
Acute and Chronic Toxicity of Selected Pesticides Used in Strawberry Greenhouse to Honeybee (Apis mellifera) Larvae
Abstract
Honey bee colonies have often collapsed when introduced in strawberry greenhouses for pollination. Toxicological risk assessments conducted to determine the likelihood how a given pesticide would impact honey bees primarily consider the survival of adult bees exposed to the pesticide. However, larvae are also exposed to pesticides as their diets can contain contaminated nectar and pollen collected by foragers. To understand the effect of pesticides in greenhouses on honey bee larvae, we investigated the effects of some insecticides used in strawberry greenhouse on A. mellifera larvae, by acute and chronic toxicity bioassay. Commercial formulations were serially diluted from the recommended concentration to 10-6 times. For acute toxicity, 30 μL of desired concentration of pesticides in diet C were fed to the three-day-old larvae. Mortality of larvae was monitored until the 6th day. For chronic exposure of larvae toxicity, residue level concentrations of pesticides were exposed from 2nd to the 5th days. Our results show toxicity order of emamectin benzoate, lufenuron, fluxametamide, acequinocyl, and Acetamiprid with LD50 values 0.000, 0.4, 8.1, 10 and 982.3 μg/larvae respectively. Among the two pesticides tested chronically, honey bee larvae were highly sensitive to emamectin benzoate that killed more than 50% in the 6th day at 35, and 73 ppb. These results show that exposure of larvae to emamectin benzoate results in negative effects on the survival of bees, possibly compromising the dynamics of the colony, contributing to the decline of bee populations.
Keywords:
Acute, Chronic test, Honey bee, Toxicology, Emamectin benzoateINTRODUCTION
A number of reports from honey bee suppliers for crop pollination reveal that honey bee colonies often collapse when they are installed in greenhouses, particularly for pollination of strawberry during winter season (Morimoto et al., 2011). Multiple factors such as pesticides, pest and disease, habitat loss, climate change and invasive species can cause the bee population decline (Goulson et al., 2015). However, the extensive use of pesticides on crops is one major responsible factor in this regard. Environmental risk assessments conducted to determine the likelihood impact of a given pesticide primarily consider the survival of adult bees (Medrzycki et al., 2013). However, larvae are also exposed to pesticides as their diets may contain contaminated nectar and pollen collected by foragers (Chauzat and Faucon, 2007; Dai et al., 2019). Similarly, in the strawberry greenhouse worker bees could forage contaminated pollen to nurse their broods, during the winter when they are normally in the middle of overwintering. Additionally, immature honey bee can be continuously exposed to pesticides and their metabolites stored in hive (Sanchez-Bayo and Goka, 2014).
Insecticides affect not only the target pest but also many other non-target animal species (Sánchez-Bayo, 2012). Several studies have demonstrated that insecticides ranging from insect growth regulators and encapsulated organophosphate formulations to systemic insecticides are more toxic to larvae than to the adult bees (Rortais et al., 2005; Zhu et al., 2014). Neonicotinoids became criticized due to their possible toxic effects on pollinators (Goulson et al., 2018), especially honeybees and wild bees. Nitro-neonicotinoids (imidacloprid and thiamethoxam) are more toxic to the honeybee with contact LD50 values in the ng/bee range than the cyano-neonicotinoids (acetamiprid and thiacloprid) with LD50 value in the 2 μg/bee range (Iwasa et al., 2004).
