
The Synergistic Effects for Apoptosis of Propolis Extracts and MG-132 on HeLa Ovarian Cancer Cell Line and Its Molecular Mechanism
Abstract
In this study, we investigated the synergistic effect of apoptosis of propolis extract and MG-132 on HeLa ovarian cancer cells. The cytotoxicity of MG-132 and propolis was confirmed on dose-dependent manner. The LD50 values of MG-132 and propolis showed at 10 μM and 100 μg/mL, respectively. Based on this concentration, in order to investigate the synergistic effect, the apoptosis effect of the mixture of low concentration of MG-132 and propolis extract was confirmed. The concentration of the mixture determined 0.5 μM MG-132 and 10 μg/mL and 25 μg/mL of propolis extracts. Cytotoxicity was measured after treating HeLa cells with a mixture of MG-132 and propolis extract. HeLa cells survived at least 90% in 0.5 μM of MG-132 and 25 μg/mL of propolis. However, it survived less than 80% when treated with a MG-132 and propolis extract. We investigated the level of expression of apoptosis-related proteins by Western blotting. When apoptosis stimulation occurred, apoptosis marker proteins PARP and caspase-3 produced 70 kDa and 19 kDa fragment, respectively. Treatment with a mixture of MG-132 and propolis extract produced PARP and caspase-3, but a single treatment did not. In the treatment of MG-132 and propolis extract, ubiquitination as another apoptosis molecule was expressed very strongly. In the mixture of MG-132 and propolis extract, the apoptosis molecule as well as proliferation protein expression level was changed. JAK showed digested fragments in mixture treatment group. Thus, the interaction of the mixture of MG-132 and propolis extract can induce apoptosis stimulation, and protein expression levels related to apoptosis and proliferation in cells are changed. Mixture of the two molecules could show potential as a cancer treatment.
Keywords:
Propolis, MG-132, HeLa, Cytotoxicity, CancerINTRODUCTION
Cancer is the most serious disease of many scientists and interesting research theme. Cancer causes cell due to genetic mutation in cells, such as the p53 gene. Abnormal mutation triggered rapid proliferation of cells and were assigned anti-apoptosis (Hollstein et al., 1991; Garrity et al., 2004). These events allowed for changes in protein expression level and functional molecules such as phosphorylation, glycosylation and structure modifications of proteins (Haldar et al., 1996; Rusa et al., 2005; Wiman, 2010; Christiansen et al., 2014). Today, many molecular scientists and physiologists are working to address mechanisms related cancer themes such as genesis, metastasis and angiogenesis etc. (Kingsley et al., 2007; Cai and Chen, 2008; Mulder et al., 2009; Mashouri et al., 2019). Many cancer treatments have been developed, including surgery, drug theraphy, and gene targeting (De Vita Jr. et al., 1975; Perez-Tomas, 2006). Among these, drug treatments related to cancer are divided into natural and synthetic drugs. Peptide-aldehyde proteasome inhibitor MG-132 (carbobenzoxyl-L-leucyl-L-leucyl-L-leucinal) is associated with more than 80% of protein degradation (Guo and Peng, 2013). Recently, it has been able to suppress the growth of various human cancer cells (Shirley et al., 2005; Wente et al., 2005; Matsuo et al., 2010). Stoll et al. (2009) have shown that MG-132 provides evidence that it plays an essential role in apoptosis. Previous studies have shown that MG-132 enhances cisplatin-induced apoptosis in various tumor cells (Dang et al., 2014; Guo et al., 2016). However, the molecular mechanisms involved in MG-132 are not fully understood.
Propolis is a resinous product produced honeybee by mixing beeswax with exudate from various trees and plants. It used to build and protect honeybees hive. Propolis is composed of various functional compounds such as flavonoids, phenyl compounds and beeswax (de Groot, 2013). It has already been used to cure wounds. Propolis contains various active substances (de Freitas et al., 2017). Recently, propolis compounds have been studied as various functional materials. Propolis includes antimicrobial (Fernandes Junior et al., 2005; Wojtyczka et al., 2013), antioxidant (Zhang et al., 2013), anti-inflammatory (Kim et al., 2018) and anti-tumor (Sforcin, 2007) activities. However, the effects of propolis have been reported in various research regions, its molecular mechanisms are insufficient. Especially, the synergistic effects of propolis molecule functions related to anti-tumor activities with other anti-cancer drugs have not been sufficiently studied.
