The Mechanism of Antiangiogenic Effects of Korean Propolis in Human Umbilical Vein Endothelial Cells
Abstract
We recently reported that the ethanol extracts of Korean propolis (EEKP) possesses antiangiogenic activity both in vitro and in vivo. However, the mechanism of angiogenesis inhibition by Korean propolis have not been fully elucidated. In this study, we investigated the antiangiogenic effects of EEKP on tube-forming human umbilical vein endothelial cells (HUVECs). We found that inhibition of tube formation by EEKP was accompanied by partial fragmentation of endothelial cells, indicating that it induced cell death. Western blotting revealed that EEKP induced inactivation of Raf/MEK/ERK pathway in a concentration-dependent manner. EEKP also induced up regulation of p38 MAPK. It was shown that EEKP induced apoptosis via activation of pro-apoptotic signaling such as caspase cascades. Furthermore, EEKP induced the reduction of vascular endothelial cadherin (VE-cadherin) and platelet endothelial cell adhesion molecule-1 (PECAM-1). These results indicate that EEKP exerts its antiangiogenic effects through induction of HUVECs apoptosis. Korean propolis may have the potential to be developed into pharmaceutical drugs for the treatment of angiogenesis-dependent human diseases such as cancer.
Keywords:
Angiogenesis, Propolis, Tube formation, Human umbilical vein endothelial cells, KoreaINTRODUCTION
Angiogenesis, the growth of new blood vessels from pre-existing ones, is an important process in the pathogenesis of malignant, tumor growth, fibro-proliferative, and inflammatory diseases, and tumor angiogenesis is a promising target for cancer prevention and treatment (Welti et al., 2013). This process is controlled by multiple growth factors and signaling pathways. In addition, the induction of apoptosis in endothelial cells is one of the major antiangiogenic mechanisms employed by various angiogenesis inhibitors (Folkman, 2003). Apoptosis is an inducible form of controlled cell death, and that is also an important phenomenon in the chemotherapeutic agent induced killing of cancer cells.
Propolis is a natural resinous substance collected by honeybees from various plant sources. The composition of propolis depends on the specific local flora at the site of collection and thus on the geographic and climatic characteristics of the site, and their biological activities also vary considerably (Kujumgiev et al., 1999; Bankova et al., 2000; Castaldo and Capasso, 2002). Scheller et al. reported that the ethanolic extract of propolis is capable of increasing survival of mice bearing Ehrlich carcinoma and suggested that immunostimulant activity of propolis may be associated