Foraging Activities of Honeybees (Apis mellifera L.) and Bumblebees (Bombus terrestris L.) on Passionfruit (Passiflora edulis f. edulis) Flower and their Pollination Effects
Abstract
We studied the activity and pollination effects of insect pollinators (Apis mellifera and Bombus terrestris) in passionfruit pollination to investigate the possibility of insect pollination in greenhouse-cultivated passionfruit. The climatic condition inside the greenhouse during the flowering of passionfruit, bee traffics and flower visit duration of two species were measured. In addition, the pollination effect was determined by investigating the physical characteristics of fruits according to each pollination methods. The bee traffic and foraging activity of A. mellifera were 3.6 and 7 times greater than those of B. terrestris, respectively. However, when this result was converted into a percentage to colony size, the ratio of B. terrestris was 19.1, and was 10.2 times higher than that of A. mellifera. In addition, the activity of A. mellifera was affected by the illumination level (R=0.593) and ultraviolet rays (R=0.647), but the activity of B. terrestris was less affected by light. Therefore, we considered B. terrestris to be more suitable for the pollination of passionfruit than A. mellifera. Regarding the pollination effect, there was no difference between the two insect pollinators; however, better fruit quality was observed with insect pollinators than with artificial pollination. Certain problems should be addressed when using an insect pollinator directly for the pollination of passionfruit. First, pollen robbing caused by the differences between flowering start time and bee activity start time should be considered. Second, the low pollination efficiency because of the presence of extrafloral nectaries in passionfruit should be considered. Lastly, the pollination effect caused by the colony decline because of the long duration of pollination should be factored in. If these problems are solved by controlling the activity time of the foraging bees, increasing the pollinator density by 30%, and adding or managing the bee colony, insect pollinators could be effectively used for passionfruit pollination.
Keywords:
Honeybee, Bumblebee, Passionfruit, PollinationINTRODUCTION
Passionfruit (Passiflora edulis Sims) is a perennial vine crop belonging to the family Passifloraceae (Dhawan and Sharma, 2004; Lim et al., 2018). It originates in southern Brazil, Paraguay, and northern Argentina and is cultivated mainly in the high altitudes of tropical and subtropical regions. Moreover, it has been introduced to five continents as an ornamental and commercial crop (Rendón et al., 2013). Passionfruit grown in Korea is mostly a hybrid of the purple passionfruit (Passiflora edulis f. edulis), and is cultivated in greenhouses for stable fruit production (RDA, 2019). Purple passionfruit blooms and sets fruit twice a year (Lederman, 1987). The spring bloom during April~May yields fruit in the summer, and the fall bloom during September~October yields fruit in the winter (Kishore et al., 2010). As it is not self-incompatible, the purple passionfruit can be self-fertilized, but owing to the unique shape of its flowers, it mainly requires pollinators, such as insects, for successful pollination (Aponte and Jáuregui, 2004; Kishore et al., 2010). In its country of origin, passionfruit is cultivated in open fields and several species of insect pollinators pollinate its flowers (Lederman, 1987). Among wild insect pollinators, carpenter bees (Xylocopa spp.) are known as the most effective passionfruit pollinators because their body is larger than that of the other bee species (Hammer, 1987; Hoffmann et al., 2000; Keasar, 2010). However, as a consequence of climate change, deforestation, and pesticide use, wild bee populations are rapidly decreasing, which is why many countries, including Brazil, where the passionfruit originates from, are conducting artificial pollination for the production of passionfruit (Silva, 2005; Yamamoto et al., 2012). In Japan and Korea, where passionfruit is cultivated in greenhouses, artificial pollination is commonly performed for the stable production of high-quality fruits (Mizuno, 2008; Lim et al., 2018). However, artificial pollination of passionfruit requires manpower, which is an expensive process. As flowers bloom and fall daily, there is a requirement of 3~5 individuals per ha daily during the blooming period that lasts approximately 90 days (Ish-Am, 2009). To solve this problem, studies on the artificial use of insect pollinators have been conducted in various passionfruit-cultivating countries. In Brazil, the method of attracting carpenter bees and using them for the pollination of passionfruit has been investigated in several studies (Freitas and Oliveira, 2003; Keasar, 2010; Silveira et al., 2012; Junqueira et al., 2016). Ish-Am (2009) reported a method of passionfruit pollination using honeybee (Apis mellifera) as a pollinator and by controlling their density to solve the problem of lowering the pollination effect because bees visit extrafloral nectaries rather than flowers. In Japan, where passionfruit is mostly cultivated in greenhouses, a study was conducted to compare the effects of Asian honeybees (Apis cerana), European honeybees (A. mellifera), and bumblebees (Bombus terrestris) as passionfruit pollinators (Shimabukuro et al., 2007; Mizuno, 2008). In Korea, a study was carried out to compare the pollination effects of two insect pollinators (A. mellifera and B. terrestris) using fan and hand pollination (Lee et al., 2013). However, in 94% of the area of passionfruit cultivation in Korea, artificial pollination is still used (Yoon et al., 2017). In the present study, we focused on the foraging activity of insect pollinators in the cultivation environment of passionfruit, which was different from previous studies focusing only on the effect of insect pollinators. In addition, we investigated the relationship between the flowering characteristics of passionfruit flowers and the activity characteristics of two insect pollinators (A. mellifera and B. terrestris), and explained the pollination effect through changes in fruit quality related to foraging activity. Moreover, based on the obtained data, we developed a method to effectively use insect pollinators in the field.
MATERIALS AND METHODS
1. Insects and crops
The experiments were conducted from May 15 to June 30, 2018, in Imun-ri, Wanju-gun, Jeollabuk-do, Korea (35°49′24″N, 127°00′43″E). We selected the passionfruit vine (Passiflora edulis var. edulis Sims) for the study. Its bloom period was from May 6 to July 5. In order to compare their pollination effects, European honeybees (Apis mellifera L., Italy, three flames, over 5,000 workers) and bumblebee (Bombus terrestris L., 70 workers) of 13 generations reared under artificial conditions [26℃, relative humidity (RH) 80%] (Yoon et al., 2008) were selected and introduced to the study sites.
2. Treatment groups