In this study, after confirming the cytotoxicity of MG-132 and propolis, the synergistic effect of the two substances was investigated. The low concentration without cell death was determined by the cytotoxic concentration of MG-132 and propolis. Then, a low concentration of MG-132 and propolis mixture was treated in HeLa ovarian cancer cell line. The cytotoxicity effects of MG-132 and propolis mixture was measured and the expression level of the mixture treated protein was confirmed. ubiquitination, PARP and caspase-3 levels were determined by Western blotting. Our data suggested that a mixture of MG-132 and propolis has a stronger activity against apoptosis and alters the expression level of the protein. As a result, propolis may be used as a drug adjuvant material for anti-cancer dugs.
MATERIALS AND METHODS
1. Reagents
The propolis was supplied by Chungju beekeeper in Korea. We have prepared the propolis extracts as described below. The proteasome inhibitor MG-132 was purchased from Sigma Aldrich (USA). Dulbecco’s modified eagle’s medium (DMEM), fetal bovine serum (FBS) and penicillin-streptomycin solution for subculture of HeLa ovarian cancer cell lines were purchased from Gibco (USA). FBS was used after serum inactivating step in 56℃ for 50 min. The EZ-Cytox MTT solution for measuring cell viability was purchased from DainBio (Korea). Janus kinase (JAK), PARP (Poly (ADP-ribose) Polymerase), ubiquitination, Stat3 (Signal Transducer and Transcriptions) and caspase-3 antibodies were purchased from Cell Signaling Technology (USA) and the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody was purchased from Santacruz biotechnology (U.S.A.).
2. Cell culture
The HeLa ovarian cancer cell lines was purchased from the Korea Cell Line Bank (KCLB) and maintained with high glucose DMEM containing 10% FBS and 100 units/mL penicillin-streptomycin as antibiotics. Subculture of the cells with 0.05% trypsin for cell detachment and maintained in an incubator set at 37℃ and 5% CO2.
3. Extraction of propolis from raw propolis
2 L of 80% ethanol (EtOH) solvent was added to 200 g of raw propolis in Chungju apiary, Korea. Propolis was extracted for 48 hrs at room temperature. Propolis extracts were filtered by non-woven fabric, and then the micro-debris of raw propolis was completely removed with a Whatman No. 2 filter. This extract was absolutely concentrated by vacuum evaporation. The evaporated propolis extract was diluted with absolute EtOH to the experimental conditions.
4. Cytotoxicity of HeLa cells for MG-132, propolis and MG-132/propolis mixture
Cytotoxicity was measured to investigate the effect of MG-132, propolis and MG-132/propolis mixture on cell viability. The cytotoxicity assay used was EZ-Cytox MTT assay kit. HeLa cells were inoculated into 96-well plate of 2×104 cells/well and incubated for 24 h. The MG-132 and propolis were dose-dependent manner: MG-132 concentrations are 0, 0.1, 0.5, 1, 5, 10, 25 and 50 μM, which is dimethyl sulfoxide (DMSO) as a control for MG-132 solvent. Propolis extract concentrations are 0, 0.1, 0.5, 1, 5, 10, 25 and 50 μg/mL. And absolute EtOH was used as a control for propolis extraction solvent. HeLa cells were treated with MG-132 and propolis, respectively. As a control for this experiment, only medium was treated without drugs and propolis extractOnly EtOH and DMSO were compared as a solvent control. After 24 hrs exposure, EZ-Cytox solution was added to the medium at 1/10 volume and the cells were incubated for 2 hrs. The optical density (O.D.) value of the medium was determined at a wavelength of 450 nm.
Based on these results, the concentration of the MG-132/propolis mixture was determined. The number of inoculated cells and culture conditions are the same as previously described. In this experiment, we divided to 6-groups as media: EtOH, DMSO, 0.5 μM of MG-132, 25 μg/mL of propolis extract and 0.5 μM of MG-132/25 μg/mL of propolis extract.
5. Western blotting
Proteins for Western blotting were extracted from HeLa cells. Cells treated with MG-132, propolis and MG-132/propolis were washed with 1X phosphate buffered saline (PBS) and lysed with 300 μL of nonidet P (NP)-40 lysis buffer (Dainbio, Korea). The cell lysate supernatant was cleared by centrifugation at 13,000 rpm for 20 min. Cell lysate concentration was determined by bicinchronic acid (BCA) assay. 20 μg of cell lysate was used for western blotting. Proteins were resolved with 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins in the gel were transferred to a polyvinylidene fluoride (PVDF) membrane. To block the non-specific binding, membranes were incubated in 2% skim milk at 1X tris-buffered Tween 20 (1X TBST) for 1 h at room temperature. The membranes were incubated with primary antibodies such as anti-JAK, anti-PARP, anti-caspase-3, anti-ubiquitination, anti-Stat3 and anti-GAPDH. All antibodies were incubated for 16 h at room temperature. The expressed protein levels were detected by secondary horseradish peroxide (HRP)-conjugated anti-goat antibodies. Protein expression was visualized with an enhanced chemiluminescence (ECL) pico detection system (GenDEPOT, Korea).
