
Impact of Confinement and Population Size on the Instrumentally Inseminated Queen’s Performance of Apis cerana Species in South Korea
Abstract
Instrumental insemination of honey bee is an attractive alternative to natural mating in breeding program as it allows mating crosses between desirable queen and specific drone. However, nursery condition that the queen is kept before and after insemination is major factor affected to the performance of instrumentally inseminated queen. In this study, we evaluated the influences of three different nursery-conditions of push-in cages, mini nuclei and normal colonies on number of spermatozoa stored in the spermatheca, body weight, onset of ovipositon and performance of instrumentally inseminated Apis cerana queen. Our results demonstrated that instrumentally inseminated queens kept in mini nuclei and in normal hives showed no significant difference in queen’s weight (159.8 and 166.2mg, respectively), number of spermatozoa in spermatheca (2.02×106 and 2.76×106, respectively), proportion of queen supersedure (33.3 and 66.7% queen survival at 11 months after oviposition, respectively) and brood production, compared to naturally mated queens. In contrast, instrumentally inseminated queens kept in push-in cages showed significant difference of those above data in comparison to queens mated naturally. Our results suggested that instrumentally inseminated queens could be kept in mini nuclei containing about 1.000 attendant bees to have desirable performance of queen whereas the push-in method should be practiced for the purpose of using queen in the length of time less than 7 months.
Keywords:
Apis cerana, Natural mating, Spermatheca, Semen, Nursery conditionINTRODUCTION
Honey bee queens of Apis cerana (Apis genus) were found to have similar mating behavior to that of Apis mellifera as they are polyandrous and mate early in life, and then store sperm in spermatheca to be used throughout their lifetimes (Sharma, 1960; Laidlaw and Page, 1984). Virgin queen emerges into a colony of sterile workers and usually initiates several orientation flights when she is about 4~6 days (Sharma, 1960; Woyke, 1975; Shah and Shah, 1980). Queens of A. cerana mate naturally by taking two mating flights (Sharma, 1960; Ruttner et al., 1972; Shah and Shah, 1980) and copulating with an average of 10 drones in one flight (Woyke, 1973). After the mating flights, about 1.3 to 2.7 million spermatozoa are stored in her spermatheca (Ruttner et al., 1972; Woyke, 1975). Once mating is completed, queen undergoes massive and permanent physiological and behavioral changes and her ovaries become fully activated and she initiates egg-laying (Tanaka and Hartfelder, 2004; Richard et al., 2007). Naturally mated queens begin laying eggs 2-4 days after the last mating flight (Shah and Shah, 1980). Mated queens will never mate again when they begin to oviposit and will remain in the colony for the rest of their lives (unless they depart during colony swarming) (Winston, 1991).
Instrumental insemination of honey bees has become an attractive alternative to natural mating. This technique allows types of specific genetic crosses that are not possible with natural mating such as mating a queen to a single drone or to a few specific drones and mating mutant queens and drones, and thus producing colonies with desired traits (Harbo, 1986). A. cerana queen can be instrumentally inseminated (Woyke, 1973). Instrumentally inseminated queens begin laying eggs 7~10 days after insemination, and can produce exclusively worker brood (Woyke, 1973; Vung et al., 2016). However, instrumentally inseminated queens have been reported to have problems with low rates of oviposition, initial number of stored spermatozoa and early supersedure (Harbo and Szabo, 1984; Harbo, 1986; Smith et al., 1991). However, Nelson and Laidlaw (1988) found no significant differences in queen weight, brood production between naturally mated and instrumentally inseminated queens. They explained that the lack of beekeeping management practices and treatment given to instrumentally inseminated queens before and after insemination during stages: from the newly emerged, to the brief receptive mating age; to post insemination and onset of ovipositon, appeared to have a more significant effect on queen performance, than the actual insemination procedure. The maintenance condition in which the queen were kept before and after insemination were major factor affecting the performance of instrumentally inseminated queen. Instrumentally inseminated queen kept in colony with larger worker bee population and constant temperature stored more spermatozoa in spermatheca, produced more worker brood, started egg-laying earlier and had longer in lifespan than the queens that were maintained in smaller bee cluster sizes or caged queens without attendant bee. The size of the colony was also affected the survival rate of queen (Woyke and Jasiński, 1990; Cobey, 2007).
