
Comparative Study on the Antimicrobial and Antioxidant Activity of Propolis
Abstract
Propolis, a mixture of resinous substances gathered by honeybees from various plants, has garnered significant attention due to its wide-ranging activities. In this study, we compare the antimicrobial and antioxidant activities of two commercially available propolis samples obtained from different geographical locations. Using two powder-type propolis samples, Power King Propolis (PKP; collected in Korea) and Beehi Propolis (BP; primarily collected in Australia), we investigated their antimicrobial and antioxidant activities. Propolis exhibited antibacterial activity and protected mammalian cells against oxidative stress, leading to increased cell viability. Furthermore, the comparison of antimicrobial and antioxidant activities between the two propolis samples indicated higher values for PKP compared to BP. These findings indicate that the difference in the antimicrobial and antioxidant activities of propolis can be attributed to variations in its composition, which are influenced by the plant sources collected from different geographical locations.
Keywords:
Honeybee, Propolis, Antimicrobial activity, Antioxidant activity, Reactive oxygen speciesINTRODUCTION
Propolis, an essential product of honeybees, has a rich history of use as a functional food for human health care. Raw propolis is a mixture collected by honeybees from various plant resources, consisting of plant resins, essential oils, beeswax, and pollen (Przybyłek and Karpiński, 2019; Šuran et al., 2021). In general, propolis contains a variety of organic compounds such as polyphenols, terpenes, and esters (Przybyłek and Karpiński, 2019; Šuran et al., 2021). Due to this composition, propolis exhibits diverse biological and pharmacological activities, including antimicrobial, antioxidant, and anti-inflammatory effects (Schnitzler et al., 2010; Ramanausjiene and Inkeniene, 2011; Franchin et al., 2016; Przybyłek and Karpiński, 2019).
The composition of propolis varies according to geographical regions, environmental factors, seasons, and the plant species collected (Bankova, 2005; Bankova et al., 2006; Souza et al., 2016; Rufatto et al., 2018). Honeybees produce propolis from various plant sources, such as poplar, birch, willow, spruce, oak, fir, pine, and acacia (Przybyłek and Karpiński, 2019). Additionally, honeybees use the secretions of Xanthorrhoea in Australia and Baccharis, Araucaria, and Eucalyptus in Brazil as sources for propolis. On the other hand, the chemical composition of propolis also depends on the types of solvents, their ratios, and the extraction procedures (Šuran et al., 2021). Due to the existence of several types of propolis with different chemical compositions, standardization for propolis has been proposed based on plant origin and chemical composition (Bankova, 2005).
While propolis is composed of over 500 bioactive molecules, which are primarily secondary metabolites derived from plants (Huang et al., 2014), and its composition is dependent on the plant sources available in different geographical locations (Wang et al., 2016), it exhibits similar biological and pharmacological activities. In this study, we compare the antimicrobial and antioxidant activities of two commercially available propolis samples, which are similar types of propolis obtained from different geographical locations.
MATERIALS AND METHODS
1. Propolis
Two commercially available powder-type propolis samples were obtained from BN Care Co., Ltd. (Icheon, Korea): Power King Propolis (PKP), collected in Korea, contains 600 mg of total flavonoids per 60 g of total propolis and Beehi Propolis (BP), primarily collected in Australia, contains 676 mg of total flavonoid per 60 g of total propolis. The powder-type propolis samples were dissolved in distilled water at 37℃ for 30 min and then filtered using a 0.45-μm Millipore filter (Sartorius Stedim Biotech., Goettingen, Germany). In this study, we used the filtered propolis samples for further assays.