6. Statistical Analyses
The statistical significance of the results obtained through the experiment was obtained using the R statistical program (3.4.1 version, NewZealnd), and the experimental mean significance was verified at p<0.05 by ANOVA test.
RESULTS AND DISCUSSION
1. MG-132 and propolis affected HeLa cell viability
We investigated the cell viability of MG-132, propolis and MG-132/propolis mixture. First, to confirm the cytotoxicity of the MG-132 and propolis, the HeLa ovarian cancer cell line was cultured dose-dependently with MG-132 and propolis. The concentrations of MG-132 are 0, 0.1, 0.5, 1, 5, 10, 25 and 50 μM. Propolis concentrations are 0, 0.1, 0.5, 1, 5, 10, 25 and 50 μg/mL. Cells were seeded in 96-well plates at 2×104 per well. After 24 h, medium containing dose-dependent MG-132 and propolis were replaced, respectively. The cells were incubated for 24 h in an incubator at 37℃, 5% CO2. EZ-cytox MTT assay solution was added to each well at 1/10 medium volume and incubated for 2 h. As shown in Fig. 1A, the LD50 value of MG-132 was 1 μM. And the LD50 value of propolis was more than 50 μg/mL (Fig. 1B). Since the purpose of our study was to investigate the synergistic effect of MG-132 and propolis, we determined the MG-132/propolis mixture concentration based on the results.
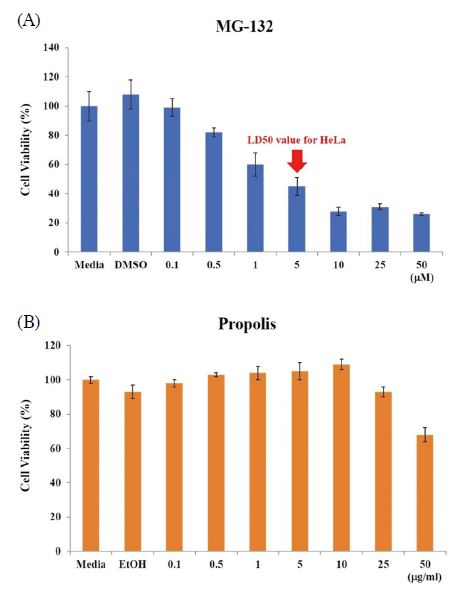
Cytotoxicity of MG-132 and propolis on HeLa cells. HeLa cells were inoculated in 96-well plates at 2×104 cells/well. After 24 h, cells were treated MG-132 (A) and propolis (B) in dose-dependent manner. Cell viability was measured by EZ-Cytox MTT assay. Error bar is standard deviation.
Next, in order to recognize the synergistic effect of MG-132 and propolis, we selected low concentration MG-132 and propolis above LD50 value. For this reason, choose a concentration of 0.5 μM of MG-132 and a propolis concentration of 25 μg/mL. When treated with MG-132 and propolis each alone, this concentration dose not affects cell viability. We compared the cytotoxicity of MG-132, propolis alone and MG-132/propolis mixture. The cell viability of 0.5 μM of MG-132 and 25 μg/mL of propolis were 87% and 96%, respectively. However, the cell viability of two mixtures was 78%. The mixture of the two materials reduced cell viability by approximately 10% (Fig. 2A). These results mean that the mixture of MG-132 and propolis has a better effect than single treatment for cancer cell apoptosis. In addition, these results showed the synergistic effect of MG-132/propolis mixture and the potential of propolis as an adjuvant for cancer treatment. Fig. 2B shows the morphology of cells for each cytotoxicity.
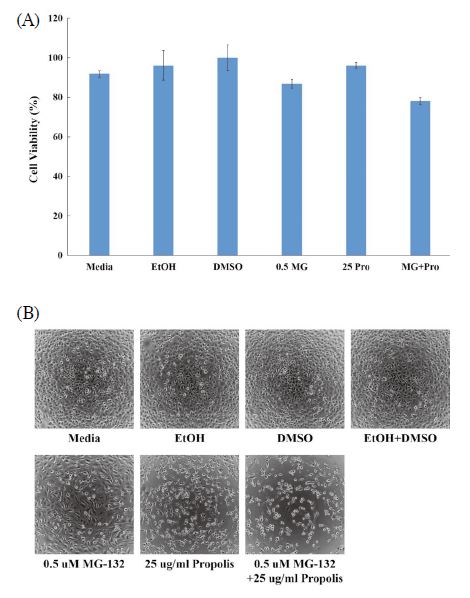
Synergistic effects of the MG-132/propolis mixture. 0.5 μM of MG-132 and 25 μg/mL of propolis were treated into 2×104 cells/well of HeLa cells. Cytotoxicity (A) and cell morphology (B) of MG-132, propolis and MG-132/propolis mixture (0.5 MG: 0.5 μM of MG-132, 25 Pro: 25 μg/mL of propolis, MG+Pro: 0.5 μM of MG-132 and 25 μg/mL of propolis mixture). Error bar is standard deviation.
2. Regulation of apoptosis-related protein expression level in HeLa ovarian cancer cell line of MG-132/propolis mixture
When MG-132/propolis mixture was treated, the expression level of apoptosis-related protein was confirmed. HeLa cells were seeded in 100 mm dish on 1×107. We tested two additional doses for a 10 μg/mL propolis and 0.5 μM MG-132/10 μg/mL of propolis mixture to see if the protein expression levels depend on the dose-dependent MG-132/propolis mixture. Only the media treatment group is a control in this experiment. And EtOH and DMSO are solvent controls for propolis and MG-132 diluent, respectively. Ubiquitination, PARP and caspase-3 are apoptosis markers in cells, and JAK and Stat3 are proliferative signaling proteins. GAPDH is the loading control for these experiments. When only MG-132 was present, the level of ubiquitination expression was higher than that of the control group, and propolis did not increase the ubiquitination level. By the way, ubiquitination is strongly expressed in MG-132/propolis mixture group. This results means that propolis is an adjuvant to MG-132 in the MG-132/propolis mixture. Increased ubiquitination leads to more abnormal protein degradation, induced apoptosis, and cell death. When the cell stimulates apoptosis signal, the representative apoptosis protein