In beekeeping practices, instrumentally inseminated A. mellifera queens were kept in various method before and after insemination, such as, confining queen in queen cage in incubator or in queenless colony, keeping queen in nuclei or regular (“normal”) colony (Woyke and Jasinski, 1979; Harbo, 1986; Laidlaw, 1987; Chuda-Mickiewicz et al., 2003). The best method to maintain the queens was to keep them before and after insemination as single individuals in regular colonies from which they were removed only for the insemination to be returned to the colony immediately once the treatment was completed (Woyke and Jasiñski, 1982; Laidlaw, 1987; Prabucki et al., 1987). Such a procedure most closely imitated natural conditions. However, it was expensive, troublesome, involved many colonies and maintenance labour so it was unfeasible in mass production of queen. Because of that, queens were kept in mini nuclei or caged in nursery queenless colonies or incubator with or without attendant bees (Mackensen, 1955; Vesely, 1970; Harbo, 1986; Laidlaw, 1987). Woyke and Jasi?ski (1982) reported that, to get normal number of spermatozoa in the spermatheca (e.g. 3.7 million), at least 350 worker bees should attend the instrumentally inseminated queen kept outdoor in mini nucleus. The practice of confining queen to individual cage in a queenless colony provided convenience by holding a large number of queens before and after insemination. This allowed flexible scheduling and was labor efficient. However, it was found that keeping the queen in a cage without attendant bees or with too low number of bees, instrumentally inseminated queens stored less spermatozoa in spermatheca compared to queens allowed free movement in colony after insemination (Vesely, 1970; Woyke, 1988), Moreover, caged queens tended to be suffered from injuries (which subsequently increase queen supersedure) by aggressive worker bees (Jasiński, 1987; Woyke, 1988). Nevertheless, the practice of caging queens was a valuable management tool, and the duration of the confining period should be minimized, for instance, reducing the time virgin queens were caged before insemination, from 10 to 4 days, reduced injury levels from 54% to 0% (Cobey, 2007).
The aim of our study was to investigate the effects of three different methods of keeping A. cerana queens before and after insemination (i.e., queen were kept in push-in cages, mini nuclei and normal hive) that had been proved to be efficient in A. mellifera. The effects of nursery condition on instrumentally inseminated queen were evaluated by the weight of egg-laying queen, spermatozoa stored in spermatheca, queen loss, onset of ovipositon, supersedure and by worker brood production.
MATERIALS AND METHODS
We conducted our study at National Institute of Agricultural Sciences, Rural Development Administration, Jeonju City, Korea (36°49’30” N, 127°2’3” E) from April 2017 to March 2018. All the honey bee colonies used as sources of bees were headed by naturally mated queens of indigenous Korean A. cerana. Drones were raised from 3 colonies in early April 2017. All the experimental queens that were raised using the grafting method (see below) were obtained from a single source colony, and thus were sisters to each other.
We raised queens from worker larvae of known age that derived from a single colony. Briefly, larvae of similar age, which were younger than 24 hrs old, were physically transferred (“grafted”) from worker cells on brood comb to cell-cups containing 10μl of fresh royal jelly and then suspended vertically in a nurse colony to be raised as queens. Populous colony crowded with nurse bees were selected as queen-rearing colony (i.e., “nursery colony”) to rear grafted larvae. The resulting queens developed inside their respective cell in nurse-colony and were later transferred to individual queen-cages (3.5×10×1.5cm) at day tenth after grafting. Queen cells were then kept in incubator (Growth chamber, Vision VS-1203PFHLN, Korea) at 34°C and 60% relative humid until emergence. Virgin queens, soon after emerging from queen cells, were introduced to cages or colonies of their experimental group. Two cohorts of queen were artificially reared on 22 and 24 April 2017.
Eighty four virgin queens were randomly allocated to four groups: 3 groups for instrumental insemination in which queen were kept in different nursering condition before and after insemination, and a group for the natural mating.
Group 1. Twenty four virgin queens were confined in individual “push-in” cages (PC) by pushing the wire-screen cage (10×8×3cm) firmly into the well-drawn wax comb around the queen (Cobey et al., 2013). The comb holding the caged queens was placed between two brood combs in queenless nursery-colony. Queens were fed by worker bees through the mesh of the cage. Thus, the queen can run freely and lay egg on restricted area of comb.
Group 2. Eighteen virgin queens were introduced to mini nuclei (MN). The mini nuclei (24×20×18cm) were stocked with 3 brood comb (22×15cm) and approximately 1,000 adults. The mini nucleus hive was equipped with a covered runway and queen excluder at the entrance to prevent queen from flying.
Group 3. Eighteen virgin queens were introduced to a modified Langstroth 10-frame hive-box (41×30×25cm) as a “normal” hive (NH) group. The normal hive contained 3 brood combs (39×23cm) and approximately 4,000 adults. The excluder was fixed at the entrance until queen initiated egg-laying.
Group 4. Twenty four virgin queens were released in normal hive (with similar bee population as described in group 3) for natural mating as control group. Queens were allowed for free flight.