Fluxametamide (Gracia, Nissan Chemical), an isoxazoline compound, was discovered, developed, and registered in January 2019 in Japan. The site of action is γ-aminobutyric acid (GABA Cl-, Glu Cl- channel inhibitor). In this classification, a large proportion of insecticides are categorized as nerve- and muscle-targeting insecticides (Sparks and Nauen, 2015). There is little influence on the bee-visiting insects (Asahi et al., 2018; Umetsu and Shirai, 2020).
Emamectin benzoate is a semisynthetic insecticide derived from the avermectin family of compounds that acts as an agonist for γ-aminobutyric acid-gated chloride channels (Fishel, 2011). The resulting irreversible activation of chloride channels causes disruption of nerve impulses resulting the rapid paralysis in a range of lepidopteran species (Ishaaya et al., 2002). Emamectin benzoate is a toxic pesticide to honey bees (Fanglin et al., 2008; Abdu-Allah and Pittendrigh, 2018; Wang et al., 2020).
Lufenuron is a benzoylphenylurea insecticide whose mode of action is known to be the inhibition of chitin synthesis in the cuticle of insects (Tomlin, 2000). Lufenuron is also sold as an agricultural pesticide for use against lepidopterans, eriophyid mites, and western flower thrips (Ghelichpour and Mirghaed, 2019). Lufenuron were less toxic to A. mellifera workers (Thomazoni et al., 2009; Ahn et al., 2013b; Carvalho et al., 2021).
Acequinocyl is an acaricide with mainly contact action belonging to naphthoquinones used for the control of several species of mites in agricultural crops (Caboni et al., 2004). Acequinocyl is moderately toxic to A. mellifera worker (Kang and Jung, 2017). Dimethoate is a broad-spectrum organophosphorus insecticide commonly used as a positive control in comparative experiments with various pesticides (Dai et al., 2019).
Chronic exposure to pesticides during the early life stage of honey bees may thus contribute to inadequate nutrition and/or direct poisoning with a resulting impact on the survival and development of bee brood (Aupinel et al., 2007; Dai et al., 2017; Al Naggar and Baer, 2019). In Korea, fluxametamide, emamectin benzoate, lufenuron, acequinocyl, and dimethoate are generally used in the strawberry cultivation in greenhouse and the effect of these pesticides on the honey bee larvae is hardly known. Therefore, in order to understand the effect of insecticides on honey bee larvae, we carried out acute and chronic toxicity of selected pesticides (acetamiprid, emamectin benzoate, fluxametamide, lufenuron, and acequinocyl) of different concentrations by hypothesizing low toxic pesticides to adult honey bees could be more toxic to larvae.
MATERIALS AND METHODS
1. Materials
Chemicals
Technical grade pesticides purchased from Korea, acetamiprid (8.0%>purity), emamectin benzoate (2.1%>purity), fluxametamide (9.0%>purity), lufenuron (5.0%>purity), acequinocyl (15.0%>purity) and dimethoate (46.0%>purity) were used for all bioassays.
2. Rearing methods of honey bee larvae
In vitro larval rearing bioassays were performed following the standard method described in Schmehl et al., 2016. First instar worker larvae were grafted into the wells of a sterile 48-well tissue culture plates (Life Science, SPL, Korea) lined with brown cell cups filled with 20 μL of diet. After grafting, the larvae in sterile tissue culture plate (STCP) kept in dark incubator at 35°C and at 94% relative humidity in desiccators (Bel-Art, 1-800-4) containing dishes filled with a saturated K2SO4 solution. During the rearing process, the larvae were fed increasing volumes of three diet compositions (A, B and C). The larval diets were composed as mentioned Table 1 (Schmehl et al., 2016). Larvae were fed daily for 6 days putting a food drop onto the well’s bottom; diet composition and amounts fed to larvae are shown in Table 1.