with macrophage activation and enhancement of macrophage phagocytic activity (Scheller et al., 1989). Matsuno reported that various components of propolis have potent anti-inflammatory and antitumor activity (Matsuno, 1995). In addition, Oršolić et al. reported that the antitumor activity of water-soluble propolis extract is not a result of direct cytotoxicity to tumor cells, while the polyphenolic compounds such as caffeic acid and CAPE present in propolis affect tumor growth through the inhibition of DNA synthesis and exert direct antitumor effects by close contact with tumor cells (Oršolić et al., 2005).
Previously, we reported the antioxidant activities and chemical compositions of propolis from all provincial regions in Korea (Choi et al., 2013). We also reported that the ethanol extract of Korean propolis (EEKP) possesses antiangiogenic activity both in vitro and in vivo (Park et al., 2014). In previously report, all EEKP samples (P1-P9) were shown to inhibit tube formation of HUVECs significantly, in a concentration-dependent manner. EEKP samples from Uijeongbu (P1) and Pyoseon (P9) had the strongest and second strongest inhibitory effects, respectively, and also inhibited proliferation of HUVECs in a concentration-dependent manner. Especially EEKP from Pyoseon (P9), a subtropical region, has a different composition from Populus-type propolis, and its plant source is currently unknown. EEKP from Pyoseon (P9) was shown to possess relatively weak antioxidant activity, but strong antiangiogenic effect in previously study. However, the mechanism of angiogenesis inhibition by Korean propolis has not been well-established.
In this study, we investigated the apoptotic effects of Korean propolis on human umbilical vein endothelial cells (HUVECs), focusing on EEKP from Uijeongbu (P1). Furthermore, to clarify the mechanism of angiogenesis inhibition by EEKP, we investigated whether or not EEKP-induced angiogenesis inhibition entailed apoptosis and analyzed changes in survival signals and the apoptotic pathway by western blot assay.
MATERIALS AND METHODS
1. Materials
MCDB-104 medium was purchased from Wako Pure Chemicals Industries (Osaka, Japan). Fetal bovine serum (FBS) from Moregate (Brisbane, Australia), Atelocollagen Bovine Dermis (type I collagen) from Koken (Tokyo, Japan), human basic fibroblast growth factor (bFGF) (Recombinant) from Austral Biologicals (San Ramon, CA, USA) and epidermal growth factor (EGF) from BD Biosciences (Bedford, MA). Polyvinylidene difluoride (PVDF) blotting membranes and Enhanced Chemiluminescence (ECL) SelectTM western blotting detecting solution were obtained from GE Healthcare (Chalfont St. Giles, UK). Unless otherwise stated, Medium199 and all chemicals were purchased from Sigma (St. Louis, MO, USA). All antibodies used in this experiment were from Cell signaling Technology (Beverly, MA).