The test plot consisted of seven net house units (155 m2/unit, mesh: 2 mm×2 mm) with 30 passionfruit vines per net house unit. The distance between plants was 1.2 m×2.0 m, the age of the passionfruit vine was four years, and the average vine height was 2.3 m. To investigate the effect of different pollination methods, three colonies of 5,000 (two frames) A. mellifera and three colonies of 100 B. terrestris were installed 1 m from the entrances of the six net houses on May 11, 2018. Artificial pollination was carried out in one net house during the experimental period. The bee colonies were removed from the greenhouses 46 days after installation. At each site, artificial pollination and control groups were set. For artificial pollination, we transferred the pollen from the anther directly onto the pistil head with a brush when the flowers bloomed at 11 a.m. every day during the study period. Immediately after artificial pollination, the flower clusters were covered with a 1 mm×1 mm mesh to prevent insect pollination. The pollination activity of insect pollinators, fruit set, and physical properties of the harvested passionfruit were investigated to compare the pollination effects.
3. Climatic conditions in passionfruit greenhouses
To investigate the climatic conditions in the greenhouses, data loggers (Illuminance UV recorder TR-74Ui; T&D Co.; Matsumoto, Nagano, Japan) were installed 5 m from the entrance of the three test plots (A. mellifera, B. terrestris, and artificial pollination) and 1.3 m above the ground. The installed data loggers recorded the temperature, relative humidity, illuminance level, and UV level inside the greenhouses at 30 min intervals from May 15 to June 30, 2018. To investigate the correlation between the climatic conditions inside the greenhouses and bee activity, a correlation analysis was conducted on the number of bees flying without pollination behavior and the number of bees visiting the flowers in relation to the climatic conditions.
4. Pollination activity and foraging characteristics
To compare the pollination activity of A. mellifera and B. terrestris, we investigated the bee traffic and bee visits on passionfruit plants. Bee traffic was defined as the number of worker bees entering and exiting the hives. To investigate it, we developed an imaging-based bee traffic measurement system (Korea Patent No. 101961670). Bee traffic was examined at 1 h intervals from 05:00 to 20:00 every day during the experimental period. In addition, the number of bees pollinating the passionfruit flowers per plant was observed at 1 h intervals from 05:00 to 20:00 for four days (May 15, 20, 25, and 30), that is, the number of foraging bees visiting passionfruit flowers at the same time intervals. In order to compare the preferences of honeybees and bumblebees for nectar from flowers and extrafloral nectaries (EFNs), the number of foraging workers on the flowers and petioles of passionfruit vines in the test plots was counted for 5 min every hour between 05:00 and 20:00 for four days. In addition, a regression analysis was performed to confirm how the bee traffic changed over time after the installation of the beehive.
5. Effect of pollination method on fruit quality
The fruits were harvested 80 days after pollination (from August 16 to September 9, 2018). Fruit quality was determined by randomly selecting 120 fruits from each treatment group and analyzing non-cleaved (weight, length, and diameter) and cleaved fruit properties (weight of the pulp flesh, number of seeds, soluble solids, titratable acidity, soluble solids/titratable acidity (SS/TA) ratio, and weight of pulp flesh/fruit weight (WP/WF) ratio). The SS/TA and WP/WF ratios were expressed as percentage of fruits with a soluble solid/titratable acidity and weight of pulp flesh/fruit weight, respectively. The relationships between fruit weight, length, diameter, number of seeds, and weight of pulp flesh were analyzed to determine the commercial value of fruits pollinated by the two insect pollinators. In addition, a regression analysis was performed to investigate the changes in fruit weight in relation to the harvest date. The fruits were weighed using an electronic balance (CB-3000, AND, Seoul, Korea), and their length and diameter were measured using Vernier calipers (500-181, Mistutoyo, Kawasaki, Japan). The juice was extracted from the flesh at the median equatorial plane of the fruit using a cheesecloth, and the concentration of soluble solids in the extracted samples was measured using a digital sugar meter (PR-32α, Atago, Tokyo, Japan). For measuring the titratable acidity, the juice was extracted from five fruits from each treatment group; 5 mL of each juice sample was diluted with 35 mL of distilled water, the pH of each sample was neutralized to 8.3 using 0.1 N NaOH, and the titratable acidity was determined using citric acid.
6. Statistical analysis
A t-test was used to compare the pollination behaviors of A. mellifera and B. terrestris (bee traffic, and foraging activity, including bee flying and bee visiting the flower), as well as to compare insect preference between the flowers and petioles of passionfruit vines. The effect of the pollination method on fruit quality was analyzed using one-way ANOVA. Significant differences identified using ANOVA and Tukey’s test were used for posthoc analysis. In correlation analysis, Pearson’s correlation was performed after normality was verified using the Kolmogorov-Smirnov test. After performing the correlation analysis, a linear regression equation was derived using regression analysis if a significant correlation was confirmed. All statistical analyses were performed using the SPSS PASW 22.0 package for Windows (IBM, Chicago, IL, USA).
RESULTS
1. Climatic conditions inside the greenhouses during the passionflower blooming period
The climatic conditions during the bee activity time (from 05:00 to 20:00) throughout the 46 days of the passionfruit blooming period (May 15 to June 30, 2018) were as follows: average temperature of 24.1±1.6℃, relative humidity of 66.4±10.3%, illuminance level of 6094.4±2295.9 lx, and UV level of 0.077±0.048 mW/cm2 (Table 1). Daily changes in the climatic conditions were as follows: the temperature began to rise at 6:00 in the morning, was relatively unchanged from 10:00 to 17:00, and then sharply decreased after 16:00. The highest temperature was recorded at 14:00 (26.8±2.7℃), and the lowest at 4:00 (18.2±4.7℃) (Fig. 1A). The relative humidity remained 80~90% from 21:00 to 07:00, began to decrease at 7:30, and then increased again after 15:30. The highest humidity (92.4±4.9%) was recorded at 5:30 and the lowest humidity (54.3±16.0%) at 14:00. In general, the relative humidity tended to be inversely related to temperature (Fig. 1B). Illuminance began to increase from 5:00 and continued increasing until 12:00, after which it began to decrease, sharply decreasing after 13:00. The highest illuminance level (17703.1±13000 lx) was recorded at 12:00 (Fig. 1C). The pattern of the UV level almost coincided with the pattern of the illuminance level. The highest UV level (0.244±0.200 mW/cm2) was recorded at 11:00. The passionfruit plants started to bloom at 10:20±25, and within 40 min from when the petals opened, the stigmas spread downward and descended. Blooming was completed within 2 h from the beginning of blooming, and pollen had already appeared during the beginning of flowering.