Daily food volumes provided to the larvae and composition of the diet according to their age from day 0 to 5 after grafting, day 0 being the grafting
To test the acute effects of honey bee broods reared in laboratory, six different concentration of emamectin benzoate, fluxametamide, lufenuron acequinocyl and acetamiprid were prepared from the recommended concentration to 10-6 by serial dilution. Treatments were formulation of five pesticides, 2% acetone (solvent control), negative control and dimethoate (positive control). 30 μL of desired concentration of pesticides mixed with diet C (Table 1) Schmehl et al. (2016), was fed to the 3 day larvae and monitored for 3 days or for 72 hrs. Three replicates were used per treatment group with each replicate composed of 12 larvae. Larva was considered as dead if there is no movement of larva and the activity of the spiracles under a dissecting microscope.
Larvae were prepared as described above. Three different concentrations based on residue level in pollen, nectar or wax (Table 2) were used. The larvae were exposed to pesticides from second to fifth day. Stock solution was prepared in acetone that account 0.5% volume of the final diets. Three replicates were used per treatment group with each replicate composed of 12 larvae. Two days old larvae replaced any larvae died before the test diets were administered. Mortality was determined by viewing larval movement and the activity of the spiracles under a dissecting microscope.
3. Data analysis
The median lethal concentration (LC50) value and the relative 95% confidence intervals of 72-hr post exposure to different pesticides were calculated by means of Probit analysis in SPSS version 26 (IBM Corp., 2011) to determine the dose-mortality response curves. Since in the test each honey bee larvae ingests a 30 μL of food, the repeated treatment cumulative median lethal dose (LD50) was obtained from the relative LC50.
The larvae survival was analyzed with the Kaplan-Meier method in SPSS version 26 (IBM Corp., 2011). The Kaplan-Meier survival curves for the tested concentration and untreated control, negative control (1% acetone) and positive control 45 ppm dimethoate (Dai et al., 2019) were drawn.
RESULTS
1. Acute toxicity
The larvae were exposed to six different concentrations that were prepared by serial dilution of pesticides taking recommended field concentration as starting. Larvae mortality 24 hr after grafting was very low (0-3 dead larvae/plate; average survival±standard deviation= 99.2%). The acute toxicity of five tested insecticides and dimethoate (positive control) to A. mellifera larvae were given in Table 3. The toxicity order of tested pesticides were emamectin benzoate, lufenuron, fluxametamide, acequinocyl and acetamiprid with LD50 value of 0.000, 0.4, 8.1, 10 and 982.3 μg/larvae respectively. Our results showed that honey bee larvae were most sensitive to emamectin benzoate and most tolerant to acetamiprid. Tested concentrations of acetamiprid (p=0.31) and acequinocyl (p=0.057) resulted no significant different with compare to negative control.
2. Chronic toxicity
Two pesticides acetamiprid (low toxic) and amamectin benzoate (high toxic) were tested for chronic toxicity on A. mellifera larvae. Solvent and negative control mortality was lower than 15% at 72 h (6 day) after feeding diet B on 2nd and diet C on (3rd, 4th and 5th) days. Acetamiprid, affected larvae survival only at highest tested concentration (3,000 ppb) that killed 90%. Overall survivals of larvae fed 3,000 ppb of acetamiprid and 35 and 73 ppb of emamectin benzoate were all significantly lower than those of larvae fed the negative and solvent control diets (p<0.001) Fig. 1A and B. Emamectin benzoate killed more than 50% of larvae in six days at a level of 35 and 73 ppb (Fig. 1), a concentration that is not toxic to adult bees in acute bioassays (Zhu et al., 2014).