At the time of instrumental insemination, semen was collected from mature drones (which were caught at hive entrance of drone. Collected semen was inseminated virgin queen using a Schley Model II insemination device by following insemination protocols which readily produce egg-laying queens (Woyke, 1975; Vung et al., 2016) Virgin queens (i.e., in the PC, MN and NH groups) were instrumentally inseminated twice at the age of five and six days old, each time with a dose of 4μl semen.
Number of queen survival and the time of the appearance of eggs in the cells (i.e., onset of oviposition) were examined daily, starting from the 7th day after queen emergence and continuing until all queens laid egg. Once the experimental queens were observed to be laying eggs, six queens in each group were anesthetized by narcotizing them with CO2 for about 5 min or until they were immobilized. They were weighed individually to the nearest 0.1mg on a digital scale, and then dissected in phosphate-buffered solution (PBS: 137mM NaCl, 2.7mM KCl, 10mM Na2HPO4, 1.8mM KH2PO4, pH 7.4) (Gençer and Kahya 2011). The spermatheca of each queen was transferred to a 1.5ml Eppendorf tube and number of spermatozoa in the spermathecae were counted by following protocol described by Cobey et al. (2013). Other six egg-laying queens in each group were introduced to new established colonies for further investigation.
In order to evaluate the performance of experimental queens, queens were introduced to new established colonies with uniform strength (e.g. equivalent number of adult and sealed brood) on 26 May 2017. To establish new colonies, egg-laying queens of donor colonies were eliminated one day prior to colony division. Sealed brood combs adhering with adults were selected to estimate the beginning quantity of sealed brood and adult. We used a wire-gridded wooden frame (which was the same size as bee-comb of 39×23cm) consisting of 28, 4.6×4.6cm square-compartments for the visual count. We first estimated number of adult in each comb by overlaying the gridded frame on both sides of comb to count adults in all square-compartment, and summed to have number of adult. Then, we counted all square-compartment containing sealed brood on both sides of comb, and extrapolated the resulting number of sealed brood by multiplying of total square-compartments by 100 (approximately 100 cells of worker brood per square-compartment for A. cerana) (Chinh et al., 2005). Since the number of adult and sealed brood in each comb were determined, new colony was made by taking four brood combs covered with adults from donor colonies to a modified Langstroth 10-frame hive. Sealed brood combs of each hive were adjusted to a near-equal quantity, and number of adult was adjusted to approximately 4,000 individuals. Egg-laying queen of each experimental group were introduced to new established hives. These colonies were then located in an apiary and randomly arranged with the distance of at least three meters to each other. The entrances of hives were oriented to different directions to limit adults to drift between colonies.
To test the effect of queen kept in different nursery condition before and after instrumental insemination on brood production, number of sealed brood in each colony headed by experimental queen were estimated every two months from June 2017 (i.e., about one month after queen initiated oviposition) to October 2017 (i.e., before colony overwintering). The amount of sealed brood produced by each colony was measured using a wire grid frame as above description. Any episode of queen loss and supersedure were recorded monthly from May 2017 to March 2018. The end of a queen’s life was considered as her natural death, supersedure or oviposition stopped. In the case of queens that died during the winter (i.e., from November 2017 to February 2018), the date of death corresponded to the first spring colony inspection in March 2018.
Data analysis
Data on weight of queen, number of spermatozoa in spermatheca, number of sealed brood and number of day to initiate egg-lagying of queen were analyzed using one-way ANOVA tests followed by Fisher’s Least Significant Difference (LSD) post hoc tests for pairwise comparison of means. The proportion of queen loss before oviposition and queen supersedure over time were compared using Kaplan-Meier estimates followed by log-rank (Mantel-Cox) post hoc tests. The time that queen survived in colony was defined by rounding up to nearest month. For all testing, differences between groups at P<0.05 were considered statistically significant. All data sets were analysed using IBM SPSS statistic version 22 for Windows (IBM Corp., NY, USA).
RESULTS
In this study, all survived queen could lay eggs after insemination. The oviposition occurred with 15 of 24 naturally mated queens and 46 of 60 instrumentally inseminated queens (Table 1). The length of time from emergence to onset of oviposition in queens among groups was significantly different (ANOVA test, F=12.73, P<0.01). Naturally mated queens initiated egg-laying significantly the earliest (LSD post hoc test: P<0.01) at average of 10.5 days after emergence. Queens from MN and NH groups began egg-laying similarity (LSD post hoc test: P>0.05) and later than naturally mated queens (LSD post hoc test: P<0.01). The queens from the PC group started oviposition the latest (LSD post hoc test: P<0.01) and were later than naturally mated queen 5.5 days. This indicated that poorer nursery condition before and after insemination delayed the onset of oviposition in queens.

