Chemical Compositional Characterization on Five Samples for Development of Artificial Bee Feed
Abstract
The western honey bee, Apis mellifera L. is an essential pollinator for high yield in agriculture and provides products of economic values. Recently, the sudden decline of the bee population by numerous causes has occurred, which is called colony collapse disorder (CCD). One of the reasons in CCD, the limited nutrition in colonies declines brood rearing and shortens the lifespan of adult workers. Beekeepers need to provide artificial bee feed regularly to maintain health colony and continuity of bee-related products on apiculture. This study focuses on development of artificial bee feed, through investigation of different contents in nutritional components on five samples, namely canola pollen, mixed pollen, bee bread, MegaBee, and Test A. Among them, Test A was developed as artificial bee feed and was compared with other samples. The five samples were analyzed on free sugar, organic acid and amino acid. Sucrose content showed the largest amounts with 46.15% and 59.60% on Megabee and Test A, respectively. The Test A showed the largest account for citric acid with 92.33%. A total of 21 amino acids were detected on five samples. Among the detected 21 amino acids, proline was accounted the highest content in canola pollen, mixed pollen and bee bread with 1159.21 mg/L, 998.90 mg/L and 783.73 mg/L, respectively. Among the five samples, Test A showed the highest amount of lysine with 471.30 mg/L. Nutritional content, balance and efficiency needs to be considered for the development of artificial bee feed. This study will contribute to provide future directions on development of artificial bee feed.
Keywords:
Artificial bee feed, Nutrition, Free sugar, Organic acid, Amino acidINTRODUCTION
The Western honey bee, Apis mellifera L., is an essential partners of pollination for high yield in agriculture. It also provides honey, pollen, bee bread, propolis, royal jelly and bee venom and these products offer economic value (Kieliszek et al., 2018). The recent declines in honey bee populations and happened the sudden loss of honey bee in a colony is called colony collapse disorder (CCD) (Vanengelsdorp et al., 2015). The causes for CCD have been attributed to pesticide, parasitic mite (Amdam et al., 2004) and disease, but perhaps the reasons for bee-population declines is inadequate nutrition (van der Steen, 2007). A steady supply of pollen stimulates brood rearing and provides growth of colonies. Whereas, low nutrition contributes to reduced brood rearing and early transition in workers from nursing to foraging (DeGrandi-Hoffman et al., 2010). This early transition in workers negatively affect the longevity of honey bees. The premature foragers die faster than normal bees. Thus, colonies with limited nutrition will decline brood rearing and a shorter lifespan in adult workers. If parasitic mites and viruses are present, the colony decline can be even more serious and could be happened CCD.
Bees usually consume pollen after it is fermented, in the form of bee bread (Morais et al., 2013) Bee bread is the result of lactic fermentation of pollen collected by bees from flowers, mixed by their digestive enzymes and honey (Kieliszek et al., 2018). It differs from freshly collected pollen, in having a lower pH and less starch content (Herbert and Shimanuki, 1978; Ellis and Hayes, 2009). It is considered to have a higher nutritional value than pollen, better digestibility, and richer chemical composition. Bee bread is the main food in the hive especially for larva and young bees and it is the main source of protein in the diet of bees, determines their good health. It is mainly composed of proteins, vitamins, minerals, amino acids, fats and carbohydrates. The bee bread is considerably larger amount of peptides and free amino acids, because the proteins in bee bread are more biological active they are easily absorbed. Because of the proportions of ingredients in bee bread, it is a perfect supplementary nutrient.
Beekeepers is the necessity to maintain honey bee colonies in good condition during times when pollen is in short supply or not available (Morais et al., 2013). To maintain continuity of bee related products, beekeepers need to provide artificial bee feed regularly. But in Korea, beekeepers normally use pollen collected by other honey bee colonies is not a good option considering the health of the hive. Because of the risk of introducing disease into the colony (van der Steen, 2007). Therefore, artificial bee feed development is of vital importance for maintaining a healthy colony and increasing the productivity in apiculture. The purpose of this study is to investigate the nutritional value of canola pollen which is representative pollens that are widely used, mixed pollen, bee bread, MegaBee (commercial bee diet supplement) and our developed product which is named Test A that are replacing pollen components. The nutritional value was analyzed contents of free sugar, organic acid, and amino acids. This study will contribute towards providing a future direction on development of better artificial bee feed.