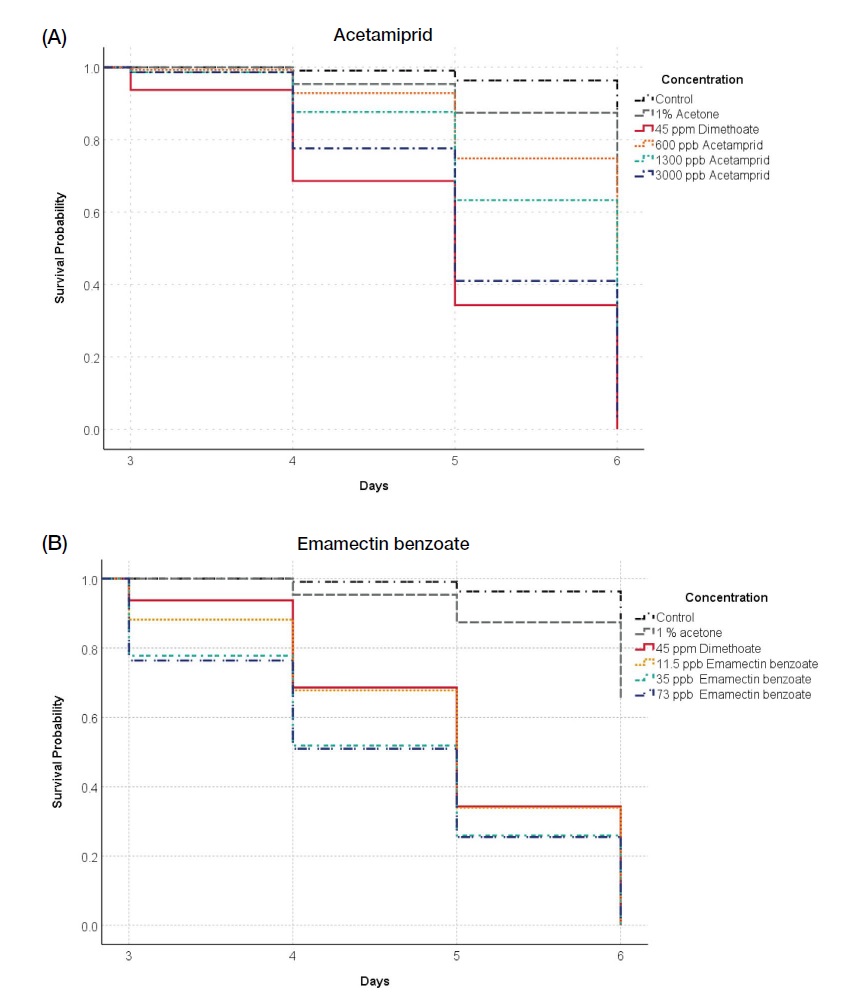
Survival of A. mellifera larvae exposed to three concentrations of acetamiprid and emamectin benzoate on 2nd day through 5th days after grafting (n=36 larvae per test substance). Larvae were fed with dimethoate-contaminated diet (45 mg/L) as a solvent control, and no contaminated diet as a negative control.
DISCUSSION
Honey bee larvae can be exposed to a wide range of pesticides via their diet which includes pollen and honey, both of which have been shown to have pesticide residues (Dai et al., 2019). The most significant increase in mortality was recorded by ememactin benzoate in both acute and chronic larvae exposure and lufenuron in acute feeding bioassay. Indicating emamectin benzoate and lufenuron were the most toxic insecticides tested. Although larvae showed tolerance to acetamiprid in both acute and chronic exposure, they are moderately affected to fluxametamide and acequinocyl.
Emamectin benzoate an agonist for gamma-aminobutyric acid-gated chloride channels (Fishel, 2011), which killed insects by disruption of nerve impulses triggered by permanent rapid paralysis (Anderson et al., 2009). The highest acute and chronic larvae toxicity were noted from emamectin benzoate. Abdu-Allah and Pittendrigh (2018) indicated that emamectin benzoate has higher penetration and low detoxification. Since contaminated larvae food is placed in the cell, the highest larvae toxicity of Emamectin benzoate could be from both feeding and contact exposure. These authors also indicated that emamectin benzoate is highly toxic to foragers with LD50 0.00006 μg/bee. The high mortality of lavae from emamectin bezoate before pupation in the present study were line with (Shurjeel et al., 2020) that stated emamectin benzoate is toxic to hioney bees at different stages.
Lufenuron chitin synthesis inhibitor in insects cuticle (Tomlin, 2000) was found to be toxic to larvae in the present study with LD50 of 0.37 μg/larva. It was reported not toxic for A. mellifera adults (Ahn et al., 2013). Another authors found that lufenuron is less toxic to A. mellifera workers (Thomazoni et al., 2009) and Polistes canadensis Canadensis (Santana-Reis et al., 2002). It has also reported lufenuron increase larvae mortality on Bombus terrestris (Mommaerts et al., 2006) and significantly disrupted larvae molting in Lepeophtheirus salmonis (Poley et al., 2018).
Our result showed both fluxametamide that act on GABA: γ-aminobutyric acid (GABA Cl-, Glu Cl- channel inhibitor) and an acaricide acequinocyl that inhibits mitochondrial complex III electron transport were moderately toxic to honey bee larvae with LD50 8.14 and 10 μg/larvae respectively (Hardstone and Scott, 2010). Study indicated that acequinocyl had high worker bee mortality when tested as formulation (Bahreini et al., 2020).
Acknowledgments
This study was partly supported by the BSRP through the National Research Foundation of Korea (NRF), Ministry of Education (NRF-2018R1A6A1A03024862) and RDA Agenda project on honeybee-pesticide interaction (PJ01577802).
References
-
Abdu-Allah, G. A. and B. R. Pittendrigh. 2018. Lethal and sub-lethal effects of select macrocyclic lactones insecticides on forager worker honey bees under laboratory experimental conditions. Ecotoxicology 27(1): 81-88.
[https://doi.org/10.1007/s10646-017-1872-6]

