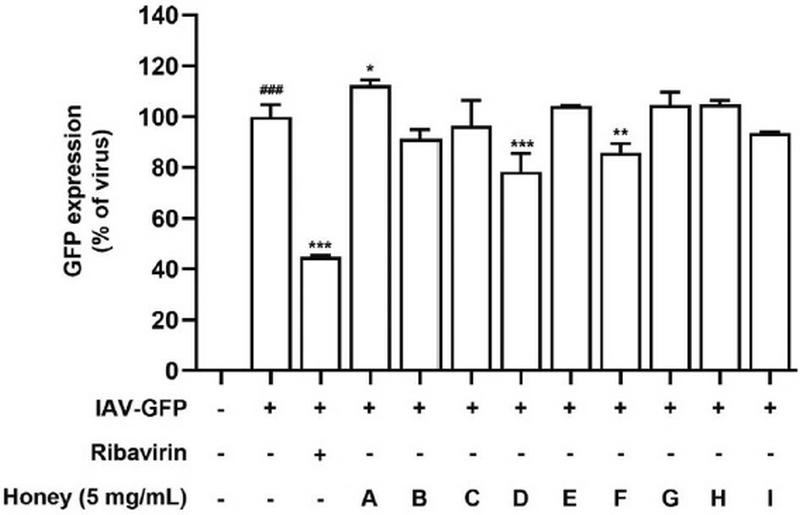
Antiviral effect of native honey under posttreatment conditions. A549 cells were infected with IAV-GFP prior to treatment with Korean native bee-honey samples or or 50 μmol/L ribavirin. Bar graph (mean±SEM) statistics were determined using a one-way ANOVA with Tukey’s post hoc test. ###P<0.001, ##P<0.01; #P<0.05, compared with the untreated group. ***P<0.001, **P<0.01; *P<0.05, compared with the IAV-GFP infection group.
Previous studies have reported the anti-Influenza A virus (IAV) effects of both Tilia amurensis honey and Castanea crenata honey. These varieties of honey demonstrated protective qualities against IAV by enhancing the innate immune system in Raw264.7 cells (Kwon et al., 2022, 2023a). Additionally, chestnut honey has exhibited an antiviral effect against Herpes Simplex Virus type 1 (HSV-1) through the regulation of the reactive oxygen species (ROS)-NLR family pyrin domain containing 3 (NLRP3) inflammasome pathway (Kwon et al., 2023b). Therefore, antiviral research using various honeys is considered necessary. Hence, our study was to investigate the antiviral effects against IAV using native bee-honeys collected from different regions.
We determined whether Korean native bee-honey exhibits antiviral effects using three viral assay conditions (pre-, co- and post). The results indicated that sample G, which harvest in Nonsan-si, Chungcheongnam-do exhibited an inhibitory effect on infection of IAV in the pretreatment assay. In addition, samples C (native bee-honey harvest in Boseong-gun, Jeollanam-do) and H (native bee-honey harvest in Muju-gun, Jeollabuk-do) showed antiviral activity under cotreatment conditions, and samples D (native bee-honey harvest in Gapyeong-gun, Gyeonggi-do) and F (native bee-honey harvest in Hamyang-gun, Gyeongsangnam-do) were inhibiting viral-replication under posttreatment conditions in IAV infection. Thus, Korean native bee-honey exhibits different types of antiviral effects under three conditions (pre-, co- and post). We plan to identify the active components and underlying mechanisms of the anti-influenza effect using domestic honey from this study. Korean native bee honey, which is known for its antiviral effects, may be a potentially useful substance for influenza prevention and treatment, although further research is necessary.
CONCLUSION
After confirming the antiviral effects of Korean native bee-honey from various regions, we established that it exhibits anti-influenza effects under simultaneous and postprocessing conditions. In addition, the antiviral effect analysis conducted under pretreatment conditions on A549 cells revealed it to be effective. In the future, we will identify the components and mechanisms responsible for the anti-influenza efficacy of Korean native bee-honey.
Acknowledgments
Cooperative Research Program for Agriculture Science & Technology Development: RS-2023-00228817.
References
- Abbas, M. and Z. U. Abidin. 2013. Proteins of Influenza Virus: A Review. J. Infect. Mol. Biol. 1: 1-7.
-
Attaway, A. H., R. G. Scheraga, A. Bhimraj, M. Biehl and U. Hatipoğlu. 2021. Severe covid-19 pneumonia: pathogenesis and clinical management. BMJ. 372.
[https://doi.org/10.1136/bmj.n436]

-
Chirsanova, C. A., T. Capcanari, A. Boiştean and E. M. I. Khanchel. 2021. Bee honey: History, characteristics, properties, benefits and adulteration in the beekeeping sector. J. Soc. Sci. IV(3): 98-114.
[https://doi.org/10.52326/jss.utm.2021.4(3).11]