Period (days, mean±SE) from emergence to onset of oviposition and weight (mg, mean±SE) of egg-laying queens from different treatments
Once the queens from all group initiated egg-laying, six queens from each group were sampled to weigh and dissected to count the sperm cells in their spematheca. The average body weight of egg-laying queens from four groups was not found to differ significantly (ANOVA test, F=2.34, P>0.05, Table 1) but the heaviest in weight was observed in naturally mated queen (169.2mg). Weight of queens from NH and MN group were insignificantly different (LSD post hoc test: P>0.05), and lower than naturally mated queen, although this differences were not significant (LSD post hoc test: P>0.05). The lightest in weight was queens from PC group (149.7mg), although their weights were not significantly different, compared to weights of queens in NH and MN group (LSD post hoc test: P>0.05).
Queen losses occurred mainly before day 11th after their emergences. The deaths of instrumentally inseminated queens were found in cages or colonies after instrumental insemination whereas queens in control group were lost with unknown reason. Lost or dead queens were taken into account of queen mortality (Fig. 1). Mortality rate of instrumentally inseminated queens started from 16.7% (NH group) to 29.2% (PC group). The highest percentage of queen loss was recorded in control group (naturally mated queen, 37.5%). Mortality rate of queen from NH group were the lowest, although the differences were not significant among group (Mantel-Cox tests, X2=3.1, P=0.38, Fig. 1).
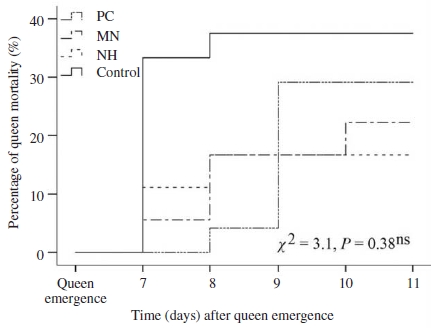
Kaplan-Meier estimates expressed the mortality rate of queens kept before and after instrumental insemination in push-in cage (PC), mini nucleus (MN) and normal hive (NH), and queens mated naturally (control). Chi-square (χ2) test of overall comparison fo1lowed by a P value shown as; ns: not significant difference at the 0.05 level.
Instrumentally inseminated queens were inseminated with the same insemination doses and amount of semen (2 times×4μl). Number of sperm cells stored in spermatheca of queen were significant different among groups (ANOVA test, F=13.78, P<0.01, Fig. 2). The naturally mated queen had, on average 2.76×106, more spermatozoa in their spermatheca than that in queens from other groups, although that was not significantly higher than number of spermatozoa stored in spermatheca of queens in MN and NH groups (LSD post hoc test, P>0.05). Caged queen from CP group stored, on average 1.27×106 (median=1.23×106), significantly less spermatozoa in their spermatheca than that of other groups (LSD post hoc test, P<0.01).
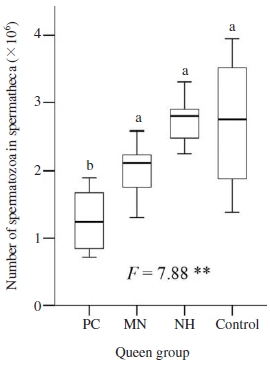
Number of spermatozoa in spermatheca of queens kept before and after instrumental insemination in push-in cage (PC), mini nucleus (MN) and normal hive (NH), and queen mated naturally (control). Boxplots demonstrate the lower quartile, median (vertical lines inside the box), and upper quartile, and whiskers represent 1.5 times the interquartile range. ANOVA test of mean comparison fo1lowed by a P value shown as **: significant difference at the 0.01 level. Fisher’s LSD post hoc test showed as different letters indicated significant differences at the 0.05 level.
Six queens from each group were introduced to new established colony to investigate their brood production. Number of sealed brood in colonies of different groups were significantly different after oviposition one and five months (ANOVA tests, at one month: F=6.92, P<0.01, at five months: F=7.56, P<0.01, Fig. 3). In these two periods (i.e. June and October), the median number of sealed brood in colonies headed by queens from NH group was similar with that of naturally mated queens. Average number of sealed brood in colonies headed by queens from MN and NH were not significantly different, although that were significantly higher compared to PC group (LSD post hoc test, P<0.01). August was dearth period, although colonies were fed during July and August, they did not prosper so that the areas of brood being measured were small. Therefore, the median number of sealed brood of all colonies in this period were less than that in June and October, and no significant difference in average number of sealed brood among groups was observed (ANOVA test, F=1.75, P>0.05).
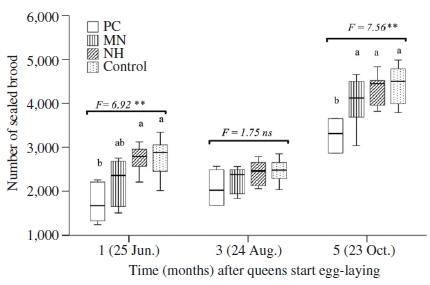
Number of sealed brood in colonies headed by instrumentally inseminated queens kept before and after insemination in push-in cage (PC), mini nucleus (MN) and normal hive (NH), and naturally mated queen (control). Boxplots demonstrate the lower quartile, median (vertical lines inside the box), and upper quartile, and whiskers represent 1.5 times the interquartile range. ANOVA test of mean comparison fo1lowed by a P value shown as ns: not significant difference; **: significant difference at the 0.01 level. Fisher’s LSD post hoc test showed as different letters in graphs indicated significant differences at the 0.05 level.
All of queen laid fertilized eggs to produce worker brood in the first three months after onset of oviposition. The number of experimental queens decreased from the month third after queen initiated oviposition. Losses of queens in control group were considered as natural for colonies, their range in line with standard apiary management. The most common cause of queen loss was queen supersedure and colony mortality during winter. Other losses, such as drone egg laying or cessation of oviposition that were observed in queens from CP group, were rare. There was significant difference in the loss of queen from different groups (Mantel-Cox tests, X2=8.4, P=0.05, Fig. 4). All queens from CP group were supersedured at 7 months after their onset of oviposition. Percentage of queen survival until March 2018 (i.e., 11 months after oviposition) in MN, NH and control amounted to 16.7, 33.3, and 66.7%, respectively.
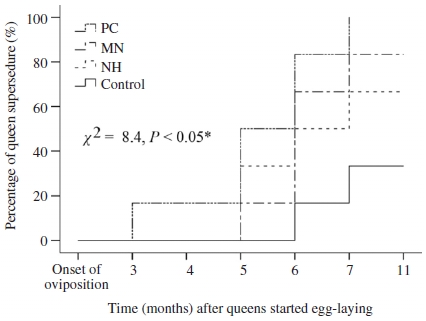
Kaplan-Meier estimates expressed the percentage of queen supersedure over time in colonies headed by queens kept before and after instrumental insemination in push-in cage (PC), mini nucleus (MN) and normal hive (NH), and queen mated naturally (control). Chi-square (χ2) test of overall comparison fo1lowed by a P value shown as; *: significant difference at the 0.05 level.
DISCUSSION
The success of insemination in queen honey bee was based upon the survivorship to production of worker brood. The naturally mated queens were missing for a variety of reasons while they flied out of their hive to take orientation and mating flight (Ruttner et al., 1972; Woyke, 1975; Gabka, 2018). Our findings showed that A. cerana queen losses during the flights (i.e., 37.5% queen loss) were in line with previous reports found in A. mellifera that naturally mated queen lost about 18-40% (Palmer and Oldroyd, 2000; Medina and Goncalves, 2001; Gabka, 2018). Instrumentally inseminated queen mainly died because of injury or infection from the insemination process, or sperm residue in the oviducts (Harbo and Szabo, 1984; Collins, 2000; Chuda-Mickiewicz et al., 2009; Gerula et al., 2016). However, our result demonstrated that survival rate of instrumentally inseminated A. cerana queen was slightly higher than that of natural mating (Fig. 1).