-
Ahn, K.-S., C. Yoon, K.-H. Kim, S.-Y. Nam, M.-G. Oh and G.-H. J. Kim. 2013. Evaluation of acute and residual toxicity of insecticides registered on strawberry against honeybee (Apis mellifera). Korean J. Pestic. Sci. 17(3): 185-192.
[https://doi.org/10.7585/kjps.2013.17.3.185]

-
Al Naggar, Y. and B. J. Baer. 2019. Consequences of a short time exposure to a sublethal dose of Flupyradifurone (Sivanto) pesticide early in life on survival and immunity in the honeybee (Apis mellifera). Sci. Rep. 9(1): 1-11.
[https://doi.org/10.1038/s41598-019-56224-1]

- Anderson, B., P. Doelling and J. J. Hetrick. 2009. Request for a New Use of the Insecticide Emamectin Benzoate (PC Code 122806). US EPA. 31s.
-
Asahi, M., M. Kobayashi, T. Kagami, K. Nakahira, Y. Furukawa and Y. J. Ozoe. 2018. Fluxametamide: A novel isoxazoline insecticide that acts via distinctive antagonism of insect ligand-gated chloride channels. Pestic. Biochem. Phys. 151: 67-72.
[https://doi.org/10.1016/j.pestbp.2018.02.002]

-
Aupinel, P., D. Fortini, B. Michaud, F. Marolleau, J. N. Tasei and J. F. Odoux. 2007. Toxicity of dimethoate and fenoxycarb to honey bee brood (Apis mellifera), using a new in vitro standardized feeding method. Pest Manag. Sci. 63(11): 1090-1094.
[https://doi.org/10.1002/ps.1446]

-
Bahreini, R., M. Nasr, C. Docherty, O. de Herdt, S. Muirhead and D. Feindel. 2020. Evaluation of potential miticide toxicity to Varroa destructor and honey bees, Apis mellifera, under laboratory conditions. Sci. Rep. 10(1): 1-14.
[https://doi.org/10.1038/s41598-020-78561-2]

-
Caboni, P., G. Sarais, M. Melis, M. Cabras and P. Cabras. 2004. Determination of acequinocyl and hydroxyacequinocyl on fruits and vegetables by HPLC-DAD. J. Agric. Food Chem. 52(22): 6700-6702.
[https://doi.org/10.1021/jf0487304]

-
Carvalho, S., G. Carvalho, C. Carvalho, J. Bueno Filho and A. J. Baptista. 2021. Toxicidade de acaricidas/inseticidas empregados na citricultura para a abelha africanizada Apis mellifera L., 1758 (Hymenoptera: Apidae). Arq. Inst. Biol. 76: 597-606.
[https://doi.org/10.1590/1808-1657v76p5972009]