2. Antimicrobial activity assay
The Gram-positive bacterium Bacillus thuringiensis 656-3 and the Gram-negative bacterium Escherichia coli DH5α were assayed in this study. The antimicrobial activity of propolis was assessed using a liquid growth inhibition method as previously described (Lee et al., 2016). Briefly, bacterial inocula (1×102 cfu per well) were incubated with serial diluted samples of propolis in a 96-well plate. The plate was then incubated in Luria-Bertani (LB) medium at 37℃ for 24 h with shaking at 220 rpm. The growth inhibition caused by the propolis samples was evaluated by measuring the absorbance at 595 nm using a microplate reader (Bio-Rad Model 3550, Bio-Rad). The minimal inhibitory concentration (MIC), which indicates 50% inhibition of bacterial growth by the propolis samples, was determined.
3. Cell viability assays
Cell viability in response to propolis samples was assessed using a murine fibroblast cell line NIH 3T3, following the protocols outlined in Park et al. (2019). The cell line was cultured in Dulbecco’s modified Eagle’s medium (DMEM, Sigma), supplemented with 10% FBS (Gibco BRL), at 37℃ in an atmosphere containing 5% CO2. For the MTT [3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay, NIH 3T3 cells (2×104 cells/well of 96-well plates) were incubated with serial dilutions of propolis samples (0, 1, 100, or 1000 μg per mL of medium) and H2O2 (50 μM) for 24 or 48 h. Subsequently, NIH 3T3 cells were treated with 50 μL of MTT reagent (BioVision, Milpitas, CA, USA) for 4 h. Cell viability was evaluated by measuring absorbance at 590 nm using a microplate reader (Bio-Rad Model 3550).
4. Measurement of reactive oxygen species (ROS) and iron levels
NIH 3T3 cells (2×104 cells/well of 96-well plates) were incubated with serial dilutions of propolis samples (0, 1, 100, or 1000 μg per mL of medium) and H2O2 (50 μM) for 24 or 48 h as described above. The levels of ROS and iron in the cell culture medium were quantified using the OxiSelect In Vitro ROS/RNS Assay Kit (Green Fluorescence; Cell Biolabs, Inc., San Diego, CA, USA) and Iron Colorimetric Assay Kit (BioVision Inc., Milpitas, CA, USA) following the manufacturers’ instructions. The experiments were performed three times using independent samples.
5. Statistical analysis
Results are presented as the mean±standard deviation (SD) of triplicates. The data were analyzed using a two-sample t-test by R package. Statistically significant differences are denoted with asterisks as follows: *, p<0.05; **, p<0.01; ***, p<0.001.
RESULTS AND DISCUSSION
To compare the antimicrobial and antioxidant activities of two commercially available powder-type propolis samples (PKP and BP), we dissolved the propolis samples in distilled water at 37℃. However, they did not completely dissolve. Therefore, we assessed the antibacterial and antioxidant activities of the two propolis samples by filtering them using a 0.45 μm Millipore filter to exclude potential contamination from specific sources. We found that the antibacterial activity was significantly higher in the propolis samples that were not filtered compared to the filtered ones (Fig. 1A). This result indicates that non-filtered propolis samples were much more effective than filtered propolis samples. In both propolis samples, regardless of filtering, the antibacterial activity was higher in the PKP samples compared to the BP samples (Fig. 1B), and both samples exhibited stronger antibacterial activity against B. thuringiensis than E. coli (Fig. 1B). Thus, our results confirmed that propolis acts as an antimicrobial agent, exhibiting stronger activity against Gram-positive bacteria than Gram-negative bacteria (Przybyłek and Karpiński, 2019).