Climatic conditions during the bee activity time throughout passionfruit blooming period in the greenhouse
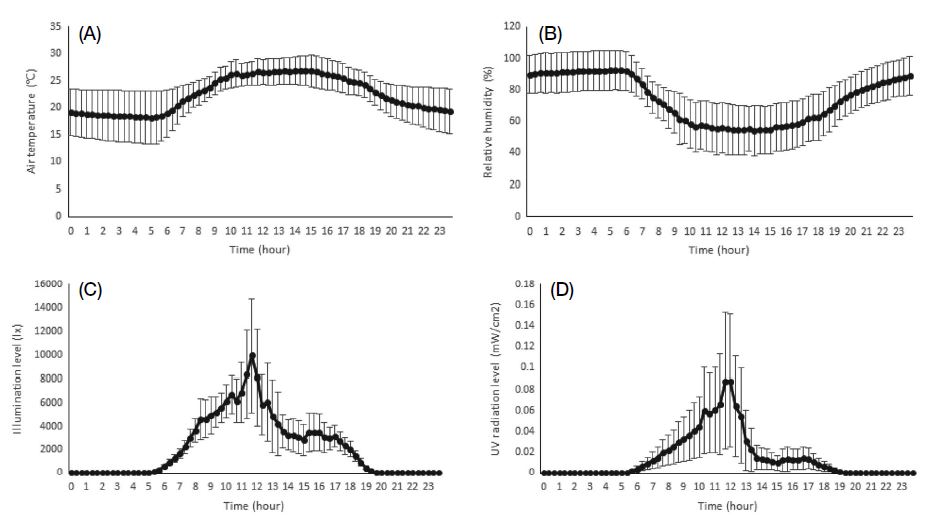
Climatic conditions during the survey period (May 17~June 17, 2017) in the passionfruit greenhouse: (A) temperature, (B) relative humidity, (C) illuminance level, and (D) UV level.
2. Pollination activity of different insect pollinators
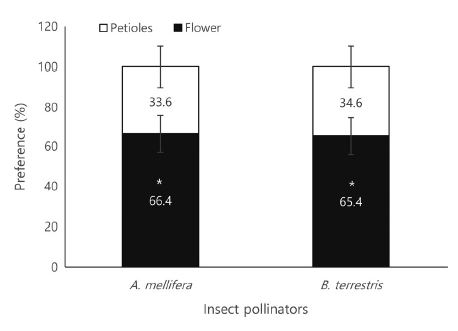
The bee traffic per hour showed significant differences between species (Table 3). Number of incoming individuals (t-test: t26=3.584, p=0.001), outgoing individuals (t26=2.770, p=0.010), and total traffic (t26=3.216, p=0.003) of A. mellifera were 4.9-fold, 2.7-fold, and 3.6-fold higher, respectively, than those of B. terrestris. However, colony size varied between the two bee species. Thus, if the previous results were converted to percentages, the rates of incoming individuals, outgoing individuals, and total traffic rates of B. terrestris were 14.6-fold (t26= -6.446, p=0.0001), 25.6-fold (t26= -6.557, p=0.0001), and 19.1-fold (t26= -13.171, p=0.0001) higher, respectively, than those of A. mellifera, and these differences were significant (Table 3). The bee traffic of A. mellifera and B. terrestris peaked at 15:00 and 08:00, respectively (Fig. 2A). Regarding foraging activity, the number of bees visiting the flowers (t30=2.473, p=0.019) and the total foraging activity (t30=2.100, p=0.044) over time showed statistically significant differences between the two species (Table 4). The number of flying bees (t-test: t16=2.473, p=0.019), number of flower visits (t16=1.955, p=0.080), and total foraging activity (t16=2.100, p=0.044) of A. mellifera were 10.6-fold, 7.7-fold, and 7.0-fold greater than those of B. terrestris, respectively. Converted to percentages, the rates of flying bees, flower visits, and total foraging activity of B. terrestris was 6.7-fold (t16= -2.858, p=0.008), 9.3-fold (t26= -3.785, p=0.001), and 10.2-fold (t26= -0.4383, p=0.0001) higher, respectively, than those in A. mellifera. The total foraging activity of A. mellifera was the highest at 12:00, followed by 13:00, 11:00, and 14:00. In contrast, B. terrestris showed the highest activity at 10:00, 17:00, and 14:00 (Fig. 2B). Meanwhile, regarding the bee traffic, A. mellifera showed a pattern where its activity was concentrated during the daytime (11:00~16:00) and then rapidly decreased, whereas B. terrestris activity was concentrated in the morning (07:00~09:00) and then gradually decreased. Regarding the foraging activity, A. mellifera was mainly active from 11:00 to 14:00, whereas B. terrestris showed a relatively even activity from 9:00 to 19:00. Both A. mellifera and B. terrestris had a higher preference for flowers than for the petioles on the passionfruit vines (Fig. 3). The percentage of honeybee flower visits per hour (66.4±10.24 bees) was 2-fold higher than that of petiole visits per hour (33.6± 10.24 bees) (t28=2.268, p=0.031). Likewise, B. terrestris visited the flowers 1.9-fold more often than they did the petioles (t36=2.299, p=0.027).
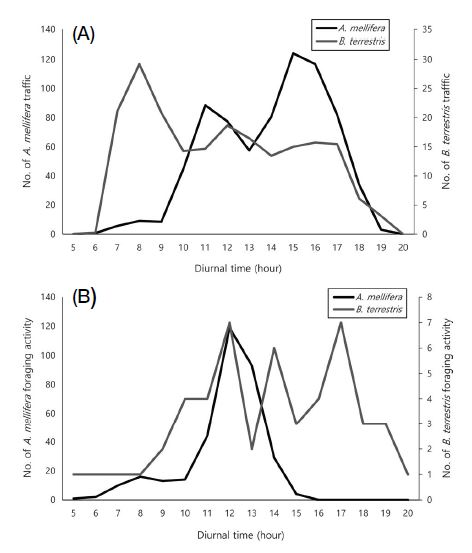
Daily bee traffic and foraging activity of Apis mellifera and Bombus terrestris in the passionfruit greenhouse.