PARP is produced as a 70 kDa fragment. The group treated with only MG-132 did not differe from the PARP fragment compared to the control group. However, cells added with 25 μg/mL of propolis appeared to have more PARP fragments. The MG-132/propolis mixed group did not differ from the propolis alone group. This means that propolis plays an essential role in PARP fragmentation. In other words, propolis is an adjuvant in ubiquitination and has been an important key to PARP protein fragmentation. However, no caspase-3 fragment is produced in the MG-132 only and propolis only groups. Active caspase-3 appeared only in the MG-132/propolis mixture group. MG-132 and propolis triggered ubiquitination and PARP fragmentation, respectively, but were unable to trigger apoptosis in cells. This means that caspase-3 activation requires simultaneous treatment of MG-132 and propolis (Fig. 3A).
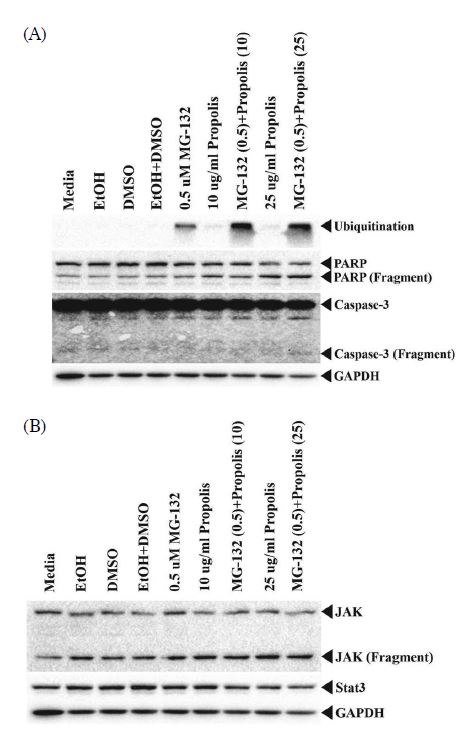
(A) Apoptosis-related protein expression by treatment with MG-132/propolis mixture. All lysates were used at 20 μg for Western blotting. Each antibody diluted at 1 : 1,000 in 2% skim milk was incubated overnight except for GAPDH. GAPDH was diluted 1 : 5,000 in 2% skim milk. (B) Proliferation-related protein expression by MG-132/propolis mixture treatment. Cell lysates were used at 20 μg for Western blotting. JAK and Stat3 antibody diluted at 1 : 1,000 and 1 : 500, respectively. Each antibody diluted in 2% skim milk and incubated for overnight.
3. Evaluation of proliferation-related protein expression after verification of apoptosis-related protein expression
The Stat3 protein is a transcriptional regulator of genes in human cells. When extracellular signaling molecules such as cytokine and growth factor stimulate the cells, the Stat3 protein is translocated to the nucleus. As a result, cells regulate the transcription of genes involved in proliferation, differentiation, growth and immune response. This Stat3 protein activation is regulated by JAK protein phosphorylation. JAK and Stat3 are molecules upstream of the signaling pathway, and their activation is an essential key for regulating cell survival or apoptosis. As shown in Fig. 3B, JAK fragmentation occurs in the group of MG-132 and propolis treatment groups. JAK protein fragmentation increased with increasing propolis concentration. And the MG-132/propolis mixed group showed more fragment levels compared to the GAPDH value. This means that MG-132 and propolis induced JAK protein degradation. Thus, the cells did not transmit signals related to proliferation and signals related to apoptosis are activated. Stat3 expression was no longer affected due to the truncated JAS protein. That is, the MG-132/propolis mixture has the JAK-Stat3 signaling cascade disabled. GAPDH is the loading control in these experiments.
CONCLUSION
Propolis is known as a useful functional material for health functional foods because of its excellent effects such as antioxidants, antibiotics, and anti-inflammatory. However, propolis studies on disease treatment are not clear. Its potential as an inflammatory protection has been reported by Kim et al. (Kim et al., 2018). To expand the use of propolis for cancer clinical adjuvant, we investigated the apoptosis mechanisms of propolis and MG-132 in HeLa ovarian cancer cells. MG-132 is known as a proteasome inhibitor, but it is also known as an anticancer agent that inhibits the growth of cancer cells. However, its usage as an anticancer drug is very limited due to cytotoxicity and side-effects. Therefore, propolis obtained from nature was used as an adjuvant for anticancer drugs.
We investigated the concentration of MG-132 cytotoxicity against HeLa ovarian cancer cells and determined the low concentration without cell death for synergistic effect based on the cell viability results. Our data suggested that propolis aids in cancer cell death during MG-132 anticancer activity. Simultaneous process of MG-132 and propolis regulates the expression levels of intracellular apoptosis and proliferation-related proteins. PARP and caspase-3 were turned into active formation and the JAK protein was degraded and cleaved. Ubiquitination is activated and increased. This process accelerates the cell death of cancer cells. Therfore, propolis is a useful adjuvant in the treatment of ovarian cancer and detailed mechanisms such as its effect on phosphorylation and glycosylation of proteins should be identified.
Acknowledgments
This study was carried out with the support from the RDA grant (PJ01387801).
References
-
Cai, W. and X. Chen. 2008. Multimodality molecular imaging of tumor angiogenesis. J. Nucl. Med. 49: 113S-128S.
[https://doi.org/10.2967/jnumed.107.045922]