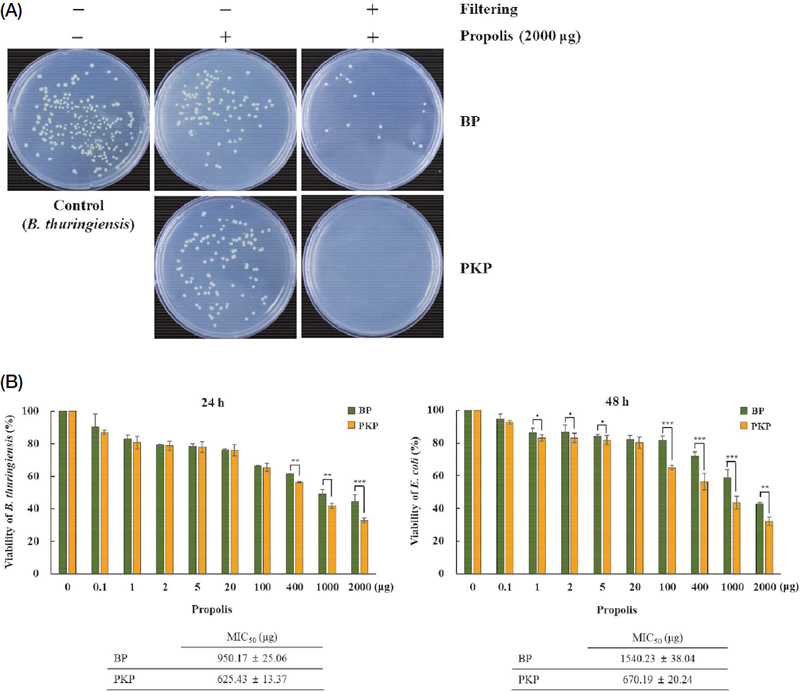
Antimicrobial activity of propolis samples. (A) Plate assay of the antibacterial activity of PKP and BP propolis samples with and without filtering. (B) Antimicrobial activity of PKP and BP propolis samples against bacteria (n=3). *, p<0.05; **, p<0.01; ***, p<0.001.
We assessed the effect of propolis samples on cell viability against H2O2-mediated toxicity in NIH 3T3 cells. Interestingly, the cell viability assays revealed a concentration-dependent cell protection effect of propolis samples, with the PKP sample showing higher viability compared to BP (Fig. 2A). Additionally, our results showed that propolis samples decreased the levels of iron and ROS in NIH 3T3 cells exposed to H2O2 (Fig. 2B and 2C), indicating that propolis exhibits an antiapoptotic effect by reducing the levels of iron and ROS, which are mediators of cell damage (Park et al., 2021). Thus, we confirmed the antioxidant activity of propolis in H2O2-exposed NIH 3T3 cells, underscoring its role as an antioxidant agent.
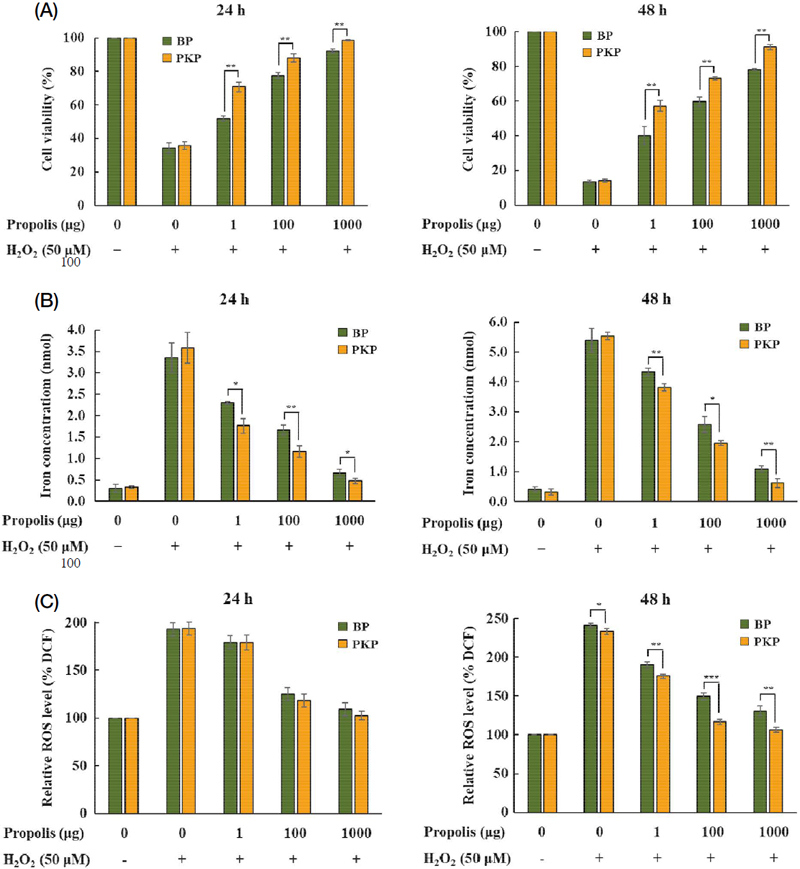
Antioxidant activity of propolis samples. NIH 3T3 cells were treated with PKP and BP propolis samples and H2O2. The percentage of cell viability (A), iron concentration (B), and relative ROS level (C) were determined at 24 and 48 h (n=3). *, p<0.05; **, p<0.01; ***, p<0.001.
The findings of this study have confirmed that propolis acts as both an antimicrobial and antioxidant agent. Despite numerous attempts to increase its solubility in water, propolis, also known as bee glue, does not completely dissolve due to its resinous nature (Marcucci, 1995). Therefore, we were interested in investigating whether filtering propolis partially dissolved would affect its biological properties. The chemical composition and biological activity of propolis vary depending on the extraction methods and procedures (Taddeo et al., 2016; Šuran et al., 2021), types of propolis (Machado et al., 2016), and collection regions (Siheri et al., 2016; Wang et al., 2016). Previous studies have demonstrated significant variations in the total phenolic contents and biological properties of ethanol-extracted propolis from 20 different regions in Korea (Wang et al., 2016). Similarly, the chemical and antimicrobial profiling of propolis collected from different regions within Libya has resulted in its classification into several groups (Siheri et al., 2016). Collectively, the biological and pharmacological activities of propolis depend on its chemical composition, which varies according to geographical locations and even within the same countries (Przybyłek and Karpiński, 2019).
In conclusion, our data demonstrate that propolis samples, when subjected to filtering, act as antimicrobial agents and antioxidants, effectively protecting against oxidative stress. These findings highlight the crucial role of the two propolis samples with filtering, ensuring their reliability in terms of antimicrobial and antioxidant effects.
Acknowledgments
This work was supported by a grant from the Nanum Bio Co., Ltd., Korea. We thank President Ryong Rim Lee, BN Care Co., Ltd. for providing propolis samples.
CONTRIBUTION OF AUTHORS
BYK, HJY, and KSL performed experiments and analyses. KSL and BRJ designed the study and drafted the manuscript. All authors helped write the manuscript.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
References
-
Bankova, V. 2005. Chemical diversity of propolis and its problem standardization. J. Ethnopharmacol. 100: 114-117.
[https://doi.org/10.1016/j.jep.2005.05.004]