Foraging activity of honeybees per hour Bee traffic of insect pollinators per hour throughout passionfruit blooming period
3. Relationship between bee activity and climatic conditions
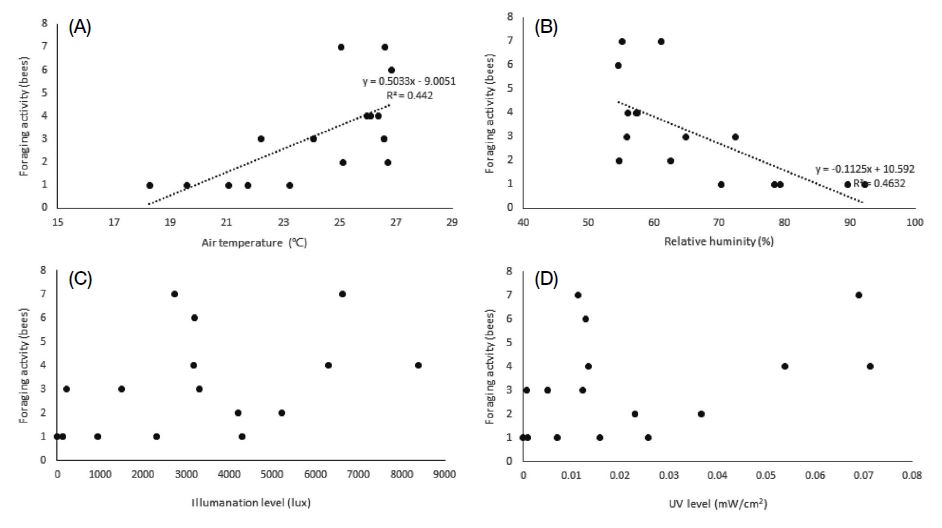
There was a significant correlation between the climatic conditions in the greenhouses and bee activity (Table 5). The foraging activity of A. mellifera was correlated with temperature (R=0.499), illuminance (0.593), and UV (0.647). In particular, illuminance and UV showed highly significant correlations with foraging activity (p<0.01). On the other hand, there was no correlation between foraging activity and relative humidity. A regression equation was derived from the regression analysis only for illuminance (ANOVA F1, 14=7.604, p=0.015, R2=0.352, DW=1.015) and ultraviolet radiation (F1, 14=10.099, p=0.007, R2=0.419, DW=0.951; Fig. 4). In the case of B. terrestris, temperature (0.665) and relative humidity (-0.681) were significantly correlated with foraging activity, whereas no correlation was observed between foraging activity and illuminance as well as between foraging activity and UV levels. Using regression analysis, significant regression equations were derived between the foraging activity of B. terrestris and temperature (F1, 14=6.757, p=0.021, R2=0.326, DW=1.961) as well as between foraging activity and relative humidity (F1, 14=7.635, p=0.017, R2=0.345, DW=2.008; Fig. 5).
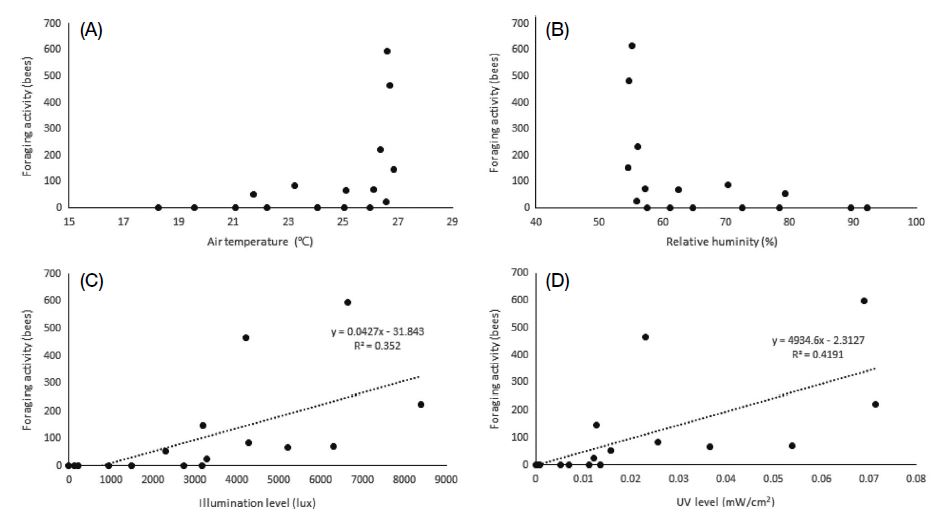
Relationship between foraging activity of Apis mellifera and the following climatic conditions: (A) temperature, (B) relative humidity, (C) illuminance level, and (D) UV level. There was no significant regression model in (A) and (B).
4. Effect of pollination methods on fruit quality
There was no significant difference in the weight and size of passionfruit fruits among the different pollination methods (Table 6). In contrast, the pulp weight, soluble solids, titratable acidity, and pulp flesh weight/ fruit weight ratio were significantly different among different pollination methods (Table 7). The fruit pulp flesh weight of plants pollinated by insect pollinators (38.7~39.4 g) was 8~9% higher than that of plants pollinated by artificial pollination (one-way ANOVA test: F2, 274=3.230, p=0.041). The soluble solid content of fruits pollinated by insect pollinators (14.6~14.7°Brix) was 1.1°Brix higher than that of fruits pollinated by artificial pollination (F2, 274=17.168, p=0.0001). The fruit titratable acidity of plants pollinated by insect pollinators (2.31~2.35%) was 0.16~0.20% higher than that of plants pollinated by artificial pollination (F2, 274=6.423, p=0.002). The pulp flesh weight/fruit weight ratio of plants pollinated by insect pollinators (0.54~0.55) was 6~8% higher than that of plants pollinated by artificial pollination (F2, 274=5.597, p=0.004). There were no significant differences in these parameters when the plants pollinated by the two insect pollinators were compared.
DISCUSSION
We investigated the activity characteristics and pollination effects of two insect pollinators (A. mellifera and B. terrestris) to investigate the possibility of insect pollination in greenhouse-cultivated passionfruit. Increasing bee foraging activity is important for obtaining effective pollination by bees (8). Climatic conditions are important factors determining the foraging activity of insect pollinators (9). As the temperature range in the passionfruit greenhouse during bee activity was 23~25℃, foraging activity of A. mellifera and B. terrestris was in accordance with their usual foraging activity. However, as the average illumination level was less than 10,000 lux, the foraging activity of B. terrestris, which is less affected by illuminance than A. mellifera, was not significantly affected by illumination, but A. mellifera may have been affected by illumination (see Figs. 4 and 5). In Korea, passionfruit vines are mostly trained to a “T”-shape (RDA, 2019). Passionfruit is a vine, which is why it is difficult to secure enough light for bees to forage in their “T”-shaped vines in greenhouses. Therefore, it would be more effective to use B. terrestris for pollination, as it is relatively less affected by illumination or ultraviolet rays than A. mellifera. Our results showed that the pollination effect by insect pollinators was the same as or greater than that of artificial pollination (Tables 6 and 7). There was no significant difference in fruit weight among the pollination methods; however, the amount of edible flesh was significantly higher in plants that were pollinated by insects than those pollinated artificially. In addition, the pulp flesh weight/fruit weight ratio, which can determine the amount of pulp in a fruit, was significantly higher in plants that were pollinated by insects than those pollinated artificially. Pulp flesh weight was correlated with sugar and acidity, which are factors determining fruit taste. Furthermore, it was confirmed that fruit weight related to fruit yield had a high correlation with pulp flesh weight (Table 8). Thus, we concluded that insect pollination was more advantageous in yielding better fruit quality than artificial pollination. The pollination effect among insect pollinators was investigated at the same level. Considering the colony size and the number of foraging bees in the present study, B. terrestris showed a higher pollination efficiency than A. mellifera. This was because, in general, compared to A. mellifera, B. terrestris is more adaptable to confined spaces, such as net houses (Katayama, 1987; Dimou et al., 2008). Therefore, future studies should compare the pollination effect of different insect pollinators in a greenhouse without a net house installed.