-
Christiansen, M. N., J. Chik, L. Lee, M. Anugraham, J. L. Abrahams and N. H. Packer. 2014. Cell surface protein glycosylation in cancer. Proteomics 14: 525-546.
[https://doi.org/10.1002/pmic.201300387]

-
Dang, L., F. Wen, Y. Yang, D. Liu, K. Wu, Y. Qi, X. Li, J. Zhao, D. Zhu, C. Zhang and S. Zhao. 2014. Proteasome inhibitor MG132 inhibits the proliferation and promotes the cisplatin-induced apoptosis of human esophageal squamous cell carcinoma cells. Int. J. Mol. Med. 33: 1083-1088.
[https://doi.org/10.3892/ijmm.2014.1678]

-
de Freitas, M. C. D., M. B. de Miranda, D. T. de Oliveira, S. A. Vieira-Filho, R. B. Caligiorne and S. M. de Figueiredo. 2017. Biological activities of red propolis: A review. Recent Pat. Endocr. Metab. Immune Drug Discov. 11: 3-12.
[https://doi.org/10.2174/1872214812666180223120316]

-
de Groot, A. C. 2013. Propolis: a review of properties, applications, chemical composition, contact allergy, and other adverse effects. Dermatitis 24: 263-282.
[https://doi.org/10.1097/DER.0000000000000011]