-
Chauzat, M. P. and J. P. Faucon. 2007. Pesticide residues in beeswax samples collected from honey bee colonies (Apis mellifera L.) in France. Pest Manag. Sci. 63(11): 1100-1106.
[https://doi.org/10.1002/ps.1451]

-
Dai, P., C. J. Jack, A. N. Mortensen, T. A. Bustamante, J. R. Bloomquist and J. D. Ellis. 2019. Chronic toxicity of clothianidin, imidacloprid, chlorpyrifos, and dimethoate to Apis mellifera L. larvae reared in vitro. Pest Manag. Sci. 75(1): 29-36.
[https://doi.org/10.1002/ps.5124]

-
Dai, P., C. J. Jack, A. N. Mortensen and J. D. Ellis. 2017. Acute toxicity of five pesticides to Apis mellifera larvae reared in vitro. Pest Manag. Sci. 73(11): 2282-2286.
[https://doi.org/10.1002/ps.4608]

- Fanglin, W., Z. Jinwen, L. Shaonan and Z. Guonian. 2008. Acute Toxicity of Emamectin Benzoate on Environmental Organism. Pestic. Sci. 3.
- Fishel, F. M. J. 2011. IRAC's Insecticide mode of Action Classification. EDIS 2011 (5/6).
- Ghelichpour, M. and A. T. Mirghaed. 2019. Effects of sublethal exposure to new pesticides lufenuron and flonicamid on common carp, Cyprinus carpio, hydromineral balance to further saltwater exposure. Int. J. Aquat. Biol. 7(4): 195-201.
-
Goulson, D., H. Frey, S. Tzinieris, M. E. Colin, P. A. Marchand, S. Bastian, F. J. Richard, R. Early, S. Herrick and R. J. Arlettaz. 2018. Call to restrict neonicotinoids. Science 360(6392).
[https://doi.org/10.1126/science.aau0432]

-
Goulson, D., E. Nicholls, C. Botías and E. L. J. Rotheray. 2015. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347(6229): 1-16.
[https://doi.org/10.1126/science.1255957]

-
Hardstone, M. C. and J. G. Scott. 2010. Is Apis mellifera more sensitive to insecticides than other insects? Pest Manag. Sci. 66(11): 1171-1180.
[https://doi.org/10.1002/ps.2001]

-
Ishaaya, I., S. Kontsedalov and A. Horowitz. 2002. Emamectin, a novel insecticide for controlling field crop pests. Pest Manag. Sci. 58(11): 1091-1095.
[https://doi.org/10.1002/ps.535]

-
Iwasa, T., N. Motoyama, J. T. Ambrose and R. M. Roe. 2004. Mechanism for the differential toxicity of neonicotinoid insecticides in the honey bee, Apis mellifera. J. Crop Prot. 23(5): 371-378.
[https://doi.org/10.1016/j.cropro.2003.08.018]

-
Kang, M. and C. Jung. 2017. Avoidance behavior of honey bee, Apis mellifera from commonly used fungicides, acaricides and insecticides in apple orchards. J. Apic. 32(4): 295-302.
[https://doi.org/10.17519/apiculture.2017.11.32.4.295]

-
Medrzycki, P., H. Giffard, P. Aupinel, L. P. Belzunces, M.-P. Chauzat, C. Claßen, M. E. Colin, T. Dupont, V. Girolami and R. J. Johnson. 2013. Standard methods for toxicology research in Apis mellifera. J. Apic. Res. 52(4): 1-60.
[https://doi.org/10.3896/IBRA.1.52.4.14]

-
Mommaerts, V., G. Sterk and G. Smagghe. 2006. Hazards and uptake of chitin synthesis inhibitors in bumblebees Bombus terrestris. Pest Manag. Sci. 62(8): 752-758.
[https://doi.org/10.1002/ps.1238]