2. Experimental sample preparation
The propolis sample were provided from honeybee farmer in Uijeongbu Korea, and we prepared the propolis extracts as described below. In brief, the crude propolis (100 mg each) were extracted with ethanol (3 mL) at room temperature for 24 h. The ethanol suspension was separated by centrifugation, and the supernatant was concentrated under reduced pressure to give EEKP. EEKP was stored under dry conditions at 4℃ until analyzed.
3. Cell culture
Cells were grown in MCDB-104 growth medium supplemented with 10% FBS, 10 ng/mL epidermal growth factor (EGF), 100 μg/mL heparin and 100 ng/mL endothelial cell growth factor as previously reported (Ahn et al., 2007). The cells were seeded on plates coated with 0.1% gelatin and allowed to grow to sub-confluence at 37°C and 5% CO2.
4. Tube formation assay
Capillary tube-like structures formed by HUVECs were prepared as previously described with slight modifications (Ahn et al., 2007; Park et al., 2014). Briefly, HUVECs (6.0×104 cells/cm2) were seeded between two layers of collagen gel (0.21% collagen) and incubated in MCDB-104 medium with 0.5% FBS supplemented with 10 ng/mL of bFGF, 8 nM phorbol 12-myristate 13-acetate, and 25 μg/mL ascorbic acid. They were treated with various concentrations of EEKP (0, 6.25, 12.5 and 25 μg/mL) for up to 24 h.
5. Apoptosis
The observation and quantification of apoptosis were conducted as previously described (Ohta et al., 2007). Briefly, tube-forming HUVEC after experimental treatment were fixed with 1% glutaraldehyde overnight at 4℃ and stained with 500 ng/mL of 4′,6-diamidino-2-phenylindole (DAPI) overnight at room temperature. Cells exhibiting chromatin condensation and/or cell nuclear fragmentation were counted as apoptotic cells. A total of more than 500 cells from six fields were counted for each treatment.
6. Western blotting
The cells were suspended three-dimensionally in collagen gel for 6, 12, 24 and 36 h. Western blotting was carried out as previously described (Ohta et al., 2007). In brief, the cellular proteins from tube-forming HUVECs in the 3-D culture model were then separated in a 6-12% SDS-PAGE, and then transferred to a polyvinylidene difluoride (PVDF) blotting membrane. Immunoreactive protein bands were visualized using the ECL or ECL plus detection system (GE Healthcare). Results were obtained from at least three independent experiments.
7. Statistical analysis