In addition, the reduced survival, as well as longevity of instrumentally inseminated queen was attributed to exposures of CO2 treatments given to the queen in the insemination process (Harbo and Szabo, 1984; Nelson and Laidlaw, 1988). The life span of instrumentally inseminated A. cerana queen was reported shorter than one year (Woyke, 1973; Woyke, 1975). Of the 18 instrumental inseminated queens were introduced to colonies in our experiment, only three queens (i.e. 17%) could overwinter to survive until 11 months after their onset of oviposition whereas the survivorship of naturally mated queen was 66% (Fig. 4).
The number of sperm cells stored in the spermatheca is a major factor determining queen longevity. Queens with insufficient numbers of stored sperm cells have higher rates of supersedure (Cobey, 2007). Sperm storage of the instrumentally inseminated queen must be sufficient to allow time for selection and to ensure breeding stock is available for propagation, and to maintain a populous colony, especially evaluation requires field selection at the colony level. Number of spermatozoa in spermatheca that we observed from naturally mated queens of Korean indigenous A. cerana (i.e., 2.76×106) were similar to the previous results found in A. cerana indica in Pakistan (i.e., 2.70×106; Ruttner et al., 1972). Woyke (1975) reported that instrumentally inseminated A. cerana queen stored less spermatozoa in spermatheca than that of naturally mated queen whereas our results revealed that this difference was not significant in comparison between queen in NM and control group (Fig. 2). Our results also agreed with reports found in A. mellifera where number of stored sperm from instrumentally inseminated queen was not significantly lower than that of naturally mated queens (Cobey, 2007; Gerula et al., 2012).
The size of the bee population where instrumentally inseminated queen was kept before and after insemination not only affected the success rate of insemination, it also affected onset of oviposition and number of sperm cells stored in the spermatheca of queen (Woyke and Jasinski, 1979; Gerula, 2007; Gerula et al., 2016). Therefore, an increase in the population of attendant worker bees increased the cluster temperature contributing to faster initiation of egg laying, and efficiency of sperm migration to store in spermatheca (Woyke and Jasiński, 1990). Our results on queen’s weight (Table 1), number of spermatozoa in spermatheca (Fig. 2), brood production (Fig. 3) of instrumentally inseminated A. cerana queen, which were kept in normal colony before and after insemination, were similar with naturally mated queens. Moreover, those data observed in queens from mini nucleus (which contained approximately 1.000 attendant worker bees) were not significant difference with that of naturally mated queens. Those results agreed with findings in A. mellifera that a population of at least 350 worker bees was necessary for outdoor mating nuclei (Woyke and Jasiński, 1990). Thus, it is suggested that A. cerana should be released and allowed free movement in colony before and after instrumental insemination. However, the length of time from emergence to onset of oviposition in instrumentally inseminated queens was significant longer than that of naturally mated ones (Table 1) as the age of queen at insemination for instrumental insemination were five or six days whereas the naturally mated queen took mating fight at about 3.6 days after emergence (Sharma, 1960).
Queen weight was one of recommended criteria to assess queen quality as it affected to reproductive potential on colony growth and performance of queen (Kaftanoglu and Peng, 1982; Rangel et al., 2013). Confined to cages, queens may not receive adequate nutrition. A protein rich diet is essential for ovaries and eggs development and lack of sufficient feeding of protein may contribute to delayed onset of oviposition. Therefore, queen that confined to cages in nursery colony stored less sperm and delayed in the onset of oviposition (Szabo and Townsend, 1974; Woyke, 1988). Our results also found that caged A. cerana queen had significantly lower in weight and delayed egg-laying, compared to queen allowed free movement in colony (Table 1). Subsequently, the brood production in colony headed by caged queen were significantly less than in colony headed by queen of other groups (Fig. 3).
Newly mated queens are very active, running on the comb often bending their abdomens, which promotes sperm migration. The movement of sperm cells from the oviducts into the spermatheca involved muscular action of the queen and sperm motility. Active, free movement of the queen and attendance by workers after insemination helps clear the semen in oviducts and increases the efficiency of sperm cell migration into the spermatheca (Woyke, 1979). Moreover, previous findings noted that the caged queens tended to retain semen in their lateral oviducts which can be harmful and sometimes fatal (Vesely, 1970; Woyke and Jasinski, 1979; Chuda-Mickiewicz et al., 2003). Thus, the caged queen of A. cerana that stored significantly less sperm in the spermatheca and had higher mortality rate than queen allowed free movement in colony might be caused by the poor nursery condition and restricted movement. In addition, the poorer rearing condition before and after insemination also affected to the survivorship of queen in colony as all caged queen were supersedured at seventh months after their onset of oviposition.
The practice of caging queen in push-in cages provided convenience in holding large number of queens. However, this method seemed not to provide optimal conditions for A. cerana queen and required improvement to make this method practically. Therefore, we recommended keeping A. cerana queen before and after insemination in bee population with at least 1,000 workers to have desirable performance of instrumentally inseminated queen.
Acknowledgments
We would like to thank staff at Sericultural & Apicultural Materials Division, Department Agricultural Biology, National Institute of Agricultural Sciences, Republic of Korea for their technical assistance in beekeeping activities. Financial support came from the PJ012526032018 of national joint research business in National Institute of Agricultural Sciences in Rural Development Administration.
References
-
Chinh, T. X., W. J. Boot, and M. J. Sommeijer, (2005), Production of reproductives in the honey bee species Apis cerana in northern Vietnam, J. Apic. Res, 44, p41-48.
[https://doi.org/10.1080/00218839.2005.11101146]