Correlations between weight of pulp flesh and fruit weight and length, diameter, number of seeds, soluble solids, and WP/WF ratio
Despite these results, there are several problems with using bees for direct passionfruit pollination. First, pollen robbing by insect pollinators occurs because of the difference between the start time of passionfruit flowering and the start time of insect pollinator activity. Pollen appears on passionfruit flowers immediately after blooming, but fertilization does not occur immediately, as the flower can be fertilized only when the stigma is spread downward and descended (McGregor, 1976; Kishore et al., 2010). In our study, passionfruit flowering started after 10:00, pollen was observed from the beginning of flowering, and the stigma spread downward and descended only 1 h after flowering (Table 2). However, a problem in pollination was related to the active time of the bees. As shown in Fig. 2, both A. mellifera and B. terrestris started to be active at 06:00. Therefore, pollen collection by bees was observed even before the flowers completely opened, meaning that during this time, bee visits to flowers could not result in pollination as the pollen could not yet reach the stigma. This type of bee behavior was described by Hammer (1987) and Yamamoto et al. (2012) as pollen robbing. To prevent this phenomenon, a method of controlling the density of the foraging bees, such as adjusting the entrance door to delay the bee activity start time, is required. Ish-Am (2009) also reported the problem of pollen robbing by bees in the pollination of passionfruit, and as a countermeasure, suggested a method of opening and closing the entrance of the beehives according to the flowering time of passionfruit. Passionfruit flowers are known to produce the highest amount of fruit when their stigmas are completely descended to increase the response of stigmas to pollen (Kishore et al., 2010). The second problem with using bees as passionfruit pollinators is that the efficiency of insect pollination decreases because of the presence of EFNs in passionfruit (Izaguirre et al., 2013). For this reason, bee foraging time is not entirely spent on collecting pollen or nectar from flowers, but also on visiting places irrelevant to pollination, which is why the labor force is dispersed. Our results also confirmed that 33~34% of foraging bees were directed to EFNs, such as those in petioles, instead of flowers (Fig. 3). Accordingly, the insect pollinator density per tree needs to be increased by more than 30%. In addition, there was no significant difference between the preference of the two insect pollinators for EFNs and flowers. However, as it is generally known that A. mellifera prefers nectar collection more than B. terrestris does (Pomeroy and Fisher, 2002), B. terrestris is considered to be more effective than A. mellifera for passionfruit pollination. Lastly, because of their relatively long pollination period, it is necessary to manage bee pollinators during passionfruit pollination. In greenhouses, passionfruit blooms in May, and has a flowering period of two months (Ish-Am, 2009; RDA, 2019). Nevertheless, the effective pollination period of B. terrestris is less than two months. This is because, in greenhouses, B. terrestris colonies usually develop for about one month and decrease thereafter (Blacquière et al., 2007). In the case of A. mellifera, colony size continues to decrease if management (such as feeding) is not carried out to maintain the oviposition of queen bees (DeGrandi-Hoffman et al., 2008). Decreasing colony size may cause problems with the production or quality of passionfruit. As shown in Fig. 6, unlike the weight of fruit obtained by artificial pollination, the weight of fruit obtained by insect pollination according to harvest period showed an increasing or decreasing pattern over time. Interestingly, this pattern was similar to