MATERIALS AND METHODS
1. Free sugar analysis
The free sugars from samples were analyzed using Dionex ultimate 3000 (Dionex). Free sugars were identified using a refractive index detector (Ri-101, Shodex) and a Sugar-pak column (Waters). The mobile phase was deionized water at a flow rate of 0.5 mL/min and the oven temperature was set to 80℃.
2. Organic acids analysis
The organic acids were analyzed using Dionex DX500 ion chromatograph (Dionex). Organic acids were identified using an electro conductivity detector and an amount to 20 μL of each sample was injected into the ICE-AS6 column. The mobile phase was 0.4 mM heptafluorobutyric acid at a flow rate of 1 mL/min with Anion-ICE micromembrane suppressor as a suppressor and 5 mM tetrabutylammonium hydroxide as a regenerant.
3. Amino acids analysis
The amino acids from each sample were analyzed using o-phthalaldehyde (OPA)/mercaptopropionic acid (MPA) and fluorenylmethyl chloroformate (FMOC) derivatization. The samples were mixed in borate buffer with OPA and FMOC. Samples were then analyzed using an Agilent 1200 series HPLC instrument (Agilent Technologies). After derivatization, an amount to 1 μL of each sample was injected into the INNO C18 column, 4.6 mm×150 mm, 5 μm (Youngjin Biochrom) at 40℃. Ultraviolet rays were detected at λ=338 nm by connected UV detectors. The emission and excitation wavelengths measured using fluorescence were 450 and 340 nm for the OPA derivative and 305 and 266 nm for the FMOC derivative. The mobile phase was comprised of solutions A (10 mM Na2HPO4 and 10 mM Na2B4O7·10H2O, pH 8.2) and B (water : acetonitrile (ACN) : methanol (MeOH)=10 : 45 : 45, v/v%) with a flow rate of 1.5 mL/min. The gradient of A: B was initially set to 100 : 0 (v/v%), 55 : 45 at 26~28 min, 0 : 100 at 28~30.5 min, and 100 : 0 from 30.5 min.
RESULTS AND DISCUSSION
1. Protein sources for development of artificial bee feed and Test A
The nutritional value of pollen often is evaluated by protein concentrations and amount of essential amino acids (Brodschneider and Crailsheim, 2010). To find protein sources for development of artficial bee feed, we searched twelve canditate protein sources which were industrial by-products. The sources were analysied for crude fiber, crude protein and essential amino acids. Depending on the results, brewer’s yeast and soybean oil meal were chosen for protein sources of substitute diet, which is named as Test A. The contents of Test A and the price of each ingredient was listed in Table 1. In test A, brewer̓s yeast and soybean oil meal was mainly used for protein suppliment. Soybean oil meal is suitable and most used feed for all livestock. The protein content is 50% after removing the skin of soybean, but 44% if the skin is not removed. Soybean oil meal is the standard for comparing the price or quality of different protein feeds. In soybean oil meal, the protein content or amino acid composition is uniform compared to other plant protein feeds. But soybean oil meal lacks of amino acids such as methionine, lysine and cystine, making it a limited source of amino acid for unit animals. Thus we mixed Brewer̓s yeast in Test A, it contain a lot of proteins, nucleic acids, carbohydrates, minerals, and vitamins.
2. Carbohydrates
Carbohydrates are primary energe source of honey bees (Brodschneider and Crailsheim, 2010). Nectar provides main source of carbohydrates for most bee species (Vaudo et al., 2015). Therefore sucrose is added in Teast A. Free sugar composition of honey accounts for an average of 38% fructose, 31% glucose which are the only monosaccharides in honey and other di and trisaccharids (Doner, 1977). Bee larvae require carbohydrates for normal development, often in the form of bee bread, however, a large quantity of carbohydrate-rich nectar is required by adult foragers (Brodschneider and Crailsheim, 2010). An adult honey bee worker require approximately 4 mg of utilizable sugars per day for survival (Barker and Lehner, 1974). Workers at foraging age have the enzymes necessary to use polysaccharides (starch) for flight metabolism (Hrassnigg et al., 2005). A lack of carbohydrates limits the number of larvae reared, when nectar sources are poor and winter storage has already depleted, or after harvesting the honey without adequate replacement of carbohydrates. Thus, carbohydrates are fed with sucrose solution to colonies in routine (Brodschneider and Crailsheim, 2010).