The results were presented as the mean±standard error (SE) of either 3 or 6 independent experiments. Data were evaluated statistically using one-way analysis of variance (ANOVA) followed by Holm-Sidak method and using Student t-test for analysis between control and treatments. Statistical significance was preset with *P<0.1, **P<0.01, ***P<0.001.
RESULTS AND DISCUSSION
1. Induction of apoptosis in tube-forming HUVECs
We first investigated the morphology of the cell nuclei to determine whether the cell death induced by EEKP was apoptosis. EEKP induced chromatin condensation and nuclear fragmentation in a concentration-dependent manner, both morphological markers of apoptosis (Fig. 1A). The rates for apoptotic cells were 20.6, 25.1, 30.4 and 36.7% at 0, 6.25, 12.5 and 25 μg/mL, respectively, which were calculated to be a 1.5-, 1.8- and 2.2-fold increase for EEKP (6.25, 12.5 and 25 μg/mL) as compared to the control (Fig. 1B). Thus, it was confirmed that treatment of EEKP from caused cell death by inducing apoptosis in tube-forming HUVECs.
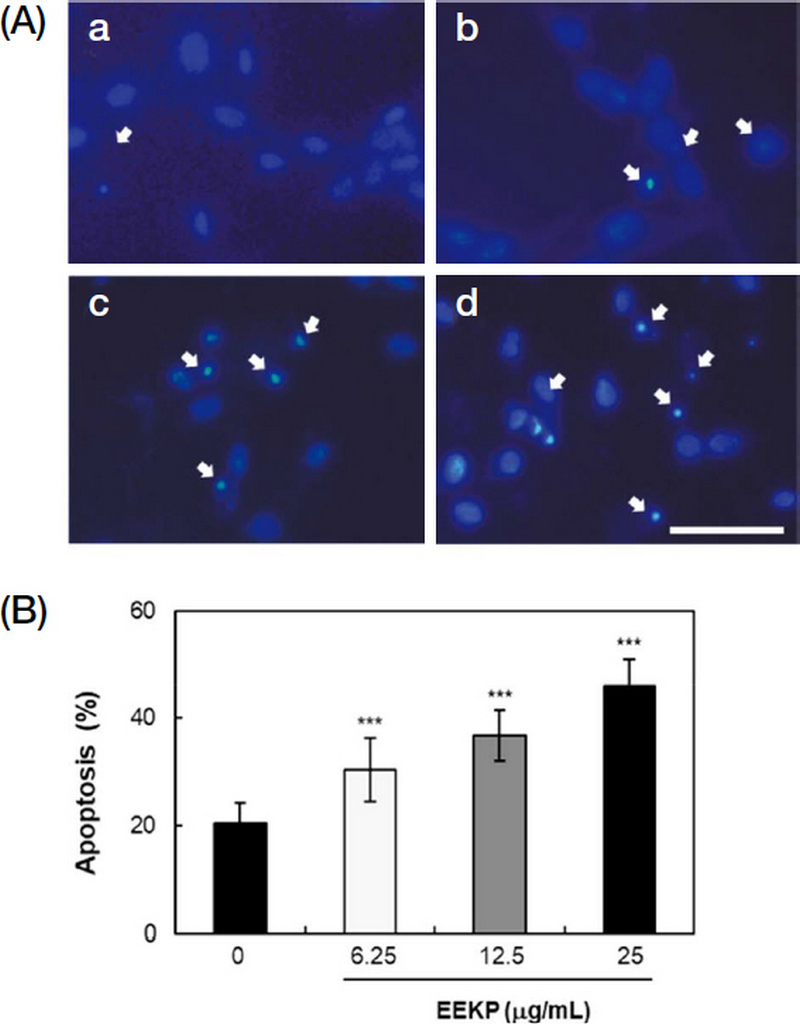
EEKP induces apoptosis in tube-forming HUVECs. (A) The tube-forming cells were treated with various concentrations of EEKP for 24 h, fixed and stained with DAPI to observe cell nuclei. (a) 0 μg/mL, (b) 6.25 μg/mL, (c) 12.5 μg/mL, (d) 25 μg/mL. Representative photos are shown. Scale bar, 50 μm. (B) Rates of apoptosis were quantified. Values are expressed as means±SE (n=6). ***P<0.001 and NS: non-significant vs. control group.
2. Changes in activation of Raf/MEK/ERK in HUVECs
To further clarify the mechanism of angiogenesis inhibition by EEKP, biochemical changes inside the tube-forming HUVECs were analyzed by Western blots. We elucidated how survival signals, Raf/MEK/ERK and p38 MAPK were affected by EEKP (Fig. 2).
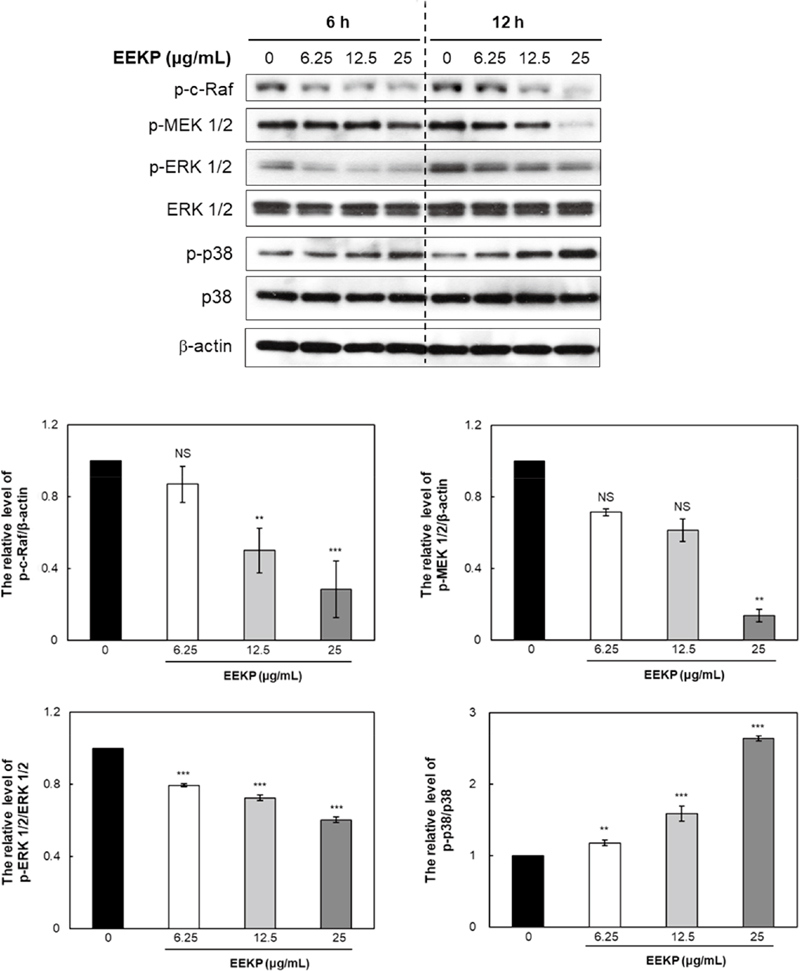
EEKP inactivates Raf/MEK/ERK in tube-forming HUVECs. Cellular proteins were collected from tube-forming HUVECs that were treated with various concentrations of EEKP (0, 6.25, 12.5, and 25 μg/mL) for 6 and 12 h. Values are expressed as means±SE (n=3). ***P<0.001, **P<0.01 and NS: non-significant vs. control group.
RAS controls several pathways that synergistically induce cellular transformation, including the well-characterized Raf/MEK/ERK cascade. Raf kinases are serine/threonine protein kinases that function in this pathway as downstream effector molecules of RAS. RAS localizes Raf to the plasma membrane, where Raf initiates a mitogenic kinase cascade that ultimately modulates gene expression via the phosphorylation of transcription factors (Kunimasa et al., 2011), which can have strong effects on cellular proliferation and oncogenesis. MEK 1/2 is known to directly phosphorylate ERK 1/2, the inhibitor effectively prevents ERK 1/2 activation and its signal transduction (Marshall, 1994). Raf/MEK/ERK signaling in endothelial cells has been shown to play a necessary role in angiogenesis both in vitro and in vivo.