the bee traffic pattern according to the period installed bee hive (Fig. 7). Based on the correlation analysis (Table 9), a negative correlation was confirmed for both A. mellifera (p=0.005) and B. terrestris (p=0.006) in daily bee traffic and beehive installed period. Moreover, the daily bee traffic of A. mellifera showed a significant correlation only with the weight of fruits obtained after pollination by A. mellifera (p=0.027), and the daily bee traffic amount of B. terrestris was correlated only with the weight of fruits obtained after pollination by B. terrestris (p=0.021). In other words, stable foraging activity of insect pollinators is required for the stable pollination of passionfruit. Thus, for stable passionfruit production, when using B. terrestris as a pollinator, a new colony must be installed when the foraging activity of the first colony decreases. In the case of A. mellifera, it is necessary to maintain the oviposition of queen bees so that the activity of the foraging bees does not decrease. If the above-described problems are recognized when using insect pollinators, a sufficient pollination effect can be obtained in passionfruit.
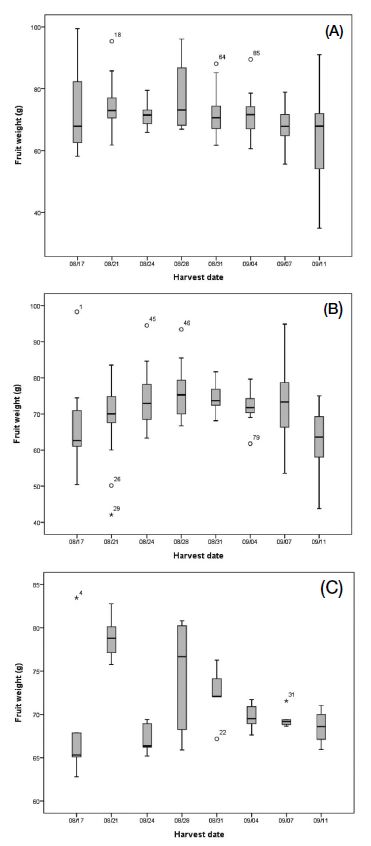
The weight of fruits (at harvest) pollinated by (A) Apis mellifera, (B) Bombus terrestris, and (C) artificial pollination. Gray boxes indicate the interquartile range; bold line in the boxes indicates the median; stars and empty cycles are the outliers.
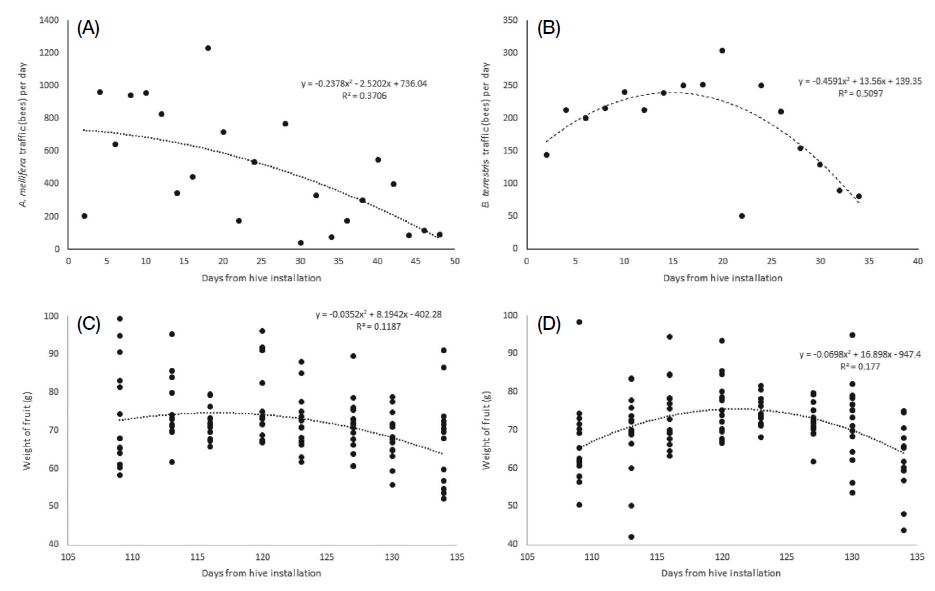
Comparison of the bee traffic pattern and fruit weight pattern according to the day from beehive installation: (A) bee traffic of A. mellifera, (B) bee traffic of B. terrestris, (C) fruit weight pollinated by A. mellifea, and (D) fruit weight pollinated by B. terrestris.
CONCLUSION
In Korea, passionfruit cultivators mostly depend on artificial pollination to obtain stable fruit sets. This type of pollination requires a high amount of labor during the flowering period, as each flower opens for only one day. Our results suggested that artificial pollination can be sufficiently replaced by insect pollination. In addition, our results confirmed that the management of insect pollinators is necessary during insect pollination, as simply placing beehives in the greenhouse is not enough for high yields. Our results provided an alternative pollination method that can be used to reduce the amount of labor needed for passionfruit cultivation. However, our study was limited as it only investigated the behavioral characteristics and effects of insect pollinators in confined spaces such as net houses. Therefore, in order to clearly determine the effects of various pollination methods on passionfruit cultivation, further studies are needed to compare the predicted yield and economic feasibility by examining the fruit set obtained using each pollination method. In addition, to effectively use insect pollinators in the field cultivation of passionfruit, it is essential to study the colony size of bees and the pollination method, while considering the total greenhouse area and the number of plants. Moreover, research on the control of foraging bee density to prevent pollen robbing by insect pollinators should also be conducted.
Acknowledgments
This work was supported by a grant from the National Institute of Agricultural Sciences, Rural Development Administration, Republic of Korea (Project No. PJ01332302).
References
- Aponte, Y. and D. Jáuregui. 2004. Algunos aspectos de la biología floral de Passiflora cincinnata Mast. Rev. Fac. Agron. 21: 211-220.
- Blacquière, T., B. Cornelissen and J. Donders. 2007. Bumble bee colony decline in greenhouses with supplemental lightning. Proc. Neth. Entomol. Soc. Meet. 18: 71-77.
-
Clarke, D. and D. Robert. 2018. Predictive modelling of honey bee foraging activity using local weather conditions. Apidologie 49: 1-11.
[https://doi.org/10.1007/s13592-018-0565-3]