-
Morimoto, T., Y. Kojima, T. Toki, Y. Komeda, M. Yoshiyama, K. Kimura, K. Nirasawa and T. Kadowaki. 2011. The habitat disruption induces immune-suppression and oxidative stress in honey bees. Ecol. Evol. 1: 201-217.
[https://doi.org/10.1002/ece3.21]

-
Poley, J. D., L. M. Braden, A. M. Messmer, O. O. Igboeli, S. K. Whyte, A. Macdonald, J. Rodriguez, M. Gameiro, L. Rufener and J. J. Bouvier. 2018. High level efficacy of lufenuron against sea lice (Lepeophtheirus salmonis) linked to rapid impact on moulting processes. Int. J. Parasitol. 8(2): 174-188.
[https://doi.org/10.1016/j.ijpddr.2018.02.007]

-
Rortais, A., G. Arnold, M.-P. Halm and F. J. A. Touffet-Briens. 2005. Modes of honeybees exposure to systemic insecticides: estimated amounts of contaminated pollen and nectar consumed by different categories of bees. Apidologie 36(1): 71-83.
[https://doi.org/10.1051/apido:2004071]

-
Sanchez-Bayo, F. and K. J. Goka. 2014. Pesticide residues and bees-a risk assessment. PloS One 9(4): e94482.
[https://doi.org/10.1371/journal.pone.0094482]

-
Sánchez-Bayo, F. J. 2012. Insecticides mode of action in relation to their toxicity to non-target organisms. J. Environ. Anal. Toxicol. S 4: S4-002.
[https://doi.org/10.4172/2161-0525.S4-002]

- Santana-Reis, V., O. Marques and J. J. COSTA. 2002. Seletividade de inseticidas ao predador Polistes canadensis canadensis (L., 1758) Hymenoptera: Vespidae. Acta Biol. Leopold. 2: 141-146.
-
Schmehl, D. R., H. V. Tomé, A. N. Mortensen, G. F. Martins and J. D. Ellis. 2016. Protocol for the in vitro rearing of honey bee (Apis mellifera L.) workers. J. Apic. Res. 55(2): 113-129.
[https://doi.org/10.1080/00218839.2016.1203530]

-
Shurjeel, H. K., M. A. Aqueel, E. Ashraf, A. Ali and A. J. Rubab. 2020. Effect of insecticides on the longevity of Apis mellifera L. (Hymenoptera: Apidae). Sarhad J. Agric. 36(3): 768-776.
[https://doi.org/10.17582/journal.sja/2017/36.3.768.776]

-
Sparks, T. C. and R. J. Nauen. 2015. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. 121: 122-128.
[https://doi.org/10.1016/j.pestbp.2014.11.014]

- Thomazoni, D., M. F. Soria, C. Kodama, V. Carbonari, R. P. Fortunato, P. E. Degrande and V. J. Valter Jr. 2009. Selectivity of insecticides for adult workers of Apis mellifera (Hymenoptera: Apidae). Rev. Colomb. Entomol. 35(2): 173-176.
- Tomlin, C. J. 2000. The pesticide manual 12th Edition. BCPC. 49: 647-648.
-
Umetsu, N. and Y. J. Shirai. 2020. Development of novel pesticides in the 21st century. J. Pestic. Sci. 45(2): 54-74.
[https://doi.org/10.1584/jpestics.D20-201]

-
Wang, Y., Y. C. Zhu and W. J. Li. 2020. Interaction patterns and combined toxic effects of acetamiprid in combination with seven pesticides on honey bee (Apis mellifera L.). Ecotoxicol. Environ. Saf. 190: 110100.
[https://doi.org/10.1016/j.ecoenv.2019.110100]

-
Zhu, W., D. R. Schmehl, C. A. Mullin and J. L. Frazier. 2014. Four common pesticides, their mixtures and a formulation solvent in the hive environment have high oral toxicity to honey bee larvae. PloS One 9(1): e77547.
[https://doi.org/10.1371/journal.pone.0077547]