EEKP was shown to inactivate Raf, MEK 1/2 and ERK 1/2 in a concentration-dependent manner (Fig. 2). Treatment of HUVECs with EEKP remarkably decreased the phosphorylation levels of c-Raf, MEK 1/2 and ERK 1/2 within 12 h. Also, phosphorylation of p38 MAPK, which is upstream of caspase-3, was induced in a concentration-dependent manner by EEKP in HUVECs (Fig. 2). This result in this study indicates that EEKP induced inhibition of angiogenesis may be primarily due to blockade of the Raf/MEK/ERK signaling pathway.
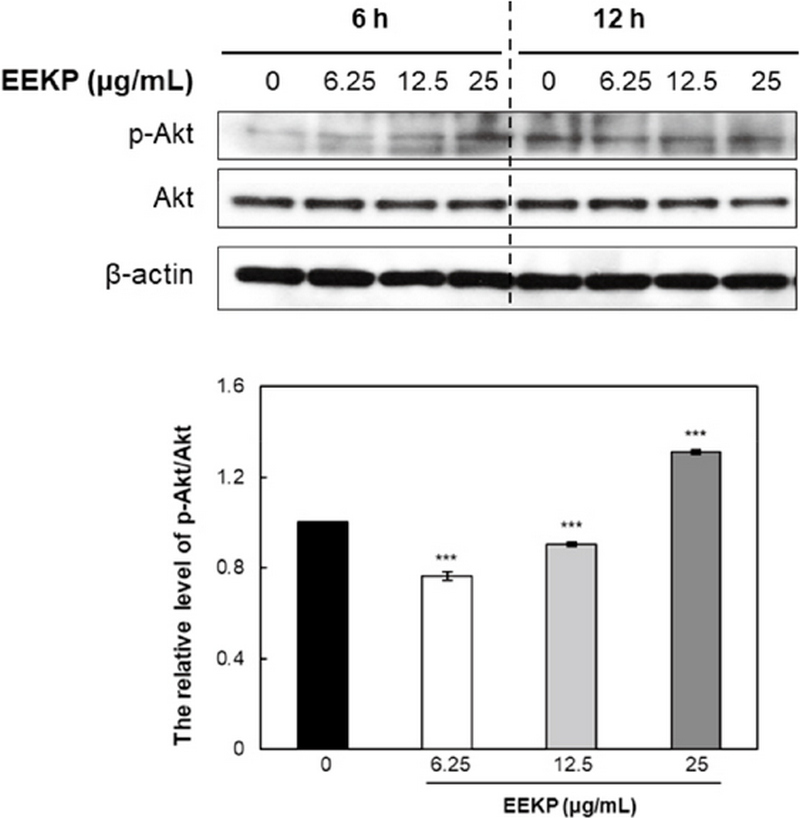
3. Changes in activation of Akt in HUVECs
Akt is a multifunctional regulator of cell survival. Phosphorylation of Akt leads to the modulation of expression of multiple downstream targets involved in apoptosis regulation. Many scientific studies have suggested that Akt controls cell survival, glycogen metabolism, cell transformation and myogenic differentiation and takes center stage in the angiogenesis signaling pathway (Hemmings, 1997; Kennedy et al., 1997). Moreover, some experiments have suggested that VEGF induced endothelial cell migration also requires activation of Akt (Dimmeler et al., 2000).
EEKP was shown to activate Akt in a concentration-dependent manner (Fig. 3). As shown in Fig. 3, treatment with EEKP at a concentration of 25 μg/mL greatly reduced the amount of Akt protein as early as after 6 h of treatment.
4. Changes in activation of the caspase cascade in HUVECs
To confirm the induction of apoptosis by EEKP in endothelial cells, HUVECs were treated with EEKP at various concentrations (0, 6.25, 12.5, and 25 μg/mL) for different time periods (12 and 24 h). After treatment of EEKP, cleavage of caspase-3 dose-dependently increased as early as 12 h after the exposure in HUVECs (Fig. 4). Also, EEKP induced cleaved forms of the caspase substrates, PARP and lamin A/C, in a dose-dependent manner. Pro-caspase-3 is known to be cleaved by upstream caspases and lamin A/C by caspase-3. A number of past reports indicated that PARP could be cleaved by proteases other than caspase-3 (Yang et al., 2004). These results demonstrated that EEKP can induce apoptosis in HUVECs through the cleavage of caspase-3 and the cleavage of PARP and lamin A/C.
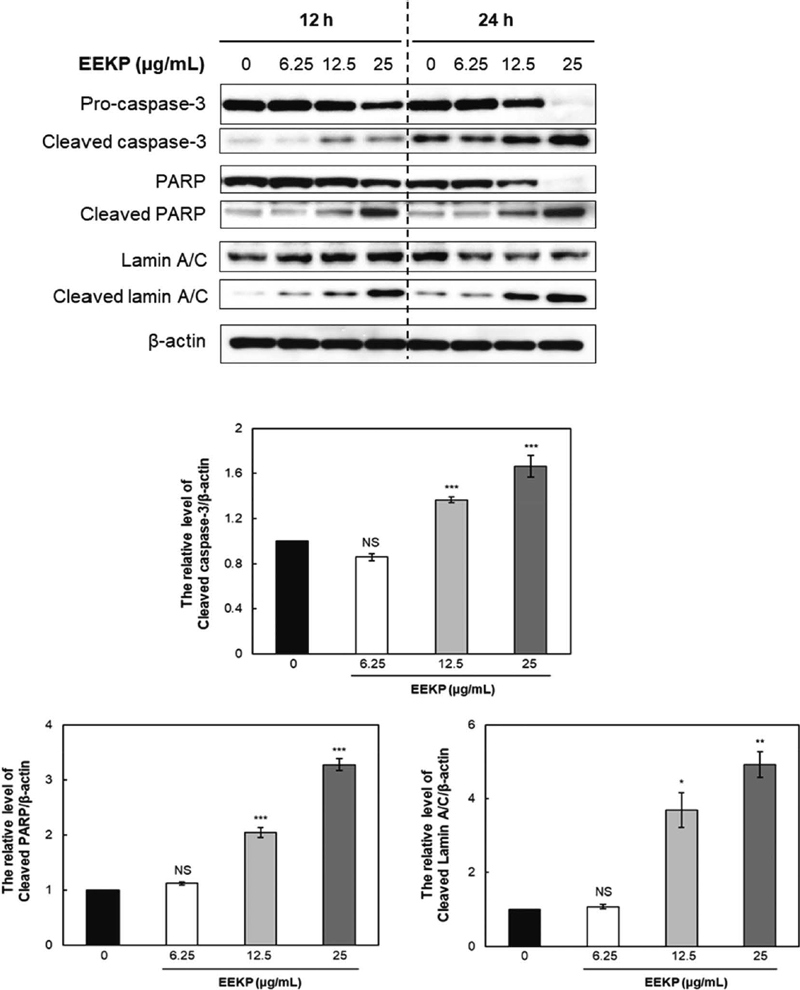
EEKP activates cleaved caspase-3 and induced cleavage of PARP and lamin A/C in tube-forming HUVECs. Cellular proteins were collected from tube-forming HUVECs that were treated with various concentrations of EEKP (0, 6.25, 12.5 and 25 μg/mL) for 12 and 24 h. Values are expressed as means±SE (n=3). ***P<0.001, **P<0.01, *P<0.1 and NS: non-significant vs. control group.