- Cox, J. E. 1957. Flowering and pollination of passion fruit. Agri. Gaz. N. S. Wales. 68: 573-576.
-
DeGrandi-Hoffman, G., G. Wardell, F. Ahumada-Segura, T. Rinderer, R. Danka and J. Petti. 2008. Comparisons of pollen substitute diets for honey bees: consumption rates by colonies and effects on brood and adult populations. J. Apic. Res. 47: 265-270.
[https://doi.org/10.1080/00218839.2008.11101473]

-
Dhawan, K. S. and A. Sharma. 2004. Passiflora: A review update. J. Ethnopharmacol. 94: 1-23.
[https://doi.org/10.1016/j.jep.2004.02.023]

-
Dimou, M., S. Taraza, A. Thrasyvoulou and M. Vasilakakis. 2008. Effect of bumble bee pollination on greenhouse strawberry production. J. Apic. Res. 47: 99-101.
[https://doi.org/10.1080/00218839.2008.11101433]

- Free, J. B. 1993. Insect pollination of crops. Academic Press, London.
-
Freitas, B. M. and J. H. Oliveira. 2003. Rational nesting boxes for carpenter bees (Xylocopa frontalis) in the pollination of passionfruit (Passiflora edulis). Ciência Rural 33: 1135-1139.
[https://doi.org/10.1590/S0103-84782003000600021]

- Hammer, I. H. 1987. The pollinator of yellow passion fruit - do they limit the success of Passiflora edulis f. flavicarpa as a tropical crop? In: Proc. Florida State Hort. Soc. 100: 283-287.
-
Hoffmann, M., T. N. S. Pereira, M. B. Mercadante and A. R. Gomes. 2000. Polinização de Passiflora edulis f. flavicarpa (Passiflorales, Passifloraceae), por abelhas (Hymenoptera, Anthophoridae) em Campos dos Goytacazes, Rio de Janeiro. Iheringia Ser. Zool. 89: 149-152 (in Portuguese).
[https://doi.org/10.1590/S0073-47212000000200002]

