
Pupa (Apis mellifera L.) Rearing Conditions to Improve Queen Weight at Emergence
Abstract
Queen quality depends on increased body weight at emergence, which is strongly affected by ecological factors during brood rearing; however, much remains undefined in terms of improving queen weight at emergence. We initially reared 12-20 h-old larvae in queenright (QR) and queenless (QL) colonies at temperatures of 28±2℃ and 70±6% relative humidity (RH) and 29±3℃ and 65±8% RH respectively, and later transferred 50% of the pupae into an incubator (34.7±0.42℃ and 80±5% RH). Queen weight at emergence was observed to be the highest in the QR and QL colonies of the incubator (217.36±5.51 mg and 199.61±4.24 mg, respectively) compared to that of QR and QL colonies (199.45±3.60 mg and 182.94±3.35 mg respectively) in the hives. Queen weight at emergence differed significantly among rearing groups (P<0.0001), whereas the difference in the emergence rate was insignificant. We observed that rearing queen bees initially from QR colonies and pupae in an incubator at an optimum and more stable temperature could be an advantage in improving queen weight at emergence. Further studies are needed to investigate their acceptance, mating, and reproductive success rates.
Keywords:
Queen quality, Weight at emergence, Pupa, Ecological factor, Honey bee queensINTRODUCTION
Queen bees are the only reproductive females and are the most important individuals in honey bee colonies. They are known for their responsibility in ensuring colony survival by laying eggs that develop into individuals of other castes. The quality of queen bees is beneficial for beekeepers in the production of hive products. While poor-quality drones are equally responsible, poor-quality queens remain the principal reason for colony failure (Kairo et al., 2016, 2017). Studies have reported poor-quality queens as the primary problem in a number of beekeeping operations, which rank among the top reasons for colony failure (Vanengelsdorp et al., 2009, 2013). Queen quality depends on several characteristics (body size, weight, thorax width, wing length, number of ovarioles, and spermatheca diameter) that have been used to describe reproductive success. Certain morphological characteristics (thoracic width, wing length, and weight) have also been used to quantify the quality of newly emerged queens (Tarpy et al., 2000, 2011; Delaney et al., 2011; De Souza et al., 2013). Additionally, queen size, weight, and thorax width are positively correlated with higher sperm counts and laying efficiency (Kahya et al., 2008; Delaney et al., 2011). Queen weight is also positively correlated with ovary weight, size, number of ovarioles, diameter of the spermatheca, and the number of stored spermatozoa (Kahya et al., 2008). These characteristics have been identified as being essential to mating success (Delaney et al., 2011; Tarpy et al., 2011), the queens’ ability to be attractive to drones during mating flights (Gilley et al., 2003; Rangel et al., 2016), and increased body weight at emergence, which is strongly linked to reproductive fitness among queens (Dedej et al., 1998; Kahya et al., 2008; Tarpy et al., 2011). Queen weight could be influenced by both environmental and human-induced factors, especially larval and pupal rearing conditions which consequently affect reproductive success. Queen weight is determined by larval rearing conditions, such as royal jelly diet (Hartfelder et al., 2015) and temperature (DeGrandi-Hoffman et al., 1993). Increased temperature during honey bee brood capping can negatively affect emerged adult bees and, consequently, mating success. Temperature is a prominent environmental and ecological factor that affects insect growth and development (Seeley and Heinrich, 1981; Shi et al., 2014; Kobori and Hanboosong, 2017). Many deformations in insect biology, such as body size (Atkinson, 1994), mating (Kuang et al., 2010) and metabolism (Wang et al., 2016; Qian et al., 2017) are associated with changes in temperature. For instance, in the pupal stage, bees raised at 34.5℃ were found to have the highest numbers of microglomeruli in the olfactory lobes compared to those raised below or above this temperature (Groh, 2004). Due to the importance of temperature in brood development, most social insects, including honey bees, have begun to regulate nest temperature (Heinrich, 1993; Stabentheiner et al., 2010). However, maintaining normal brood development is costly for honey bees, as considerable energy is spent in regulating brood temperature within the range of 32-36℃ (Seeley and Heinrich, 1981; Kronenberg and Heller, 1982). Therefore, the most important output in selecting and maintaining good brood rearing conditions is to increase queen weight at emergence for better reproductive success. According to De Souza et al. (2013) and Rangel et al. (2013), queens weighing over 200 mg headed colonies with higher brood populations, stored more pollen, and had greater population growth than those weighing less than 180 mg at emergence. Therefore, queen weight is the most reliable parameter for measuring queen reproductive quality (Amiri et al., 2017).