3. Sugar and organic acid
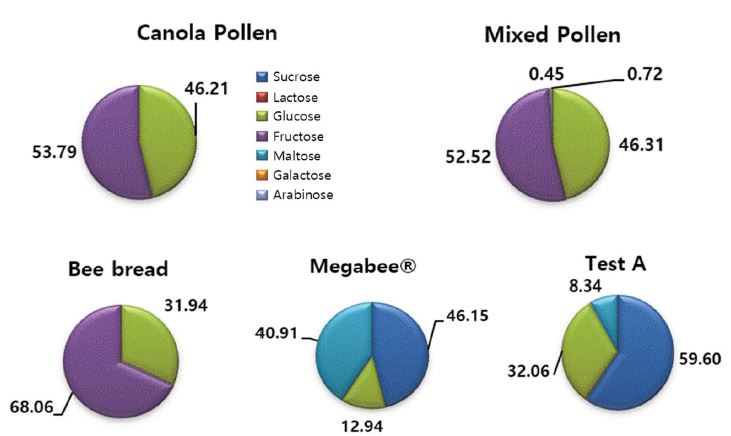
Canola pollen, mixed pollen, bee bread, Megabee and Test A were analysed for free sugar and organic acid concentration (Fig. 1 and Fig. 2). In Megabee and Test A, sucrose content showed the largest amounts with 46.15% and 59.60%, respectively. Sucrose was not found in canola pollen, mixed pollen and bee bread. Sucrose can not be used by honey bees and it’s molecule must be broken into two simple free sugars of glucose and fructose. This is done by an enzyme called sucrase, found in the digestive cavity stomach. Glusoce, as free sugar, plays a vital role in the functioning of brain, providing muscle energy for locomotion and body cells funtioning. Canola pollen and mixed pollen has a high content of glucose with 46.21% and 46.31%, respectively. Fructose, although an important free sugar for providing energy, can cause a problem like hydroxymethylfurfural (HMF) which is kown to be toxic for honey bees. Acids, like acetic acid (vinegar), citric acid (lemon juice) and tartaric acid (cream of tartar), produces HMF when mixed fructose. Bee bread showed the largest content of fructose with 68.06% among the 5 samples. In canola pollen and mixed pollen accounted for the largest content of fructose with 53.79% and 52.52%, respectively. However, fructose was not found in Megabee and Test A.
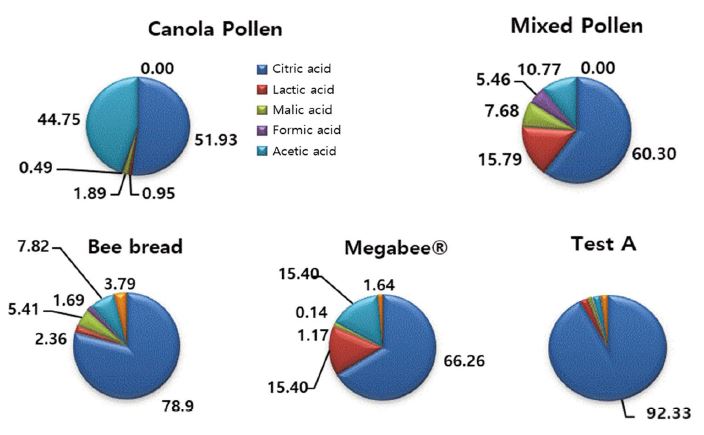
In the result of organic acid analysis, ctric acid accounted for the largest content in all five samples. Among them, Test A showed the highest amount of citric acid with 92.33%. Citric acid is a white powder used to give a sour taste to beverages and food products. It is also used as a preservative to prevent spoilage because it increases the acidity of products and restricts the bacterial growth responsible for food spoilage. It is also used in dietary supplements, as it enhaces the bioavailablity of the minerals.
4. Amino acids
De Groot reported in 1953 that arginine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan and valine are the essential amino acids for honeybees (De Groot, 1953). Table 2 enlists the functions and requirements of each essential amino acid for honey bee. Among them, isoleucine accounted the highest content with 4.5%. Dietary sources of essential amino acids are used for growth, somatic maintenance and reproduction. Nicolson and Human in 2013, reported the essential amino acid concentrations in bee-pollens, expressed as the percentage of the total amino acids ranges from 34.59% to 48.49%. The most abundant amino acids in pollen proteins are proline, leucine, lysine, glutamic acid and aspartic acid (Nicolson and Human, 2013). The results of amino acid content in five samples are listed in Table 3. Canola pollen, mixed pollen and bee bread accounted for the highest content of proline with 1159.21 mg/L, 998.90 mg/L and 783.73 mg/L, respectively. Proline, a non-essential amino acid, is an important amino acid for the laying of queen bees and also necessary for wing muscles development in insects. It is swiftly metabolized and produces several nicotinamide adenine dinucleotide phosphate. Thus, prolin is considered as a fovourable ingredient of honeybees (Kim et al., 2020). Among the five samples, Test A showed the highest amount of lysine with 471.30 mg/L. Lysine is directly involved in nitric oxide synthesis, a known neurotransmitter to affect memory in bees and moths (Gage et al., 2020).
CONCLUSION
Development of artificial bee feed is important in order to build and maintain healthy colonies and consequently increase the productivity. In bee bread nutritional contents such as free sugar, organic acid, and amino acids are of vital importance. Along with the nutritional content, economic efficiency also needs to be considered for the development of artificial bee feed. However, the development of a super artficial bee feed, depends on considering several aspects and requires multicentric studies.
Acknowledgments
This work was carried out with the support of “Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ0147622020)” Rural Development Administration, Republic of Korea and the Center for Women In Science, Engineering and Technology (WISET) Grant funded by the Ministry of Science and ICT (MSIT) under the Program for Returners into R&D (Project No. WISET-2020-495). Also, this research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (2016R1A2B3011742).
References
-
Amdam, G. V., K. Hartfelder, K. Norberg, A. Hagen and S. W. Omholt. 2004. Altered Physiology in Worker Honey Bees (Hymenoptera: Apidae) Infested with the Mite Varroa destructor (Acari: Varroidae): A Factor in Colony Loss During Overwintering? J. Econ. Entomol. 97: 741-747.
[https://doi.org/10.1093/jee/97.3.741]