-
Ish-Am, G. 2009. Directed honeybees: a preliminary investigation of an innovative solution for passionfruit pollination in Israel. Isr. J. Plant Sci. 57: 243-251.
[https://doi.org/10.1560/IJPS.57.3.243]

-
Izaguirre, M. M., C. A. Mazza, M. S. Astigueta and A. M. Ciarla. 2013. No time for candy: passionfruit (Passiflora edulis) plants down-regulate damage-induced extra floral nectar production in response to light signals of competition. Oecologia 173: 213-221.
[https://doi.org/10.1007/s00442-013-2721-9]

-
Junqueira, C. N. and S. C. Augusto. 2016. Bigger and sweeter passion fruits: effect of pollinator enhancement on fruit production and quality. Apidologie 48: 131-140.
[https://doi.org/10.1007/s13592-016-0458-2]

- Katayama, E. 1987. Utilization of honey bees as pollinators for strawberries in plastic greenhouse. Honeybee Science 8: 147-150 (in Japanese).
-
Keasar, T. 2010. Large carpenter bees as agricultural pollinators. Psyche 2010: 1-7.
[https://doi.org/10.1155/2010/927463]

- Kishore, K., K. A. Pathak, R. Shukla and A. Bharali. 2010. Studies on floral biology of passion fruit (Passiflora spp.). Pakistan J. Bot. 42: 21-29.
- Lederman, I. E. 1987. The involvement of ethylene in fruit development, maturation and ripening of the passionfruit (Passiflora edulis sims.). Ph.D. thesis, Hebrew Univ. Rehovot (in Hebrew).
- Lee, S., M. Byun, J. Park, Y. Cho, H. Cho, D. Lim, B. Jeong and K. J. Choi. 2013. Comparison of several pollination methods for the commercial production of purple passion fruit (Passiflora edulis Sims) in greenhouse condition. 99th Korean Society for Horticultural Science Conference. 2013. Iksan. Korea. p. 122.
-
Lim, C. K., S. Oh and S. C. Koh. 2018. Shoot growth, flowering, and fruit development of passion Fruit (Passiflora edulis var. edulis Sims.) in response to cold-temperature cutoff. Hortic. Sci. Technol. 36: 810-819.
[https://doi.org/10.12972/kjhst.20180079]

- McGregor, S. E. 1976. Insect pollination of cultivated crop plants. USDA Agriculture Handbook No. 496.
- Mizuno, S. 2008. Pollination efficiency of honeybees on tropical fruit grown in plastic Greenhouse. J. Sustain. Trop. Agric. Res. 1: 73-77.
-
Pomeroy, N. and R. M. Fisher. 2002. Pollination of kiwifruit (Actinidia deliciosa) by bumblebees (Bombus terrestris): effects of bee density and patterns of flower visitation. New Zeal. Entomol. 25: 41-49.
[https://doi.org/10.1080/00779962.2002.9722093]

- Rendón, J. S., J. Ocampo and R. Urrea. 2013. Study of pollination and floral biology of Passiflora edulis f. edulis Sims as a basis for pre-breeding. Acta. Agron. 62: 232-241.
- Rural Development Administration (RDA). 2019. Passionfruit Cultivation Manual. RDA press. Jeonju, Korea.
- Shimabukuro, K. and N. Matsuda (Okinawanoken), and M. Matsumura. 2007. Establishment of passion fruit cultivation technology (4th report) Effect of insect pollination on result rate and fruit quality. Autumn Conference and Symposium of the Japanese Society for Horticultural Science. Kagawa. Japan. p. 615.
- Silva, J. R. 2005. A cultura do maracujazeiro (Passiflora edulis Sims. f. flavicarpa Deg.) na Região do Triângulo Mineiro: Aspectos práticos, Relatório técnico. Isr. J. Plant Sci. 57: 243-251.
-
Silveira, M. V., A. R. Abot, J. N. Nascimento, E. T. Rodrigues, S. R. Rodrigues and A. Puker. 2012. Is manual pollination of yellow passion fruit completely dispensable?. Sci. Hortic. 146: 99-103.
[https://doi.org/10.1016/j.scienta.2012.08.023]

-
Yamamoto, M., C. I. Silva, S. C. Augusto, A. A. A. Barbosa and P. E. Oliveira. 2012. The role of bee diversity in pollination and fruit set of yellow passion fruit (Passiflora edulis forma flavicarpa, Passifloraceae) crop in Central Brazil. Apidologie 43: 51-62.
[https://doi.org/10.1007/s13592-012-0120-6]

-
Yoon, H. J., K. Y. Lee, H. S. Lee, M. Y. Lee, Y. S. Choi, M. L. Lee and G. H. Kim. 2017. Survey of insect pollinators use for horticultural crops in Korea, 2016. J. Apic. 32: 223-235.
[https://doi.org/10.17519/apiculture.2017.09.32.3.223]

- Yoon, H. J., S. E. Kim, K. Y. Lee, S. B. Lee and I. G. Park. 2008. The effect of temperature treatment on the production of worker or queen Bumblebees. J. Api. 23: 283-287.
-
Young, H. J., D. W. Dunning and K. W. Von Hasseln. 2007. Foraging behavior affects pollen removal and deposition in Impatiens capensis (Balsaminaceae). Am. J. Bot. 94: 1267-1271.
[https://doi.org/10.3732/ajb.94.7.1267]