5. Changes in the amount of VE-cadherin and PECAM-1 in HUVECs
To further acquire clues about the inhibitory mechanisms, we examined whether EEKP modulates the protein levels of angiogenesis-related cell adhesion molecules. There were significantly concentration-dependent decrease in VE-cadherin and PECAM-1 protein levels after EEKP treatment (Fig. 5).
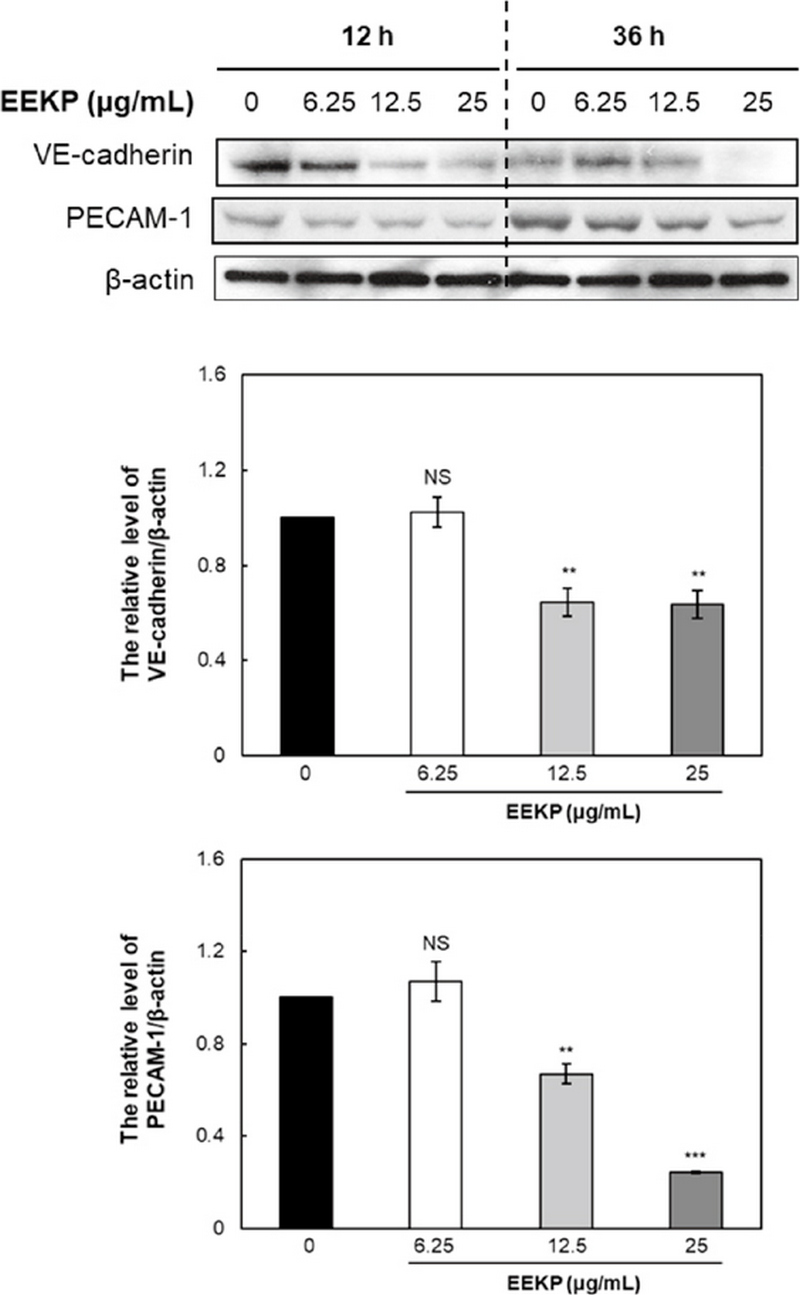
EEKP inactivates VE-cadherin and PECAM-1 in tube-forming HUVECs. Cellular proteins were collected from tube-forming HUVECs that were treated with various concentrations of EEKP (0, 6.25, 12.5 and 25 μg/mL) for 12 and 36 h. Values are expressed as means±SE (n=3). ***P<0.001, **P<0.01 and NS: non-significant vs. control group.
Interendothelial junctions play an important role in the regulation of endothelial functions, such as vasculo-genesis, angiogenesis, and vascular permeability. VE-cadherin is known to the endothelial-specific, and the major component for the adherens junctions between endothelial cells (Li et al., 2010). Liu et al. indicated that VE-cadherin was associated with the cellular apoptosis in the vascular endothelial cells (Liu et al., 2015). PECAM-1 is an important modulator of angiogenic activity of endothelial cell such that in its absence angiogenesis is attenuated both in vitro and in vivo (Park et al., 2010).
CONCLUSION
In conclusion, in this study we confirmed that the antiangiogenic effects of the ethanol extracts of Korean propolis (EEKP) were exerted via induction of apoptosis in tube-forming endothelial cells. Especially, the results indicated that EEKP was a potent apoptosis-inducing agent as well as an angiogenesis inhibitor; its action was accompanied the activation of p38 MAPK, the cleavage of caspase-3 and the cleavage of PARP and lamin A/C. Also, the inhibitory effect of EEKP on tube formation appeared to be mediated by the inactivation of the Raf/MEK/ERK survival signal. Furthermore, EEKP induced the reduction of VE-cadherin and PECAM-1. Therefore, Korean propolis may have a potential to be developed into pharmaceutical drugs for treatment of angiogenesis-dependent human diseases such as tumors.
Acknowledgments
This work was supported by the Dong-A University.
References
-
Ahn, M. R., K. Kunimasa, T. Ohta, S. Kumazawa, M. Kamihira, K. Kaji, Y. Uto, H. Hori, H. Nagasawa and T. Nakayama. 2007. Suppression of tumor-induced angiogenesis by Brazilian propolis: Major component artepillin C inhibits in vitro tube formation and endothelial cell proliferation. Cancer Lett. 252: 235-243.
[https://doi.org/10.1016/j.canlet.2006.12.039]