-
Bankova, V., M. Popova and B. Trusheva. 2006. Plant sources of propolis: An update from a chemist’s point of view. Nat. Prod. Commun. 1: 1023-1028.
[https://doi.org/10.1177/1934578X0600101118]

-
Franchin, M., D. F. Cólon, F. V. Castanheira, M. G. da Cunha, B. Bueno-Silva, S. M. Alencar, T. M. Cunha and P. L. Rosalen. 2016. Vestitol isolated from Brazilian red propolis inhibits neutrophils migration in the inflammatory process: Elucidation of the mechanism of action. J. Nat. Prod. 79: 954-960.
[https://doi.org/10.1021/acs.jnatprod.5b00938]

-
Huang, S., C. P. Zhang, K. Wang, G. Q. Li,and F. L. Hu. 2014. Recent advaces in the chemical composition of propolis. Molecules 19: 19610-19632.
[https://doi.org/10.3390/molecules191219610]

-
Lee, K. S., B. Y. Kim, H. J. Yoon, Y. S. Choi and B. R. Jin. 2016. Secapin, a bee venom peptide, exhibits anti-fibrinolytic, anti-elastolytic, and anti-microbial activities. Dev. Comp. Immunol. 63: 27-35.
[https://doi.org/10.1016/j.dci.2016.05.011]

-
Machado, C. S., J. B. Mokochinski, T. O. de Lira, F. C. E. de Oliveira, M. V. Cardoso, R. G. Ferreira, A. C. H. F. Sawaya, A. G. Ferreira, C. Pessoa, O. Cuesta-Rubio, M. C. Monteiro, M. S. de Campos and Y. R. Torres. 2016. Comparative study of chemical composition and biological activity of yellow, green, brown, and red Brazilian propolis. Evid. Based Complement. Alternat. Med. 2016: 6057650.
[https://doi.org/10.1155/2016/6057650]