-
Hussain, M., H. D. Galvin, T. Y. Haw, A. N. Nutsford and M. Husain. 2017. Drug resistance in influenza A virus: the epidemiology and management. Infect. Drug Resist. 10: 121-134.
[https://doi.org/10.2147/IDR.S105473]

-
Javanian, M., M. Barary, S. Ghebrehewet, V. Koppolu, V. Vasigala and S. Ebrahimpour. 2021. A brief review of influenza virus infection. J. Med. Virol. 93(8): 4638-4646.
[https://doi.org/10.1002/jmv.26990]

-
Kamal, M. M., M. H. U. Rashid, S. C. Mondal, H. F. El Taj and C. Jung. 2019. Physicochemical and microbiological characteristics of honey obtained through sugar feeding of bees. J. Food Sci. Technol. 56: 2267-2277.
[https://doi.org/10.1007/s13197-019-03714-9]

-
Kwon, E. B., Y. S. Kim, S. M. Han, S. G. Kim and J. G. Choi. 2022. The protective effect of Tilia amurensis honey on influenza A virus infection through stimulation of interferon-mediated IFITM3 signaling. Biomed. Pharmacother. 153: 113259.
[https://doi.org/10.1016/j.biopha.2022.113259]

-
Kwon, E. B., S. G. Kim, Y. S. Kim, B. Kim, S. M. Han, H. J. Lee, H. M. Choi and J. G. Choi. 2023a. Castanea crenata honey reduces influenza infection by activating the innate immune response. Front. Immunol. 14: 1157506.
[https://doi.org/10.3389/fimmu.2023.1157506]

-
Kwon, E. B., Y. C. Oh, Y. H. Hwang, W. Li, S. M. Park, R. Kong, Y. S. Kim and J. G. Choi. 2021. A herbal mixture formula of OCD20015-V009 prophylactic administration to enhance interferon-mediated antiviral activity against influenza A virus. Front. Pharmacol. 12: 764297.
[https://doi.org/10.3389/fphar.2021.764297]

-
Kwon, E. B., Y. S. Kim, B. Kim, S. G. Kim, S. J. Na, Y. H. Go, H. M. Choi, H. J. Lee, S. M. Han and J. G. Choi. 2023b. Korean Chestnut Honey Suppresses HSV-1 Infection by Regulating the ROS-NLRP3 Inflammasome Pathway. Antioxidants 12(11): 1935.
[https://doi.org/10.3390/antiox12111935]

-
Liyanage, D. and B. Mawatha. 2017. Health benefits and traditional uses of honey: A review. J. Apith. 2(1): 9-14.
[https://doi.org/10.5455/ja.20170208043727]

-
Namin, S. M., M. J. Kim, M. Son and C. Jung. 2022. Honey DNA metabarcoding revealed foraging resource partitioning between Korean native and introduced honey bees (Hymenoptera: Apidae). Sci. Rep. 12(1): 14394.
[https://doi.org/10.1038/s41598-022-18465-5]

-
Nikhat, S. and M. Fazil. 2022. History, phytochemistry, experimental pharmacology and clinical uses of honey: A comprehensive review with special reference to Unani medicine. J. Ethnopharmacol. 282: 114614.
[https://doi.org/10.1016/j.jep.2021.114614]

-
Ramos, I. and A. Fernandez-Sesma. 2015. Modulating the innate immune response to influenza A virus: potential therapeutic use of anti-inflammatory drugs. Front. Immunol. 6: 361.
[https://doi.org/10.3389/fimmu.2015.00361]

-
Requier, F., L. Garnery, P. L. Kohl, H. K. Njovu, C. W. Pirk, R. M. Crewe and I. Steffan-Dewenter. 2019. The conservation of native honey bees is crucial. Trends Ecol. Evol. 34(9): 789-798.
[https://doi.org/10.1016/j.tree.2019.04.008]

-
Schaffer, W. M., D. W. Zeh, S. L. Buchmann, S. Kleinhans, M. V. Schaffer and J. Antrim. 1983. Competition for nectar between introduced honey bees and native North American bees and ants. Ecology 64(3): 564-577.
[https://doi.org/10.2307/1939976]

-
Shao, W., X. Li, M. U. Goraya, S. Wang and J. L. Chen. 2017. Evolution of influenza a virus by mutation and re-assortment. Int. J. Mol. Sci. 18(8): 1650.
[https://doi.org/10.3390/ijms18081650]

-
Sooryanarain, H. and S. Elankumaran. 2015. Environmental role in influenza virus outbreaks. Annu. Rev. Anim. Biosci. 3(1): 347-373.
[https://doi.org/10.1146/annurev-animal-022114-111017]

-
Winfree, R., N. M. Williams, J. Dushoff and C. Kremen. 2007. Native bees provide insurance against ongoing honey bee losses. Ecol. Lett. 10(11): 1105-1113.
[https://doi.org/10.1111/j.1461-0248.2007.01110.x]