-
Bankova, V. S., S. L. D. Castro and M. C. Marcucci. 2000. Propolis: recent advances in chemistry and plant origin. Apidologie 31: 3-15.
[https://doi.org/10.1051/apido:2000102]

-
Castaldo, S. and F. Capasso. 2002. Propolis, an old remedy used in modern medicine. Fitoterapia 73: S1-S6.
[https://doi.org/10.1016/S0367-326X(02)00185-5]

-
Choi, S. J., K. Shimomura, S. Kumazawa and M. R. Ahn. 2013. Antioxidant properties and phenolic composition of propolis from diverse geographic regions in Korea. Food Sci. Technol. Res. 19: 211-222.
[https://doi.org/10.3136/fstr.19.211]

-
Dimmeler, S., E. Dernbach and A. M. Zeiher. 2000. Phosphorylation of the endothelial nitric oxide synthase at Ser-1177 is required for VEGF-induced endothelial cell migration. FEBS Lett. 477: 258-262.
[https://doi.org/10.1016/S0014-5793(00)01657-4]

-
Folkman, J. 2003. Angiogenesis and apoptosis. Semin. Cancer Biol. 13: 159-167.
[https://doi.org/10.1016/S1044-579X(02)00133-5]

-
Hemmings, B. A. 1997. Akt signaling: linking membrane events to life and death decisions. Science 275: 628-630.
[https://doi.org/10.1126/science.275.5300.628]