- Chuda-Mickiewicz, B., J. Prabucki, and J. Samborski, (2003), Onset of oviposition in honeybee queens kept in boxes with non-free flying bees, Journal of Apicultural Science, 47, p27-30.
- Chuda-Mickiewicz, B., J. Prabucki, J. Samborski, and P. Rostecki, (2009), The role of phytohormones in instrumental insemination of queen bees, Journal of Apicultural Science, 53, p91-96.
-
Cobey, S. W., (2007), Comparison studies of instrumentally inseminated and naturally mated honey bee queens and factors affecting their performance, Apidologie, 38, p390-410.
[https://doi.org/10.1051/apido:2007029]

-
Cobey, S. W., D. R. Tarpy, and J. Woyke, (2013), Standard methods for instrumental insemination of Apis mellifera queens, J. Apic. Res, 52, p1-18.
[https://doi.org/10.3896/ibra.1.52.4.09]

-
Collins, A. M., (2000), Relationship between semen quality and performance of instrumentally inseminated honey bee queens, Apidologie, 31, p421-429.
[https://doi.org/10.1051/apido:2000132]

- Gabka, J., (2018), Drifting of honey bee queens returning from flights, J. Apic. Res, 57, p580-585.
- Gerula, D., (2007), Observations of body injuries of artificially inseminated honey bee queens inflicted in the subsequent stages of rearing and during their introduction into colonies, Journal of Apicultural Science, 51, p5-17.
-
Gerula, D., B. Panasiuk, P. Wegrzynowicz, and M. Bieńkowska, (2012), Instrumental insemination of honey bee queens during flight activity predisposition period 2. Number of spermatozoa in spermatheca, Journal of Apicultural Science, 56, p159-167.
[https://doi.org/10.2478/v10289-012-0016-8]