-
Barker, R. J. and Y. Lehner. 1974. Acceptance and sustenance value of naturally occurring sugars fed to newly emerged adult workers of honey bees (Apis mellifera L.). J. Exp. Zool. 187: 277-285.
[https://doi.org/10.1002/jez.1401870211]

-
Brodschneider, R. and K. Crailsheim. 2010. Nutrition and health in honey bees. Apidologie 41: 278-294.
[https://doi.org/10.1051/apido/2010012]

-
DeGrandi-Hoffman, G., Y. Chen, E. Huang and M. H. Huang. 2010. The effect of diet on protein concentration, hypopharyngeal gland development and virus load in worker honey bees (Apis mellifera L.). J. Insect. Physiol. 56: 1184-1191.
[https://doi.org/10.1016/j.jinsphys.2010.03.017]

- De Groot, A. P. 1953. Protein and amino acid requirements of the honeybee (Apis mellifica L.). Physiol. Comp. Oecol. 3: 197-285.
-
Doner, L. W. 1977. The sugars of honey - a review. J. Sci. Food Agric. 28: 443-456.
[https://doi.org/10.1002/jsfa.2740280508]

-
Ellis, A. M., G. W. Hayes Jr. 2009. An evaluation of fresh versus fermented diets for honey bees (Apis mellifera). J. Apic. Res. 48, 215-216.
[https://doi.org/10.3896/IBRA.1.48.3.11]

-
Gage, S. L., S. Calle, N. Jacobson, M. Carroll and G. DeGrandi-Hoffman. 2020. Pollen Alters Amino Acid Levels in the Honey Bee Brain and This Relationship Changes With Age and Parasitic Stress. Front. Neurosci. 14: 231.
[https://doi.org/10.3389/fnins.2020.00231]

-
Herbert, E. W. and H. Shimanuki. 1978. Chemical composition and nutritive value of bee-collected and bee-stored pollen. Apidologie 9: 33-40.
[https://doi.org/10.1051/apido:19780103]

-
Hrassnigg, N. and K. Crailsheim. 2005. Differences in drone and worker physiology in honeybees (Apis mellifera L.). Apidologie 36: 255-277.
[https://doi.org/10.1051/apido:2005015]

-
Kieliszek, M., K. Piwowarek, A. M. Kot, S. Błażejak, A. Chlebowska-Śmigiel and I. Wolska. 2018. Pollen and bee bread as new health-oriented products: A review. Trends Food Sci. Technol. 71: 170-180.
[https://doi.org/10.1016/j.tifs.2017.10.021]

-
Kim, Y. K., S. Lee, J. H. Song, M. J. Kim, U. Yunusbaev, M. L. Lee, M. S. Kim and H. W. Kwon. 2020. Comparison of Biochemical Constituents and Contents in Floral Nectar of Castanea spp. Molecules 25: 4225.
[https://doi.org/10.3390/molecules25184225]

-
Morais, M. M., A. P. Turcatto, R. A. Pereira, T. M. Francoy, K. R. Guidugli-Lazzarini, L. S. Goncalves, J. M. V. de Almeida, J. D. Ellis and D. De Jong. 2013. Protein levels and colony development of Africanized and European honey bees fed natural and artificial diets. Genet. Mol. Res. 12: 6915-6922.
[https://doi.org/10.4238/2013.December.19.10]

-
Nicolson, S. W. and H. Human. 2013. Chemical composition of the ‘low quality’ pollen of sunflower (Helianthus annuus, Asteraceae). Apidologie 44: 144-152.
[https://doi.org/10.1007/s13592-012-0166-5]

-
Van der Steen, J. 2007. Effect of a home-made pollen substitute on honey bee colony development. J. Apic. Res. 46: 114-119.
[https://doi.org/10.1080/00218839.2007.11101377]

-
Vaudo, A. D., J. F. Tooker, C. M. Grozinger and H. M. Patch. 2015. Bee nutrition and floral resource restoration. Curr. Opin. Insect Sci. 10: 133-141.
[https://doi.org/10.1016/j.cois.2015.05.008]