-
Kennedy, S. G., A. J. Wagner, S. D. Conzen, J. Jordan, A. Bellacosa, P. N. Tsichlis and N. Hay. 1997. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes. Dev. 11: 701-713.
[https://doi.org/10.1101/gad.11.6.701]

-
Kujumgiev, A., I. Tsvetkova, Y. Serkedjieva, V. Bankova, R. Christov and S. Popov. 1999. Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J. Ethnopharmacol. 64: 235-240.
[https://doi.org/10.1016/S0378-8741(98)00131-7]

-
Kunimasa, K., M. R. Ahn, T. Kobayashi, R. Eguchi, S. Kumazawa, Y, Fujimori, T. Nakano, T. Nakayama, K. Kaji and T. Ohta. 2011. Brazilian Propolis Suppresses Angiogenesis by Inducing Apoptosis in Tube-Forming Endothelial Cells through Inactivation of Survival Signal ERK1/2. Evid Based. Complement. Alternat. Med. 2011: 870753.
[https://doi.org/10.1093/ecam/nep024]

-
Li, H., W. Shi, J. Liu, C. Hu, X. Zhang, H. Liu, J. Jin, P. Opolon, J.-P. Vannier, M. Perricaudet, A. Janin, C. Soria and H. Lu. 2010. The soluble fragment of VE-cadherin inhibits angiogenesis by reducing endothelial cell proliferation and tube capillary formation. Cancer Gene Ther. 17: 700-707.
[https://doi.org/10.1038/cgt.2010.26]

- Liu, G. Q., H. Y. Wu, J. Xu, M. J. Wang, P. R. Lu and X. G. Zhang. 2015. Anti-apoptosis effects of vascular endothelial cadherin in experimental corneal neovascularization. Int. J. Ophthalmol. 8: 1083-1088.
-
Marshall, C. J. 1994. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr. Opin. Genet. Dev. 4: 82-89.
[https://doi.org/10.1016/0959-437X(94)90095-7]

-
Matsuno, T. 1995. A new clerodane diterpenoid isolated from propolis. Z. Naturforsch. 50c: 93-97.
[https://doi.org/10.1515/znc-1995-1-214]

-
Ohta, T., R. Eguchi, A. Suzuki, S. Miyakaze, R. Ayuzawa and K. Kaji. 2007. Hypoxia-induced apoptosis and tube breakdown are regulated by p38 MAPK but not by caspase cascade in an in vitro capillary model composed of human endothelial cells. J. Cell Physiol. 211: 673-681.
[https://doi.org/10.1002/jcp.20975]

-
Oršolić, N., S. Terzić, Ž. Mihaljević, L. Šver and I. Bašić. 2005. Effects of local administration of propolis and its polyphenolic compounds on tumor formation and growth. Biol. Pharm. Bull. 28: 1928-1933.
[https://doi.org/10.1248/bpb.28.1928]

-
Park, S., T. A. DiMaio, E. A. Scheef, C. M. Sorenson and N. Sheibani. 2010. PECAM-1 regulates proangiogenic properties of endothelial cells through modulation of cell-cell and cell-matrix interactions. Am. J. Physiol. Cell. Physiol. 299: 1468-1484.
[https://doi.org/10.1152/ajpcell.00246.2010]

-
Park, S. I., T. Ohta, S. Kumazawa, M. Jun and M. R. Ahn. 2014. Korean propolis suppresses angiogenesis through inhibition of tube formation and endothelial cell proliferation. Nat. Prod. Commun. 9: 555-560.
[https://doi.org/10.1177/1934578X1400900434]

-
Scheller, S., W. Krol, J. Swiacik, S. Owczarek, J. Gabrys and J. Shani. 1989. Antitumoral property of ethanolic extract of propolis in mice-bearing Ehrlich carcinoma, as compared to bleomycin. Z. Naturforsch. 44c: 1063-1065.
[https://doi.org/10.1515/znc-1989-11-1231]

-
Welti, J., S. Loges, S. Dimmeler and P. Carmeliet. 2013. Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J. Clin. Invest. 123: 3190-3200.
[https://doi.org/10.1172/JCI70212]

-
Yang, Y., S. Zhao and J. Song. 2004. Caspase-dependent apoptosis and -independent poly (ADP-ribose) polymerase cleavage induced by transforming growth factor β1. Int. J. Biochem. Cell Biol. 36: 223-234.
[https://doi.org/10.1016/S1357-2725(03)00215-2]