-
De Vita Jr V. T. MD, R. C. Young MD and G. P. canellos MD. 1975. Combination versus single agent chemotherapy: A review of the basis for selection of drug treatment of cancer. Cancer: 98-110.
[https://doi.org/10.1002/1097-0142(197501)35:1<98::AID-CNCR2820350115>3.0.CO;2-B]

-
Fernandes Junior, A., E. C. Balestrin, J. E. Betoni, O. Orsi Rde, L. da Cunha Mde and A. C. Montelli. 2005. Propolis: anti-Staphylococcus aureus activity and synergism with antimicrobial drugs. Mem. Inst. Oswaldo Cruz. 100: 563-566.
[https://doi.org/10.1590/S0074-02762005000500018]

-
Garrity, M. M., L. J. Burgart, M. R. Mahoney, H. E. Windschitl, M. Salim, M. Wiesenfeld, J. E. Krook, J. C. Michalak, R. M. Gldberg, M. J. O’Connell, A. F. Furth, D. J. Sargent, L. M. Murphy, E. Hill, D. L. Riehle, C. H. Meyers and T. E. Witzig. 2004. Prognostic value of proliferation, apoptosis, defective DNA mismatch repair, and p53 overexpression in patients with Resected Dukes’ B2 or C colon cancer: A north central cancer treatment group study. J. Clin. Oncol. 22: 1572-1582.
[https://doi.org/10.1200/JCO.2004.10.042]

-
Guo, N. and Z. Peng. 2013. MG132, a proteasome inhibitor, induces apoptosis in tumor cells. Asia Pac. J. Clin. Oncol. 9: 6-11.
[https://doi.org/10.1111/j.1743-7563.2012.01535.x]

-
Guo, N., Z. Peng and J. Zhang. 2016. Proteasome inhibitor MG132 enhances sensitivity to cisplatin on ovarian carcinoma cells in vitro and in vivo. Int. J. Mol. Med. 33: 1083-1088.
[https://doi.org/10.1097/IGC.0000000000000703]

- Haldar, S., J. Chintapalli and C. M. Croce. 1996. Taxol induces bcl-2 phosphorylation and death of prostate cancer cells. Cancer Res. 56: 1253-1255.
-
Hollstein, M., D. Sidransky, B. Vogelstein and C. C. Harris. 1991. P53 mutation in human cancers. Sicence 253: 49-53.
[https://doi.org/10.1126/science.1905840]

-
Kim, S. K., S. O. Woo, S. M. Han, S. G. Kim, K. W. Bang, H. R. Jang, H. J. Moon and H. J. Kim. 2018. Anti-inflammatory effects of Korean propolis extracts on Raw264.7 macrophage cells. Korean J. Apic. 33: 187-194.
[https://doi.org/10.17519/apiculture.2018.09.33.3.187]