-
Gerula, D., P. Wegrzynowicz, B. Panasiuk, M. Bieńkowska, and W. Skowronek, (2016), Productivity and longevity of honey bee queens inseminated with freshly collected and diluted semen, J. Apic. Res, 55, p130-136.
[https://doi.org/10.1080/00218839.2016.1200880]

- Harbo, J. R., (1986), Propagation and instrumental insemination, in: Rinderer, T. E. (Ed.), Bee genetic and breeding, Academic Press, Inc., Orlando, FL.
-
Harbo, J. R., and T. I. Szabo, (1984), A comparison of instrumentally inseminated and naturally mated queens, J. Apic. Res, 23, p31-36.
[https://doi.org/10.1080/00218839.1984.11100606]

- Jasiński, Z., (1987), Injuries of queens caged in queenless colonies, Proc. 31st Intern. Apic. Cong, Warsaw, Apimondia Pub. House, p67-68.
-
Kaftanoglu, O., and Y. S. Peng, (1982), Effects of insemination on the initiation of oviposition in the queen honeybee, J. Apic. Res, 21, p3-6.
[https://doi.org/10.1080/00218839.1982.11100508]

-
Laidlaw, H. H., (1987), Instrumental insemination of honeybee queens: its origin and development, Bee World, 68, p17-36.
[https://doi.org/10.1080/0005772x.1987.11098916]

- Laidlaw, H. H., and R. E. Page, (1984), Polyandry in honey bees (Apis mellifera L.): sperm utilization and intracolony genetic relationships, Genetics, 108, p985-997.
-
Mackensen, O., (1955), Experiments in the technique of artificial insemination of queen bees, J. Econ. Entomol, 48, p418-421.
[https://doi.org/10.1093/jee/48.4.418]