-
Marcucci, M. C. 1995. Propolis: chemical composition, biological properties and therapeutic activity. Apidologie 26: 83-99.
[https://doi.org/10.1051/apido:19950202]

-
Park, H. G., B. Y. Kim, J. M. Kim, Y. S. Choi, H. J. Yoon, K. S. Lee and B. R. Jin. 2021. Upregulation of transferrin and major royal jelly proteins in the spermathecal fluid of mated honeybee (Apis mellifera) queens. Insects 12: 690.
[https://doi.org/10.3390/insects12080690]

-
Park, M. J., B. Y. Kim, H. G. Park, Y. Deng, H. J. Yoon, Y. S. Choi, K. S. Lee and B. R. Jin. 2019. Major royal jelly protein 2 acts as an antimicrobial agent and antioxidant in royal jelly. J. Asia-Pac. Entomol. 22: 684-689.
[https://doi.org/10.1016/j.aspen.2019.05.003]

-
Przybyłek, I. and T. Karpiński. 2019. Antibacterial properties of propolis. Molecules 24: 2047.
[https://doi.org/10.3390/molecules24112047]

-
Ramanauskiene, K. and A. Inkeniene. 2011. Propolis oil extract: Quality analysis and evaluation of its antimicrobial activity. Nat. Prod. Res. 25: 1463-1468.
[https://doi.org/10.1080/14786419.2010.529440]

-
Rufatto, L. C., P. Luchtenberg, C. Garcia, C. Thomassigny, S. Bouttier, J. A. P. Henriques, M. Roesch-Ely, F. Dumas and S. Moura. 2018. Brazilian red propolis: Chemical composition and antibacterial activity determined using bioguided fractionation. Microbiol. Res. 214: 74-82.
[https://doi.org/10.1016/j.micres.2018.05.003]

-
Schnitzler, P., A. Neuner, S. Nolkemper, C. Zundel, H. Nowack, K. H. Sensch and J. Reichling. 2010. Antiviral activity and mode of action of propolis extracts and selected compounds. Phytother. Res. 24(Suppl. 1): S20-S28.
[https://doi.org/10.1002/ptr.2868]

-
Siheri, W., T. Zhang, G. U. Ebiloma, M. Biddau, N. Woods, M. Y. Hussain, C. J. Clements, J. Fearnley, R. E. Ebel, T. Paget, S. Muller, K. C. Carter, V. A. Ferro, H. P. De Koning and D.G. Watson. 2016. Chemical and antimicrobial profiling of propolis from different regions within Libya. PLoS One 11: e0155355.
[https://doi.org/10.1371/journal.pone.0155355]

-
Souza, E. A., R. Zaluski, N. Veiga and R. O. Orsi, 2016. Effects of seasonal variations and collection methods on the mineral composition of propolis from Apis mellifera Linnaeus beehives. Braz. J. Biol. 76: 396-401.
[https://doi.org/10.1590/1519-6984.16714]

-
Šuran, J., I. Cepanec, T. Mašek, B. Radić, S. Radić, I. T. Gajger and J. Vlainić, 2021. Propolis extract and its bioactive compounds - From traditional to modern extraction technologies. Molecules 26: 2930.
[https://doi.org/10.3390/molecules26102930]

-
Taddeo, V. A., F. Epifano, S. Fiorito, S. Genovese. 2016. Comparison of different extraction methods and HPLC quantification of prenylated and unprenylated phenylpropanoids in raw Italian propolis. J. Pharm. Biomed. Anal. 129: 219-223.
[https://doi.org/10.1016/j.jpba.2016.07.006]

-
Wang, X., K. Sankarapandian, Y. Cheng, S. O. Woo, H. W. Kwon, H. Perumalsamy and Y. J. Ahn. 2016. Relationship between total phenolic contents and biological properties of propolis from 20 different regions in South Korea. BMC Complement. Altern. Med. 16: 65.
[https://doi.org/10.1186/s12906-016-1043-y]