-
Kingsley, L. A., P. G. J. Fournier, J. M. Chirgwin and T. A. Guise. 2007. Molecular biology of bone metastasis. Mol. Cancer Ther. 6: 2609-2617.
[https://doi.org/10.1158/1535-7163.MCT-07-0234]

-
Mashouri, L., H. Yousefi, A. R. Aref, A. M. Ahadi, F. Molaei and S. K. Alahari. 2019. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol. Cancer 18: 75.
[https://doi.org/10.1186/s12943-019-0991-5]

-
Matsuo, Y., H. Sawai, N. Ochi, A. Yasuda, M. Sakamoto, H. Takahashi, H. Funahashi, H. Takeyama and S. Guha. 2010. Proteasome inhibitor MG132 inhibits angiogenesis in pancreatic cancer by blocking NF-kappaB activity. Dig. Dis. Sci. 55: 1167-1176.
[https://doi.org/10.1007/s10620-009-0814-4]

-
Mulder, W. J., K. Castermans, J. R. van Beijnum, M. G. Oude Egbrink, P. T. Chin, Z. A. Fayad, C. W. Lowik, E. L. Kaijzel, I. Que, G. Storm, G. J. Strijkers, A. W. Griffioen and K. Nicolay. 2009. Molecular imaging of tumor angiogenesis using alphavbeta3-integrin targeted multimodal quantum dots. Angiogenesis 12: 17-24.
[https://doi.org/10.1007/s10456-008-9124-2]

-
Perez-Tomas, R. 2006. Multidrug resistance: Retrospect and prospects in anti-cancer drug treatment. Curr. Med. Chem. 13: 1859-1876.
[https://doi.org/10.2174/092986706777585077]

-
Rusa, J., A. Moritz, K. A. Lee, A. Guo, V. L. Goss, E. J. Spek, H. Zhang, X.-M. Zha, R. D. Polakiewicz and M. J. Comb. 2005. Immunoaffinity profiling of tyrosine phosphorylation in cancer cells. Nat. Biotechnol. 23: 94-101.
[https://doi.org/10.1038/nbt1046]

-
Sforcin, J. M. 2007. Propolis and the immune system: a review. J. Ethnopharmacol. 113: 1-14.
[https://doi.org/10.1016/j.jep.2007.05.012]

-
Shirley, R. B., I. Kaddour-Djebbar, D. M. Patel, V. Lakshmikanthan, R. W. Lewis and M. V. Kumar. 2005. Combination of proteasomal inhibitors lactacystin and MG132 induced synergistic apoptosis in prostate cancer cells. Neoplasia 7: 1104-1111.
[https://doi.org/10.1593/neo.05520]

-
Stoll, S. J., C. P. Susan and C. Herbert. 2009. Follicular thyroid cancer cell growth inhibition by proteasome inhibitor MG132. J. Surg. Res. 156: 39-44.
[https://doi.org/10.1016/j.jss.2009.03.070]

-
Wente, M. N., G. Eibl, H. A. Reber, H. Friess, M. W. Büchler and O. J. Hines. 2005. The proteasome inhibitor MG132 induces apoptosis in human pancreatic cancer cells. Oncol. Rep. 14: 1635-1638.
[https://doi.org/10.3892/or.14.6.1635]

-
Wiman K. G. 2010. Pharmacological reactivation of mutant p53: from protein structure to the cancer patient. Oncogene 29: 4245-4252.
[https://doi.org/10.1038/onc.2010.188]

-
Wojtyczka, R. D., A. Dziedzic, D. Idzik, M. Kepa, R. Kubina, A. Kabala-Dzik, J. Smolen-Dzirba, J. Stojko, M. Sajewicz and T. J. Wasik. 2013. Susceptibility of Staphylococcus aureus clinical isolates to propolis extract alone or in combination with antimicrobial drugs. Molecules 12: 9623-9640.
[https://doi.org/10.3390/molecules18089623]

- Zhang, J. L., K. Wang and F. L. Hu. 2013. Advance in studies on antioxidant activity of propolis and its molecular mechanism. Zhongguo zhong yao Za Zhi. 38: 2645-2652.