- Medina, L. M., and L. S. Goncalves, (2001), Effect of weight at emergence of Africanized (Apis mellifera L.) virgin queens on their acceptance and beginning of oviposition, American Bee Journal, 141, p213-215.
- Nelson, D. L., and H. H. Laidlaw, (1988), An evaluation of instrumentally inseminated queens shipped in packages, American Bee Journal, 128, p279-280.
-
Palmer, K. A., and B. P. Oldroyd, (2000), Evolution of multiple mating in the genus Apis, Apidologie, 31, p235-248.
[https://doi.org/10.1051/apido:2000119]

- Prabucki, J., Z. Jasinski, and B. Chuda-Mickiewicz, (1987), The results of mass insemination of bee queen inseminated one fold and twofold and stocked in different ways, Proc. 31st Intern. Apic. Cong, Warsaw, Apimondia Pub. House, p169-174.
-
Rangel, J., J. Keller, and D. Tarpy, (2013), The effects of honey bee (Apis mellifera L.) queen reproductive potential on colony growth, Insectes sociaux, 60, p65-73.
[https://doi.org/10.1007/s00040-012-0267-1]

-
Richard, F. J., D. R. Tarpy, and C. M. Grozinger, (2007), Effects of insemination quantity on honey bee queen physiology, PloS one, 2, e980.
[https://doi.org/10.1371/journal.pone.0000980]

- Ruttner, F., J. Woyke, and N. Koeniger, (1972), Reproduction in Apis cerana 1, Mating behaviour. J. Apic. Res, 11, p141-146.
-
Shah, F. A., and T. A. Shah, (1980), Early life, mating and egg laying of Apis cerana queens in Kashmir, Bee World, 61, p137-140.
[https://doi.org/10.1080/0005772x.1980.11097796]

-
Sharma, P. L., (1960), Observations on the swarming and mating habits of the Indian honeybee, Bee World, 41, p121-125.
[https://doi.org/10.1080/0005772x.1960.11096778]

- Smith, R. K., M. Spivak, and O. R. Taylor, (1991), Chemical differences between naturally mated and instrumentally inseminated queens, Proc. Am. Bee Res., Conf. Am. Bee J, p781.
-
Szabo, T. I., and G. F. Townsend, (1974), Behavioural studies on queen introduction in the honeybee 1. effect of the age of workers (from a colony with a laying queen) on their behaviour towards an introduced virgin queen, J. Apic. Res, 13, p19-25.
[https://doi.org/10.1080/00218839.1974.11099754]

-
Tanaka, E. D., and K. Hartfelder, (2004), The initial stages of oogenesis and their relation to differential fertility in the honey bee (Apis mellifera) castes, Arthropod Structure & Development, 33, p431-442.
[https://doi.org/10.1016/j.asd.2004.06.006]

- Vesely, V., (1970), Retention of semen in the lateral oviducts of artificially inseminated honey-bee queens (Apis mellifera L.), Acta Entomologica Bohemoslovaca, 67, p83-92.
- Vung, N. N., M. L. Lee, H. K. Kim, K. H. Byeon, and Y. S. Choi, (2016), Efficiency of artificial insemination for breeding Apis cerana in Korea, Korean J. Apiculture, 31, p323-330.
- Winston, M. L., (1991), The biology of the honey bee, Harvard university press.
-
Woyke, J., (1973), Instrumental insemination of Apis cerana indica queens, J. Apic. Res, 12, p151-158.
[https://doi.org/10.1080/00218839.1973.11099743]

-
Woyke, J., (1975), Natural and instrumental insemination of Apis cerana indica in India, J. Apic. Res, 14, p153-159.
[https://doi.org/10.1080/00218839.1975.11099820]

-
Woyke, J., (1979), Effect of the access of worker honeybees to the queen on the results of instrumental insemination, J. Apic. Res, 18, p136-143.
[https://doi.org/10.1080/00218839.1979.11099957]

- Woyke, J., (1988), Problems with queen banks, American Bee Journal.
-
Woyke, J., and Z. Jasinski, (1979), Number of worker bees necessary to attend instrumentally inseminated queens kept in an incubator, Apidologie, 10, p149-155.
[https://doi.org/10.1051/apido:19790205]

-
Woyke, J., and Z. Jasiński, (1990), Effect of the number of attendant worker bees on the initiation of egg laying by instrumentally inseminated queens kept in small nuclei, J. Apic. Res, 29, p101-106.
[https://doi.org/10.1080/00218839.1990.11101204]

- Woyke, J., and Z. Jasiñski, (1982), Comparison of the number of spermatozoa entering the spermatheca of instrumentally inseminated queens kept in nuclei and in normal honeybee colonies, Pszczelnicze Zeszyty Naukowe (Poland), p29-34.