The development of control systems to improve queen quality by improving their weight at emergence has been a driving factor for researchers and beekeepers (Amiri et al., 2017). Although the weight of first-instar larvae (larval age) plays an important role in addressing queen quality, much remains undefined in improving queen weight at emergence. In this study, we aimed to investigate the effects of pupal rearing conditions on queen weight at emergence, pupal mortality, and emergence rates in queenright and queenless colonies.
MATERIALS AND METHODS
This study was conducted in an apiary at the honey bee breeding laboratory at the Department of Agricultural Biology, National Institute of Agricultural Science (NIAS), Republic of Korea.
1. Colony selection and grafting of larvae
The larvae in a single strong colony (six combs, good brood, approximately 80% worker bee population, and good performance) bred in 2021 were selected and used in this study. Two empty built combs of honey bees (Apis mellifera L.) were marked and inserted into the selected queenright colony for the queen to deposit eggs. After 24 h, the presence of eggs was checked and recorded as day 1. Within a period of 3 days when eggs are expected to hatch into larvae, the combs were removed to transfer the first-instar (12-20 h-old) larvae into artificial queen cell cups (Doolittle, 1915). Queen cell cups were attached to rearing frames, and ≤20 h-old larvae were transferred into each cup containing one drop (5 mL syringe) of diluted royal jelly in water at a of ratio 1 : 1 (v/v) using the Chinese grafting tool (Fig. 1a). Prior to queen rearing, four strong colonies of A. mellifera were sorted and digital thermosensors (ONSET, HOBO ext temp/RH logger, UX100-023A) were inserted to monitor hive temperature and relative humidity (RH) over 3 days. Colonies (one queenright and one queenless) with similar conditions (temperature and relative humidity) were selected for queen rearing under the same environmental conditions. No adaptations were made to regulate the hive temperature.
2. Larvae rearing and transfer of pupae into queen rearing cages
After selecting the rearing colonies, the queen bee in the queenright colony was excluded using a queen excluder (Fig. 1b). Rearing frames with grafted larvae were placed into each rearing colony and fed sugar syrup (powdered sugar dissolved in water at a ratio of 1 : 1, v/v). Digital thermosensors placed in each colony showed that the average temperature and RH for queenright (QR) and queenless (QL) colonies were 28±1.87℃ and 70±6% RH, and 29±2.28℃ and 65±8% RH, respectively. The percentage of accepted larvae was verified 2 days after grafting to estimate the number of pupae to be transferred to the rearing cages. Equal numbers of capped queen cells were transferred into queen rearing cages and labeled as QR and QL (Fig. 1c). Additionally, the cages to be placed in the incubator and hives were differentiated with letters i and h, respectively (Fig. 1c). The height of each capped queen cell was measured before transfer to the rearing cages. Pupae were reared in QR, QL, and an incubator. A desiccator set at 34.7±0.42℃ and 80±5% RH and placed in an incubator was used to rear the pupae (Fig. 1d).
3. Experimental setup
Freshly hatched larvae (≤20 h-old) were grafted and reared in QR and QL colonies as described above. The number of larvae that developed into pre-pupae and pupae was recorded. A total of 150 pupae were extracted from initial rearing frames (50% each from QR and QL colonies) and inserted into queen rearing cages (Fig. 1c). The pupal rearing conditions were described as QR, QL, and incubator. Fifty queen cages containing pupae were inserted; however, QR and QL colonies provided 25 pupae each to measure up to the 50 pupae reared in the incubator. The experimental units were monitored twice a day (9:30 am and 5:30 pm) to record the date, number, and weight of the emerged queens. The queen emergence rate was recorded within 48 h, from the late hours of day 15 to the early hours of day 17. This was described as day 1 (early hours of day 15), day 2 (early and late hours of day 16), and day 3 (early hours of day 17). Pupae that did not develop and emerge into adults were removed and uncapped to observe their states. The number of emerging adults was used to calculate the pupal mortality (Cui et al., 2018). Queen weight (mg) at emergence was recorded, and weighed queen bees were reinserted into their cages with equal amounts of artificial food and placed in their respective rearing media. Queen bees were fed once every 3 days for 32 days. The queen mortality rate was recorded daily for 32 days after emergence.
4. Data analysis
The data were characterized using Descriptive Statistics. One-way analysis of variance (ANOVA) was used to compare the means of more than two treatments. When a significant difference was detected through ANOVA, we conducted multiple pairwise comparisons of means among treatments using the Newman-Keuls test. Pearson’s correlation was used to evaluate relationships between parameters, while the two-sample Student’s t-test was used to compare the means of two treatments. XLSTAT statistical software version 2007.8.04 was used to conduct the analysis with levels of significance set at 5%.
RESULTS AND DISCUSSION
1. Effects of rearing conditions on pupal mortality rate
The number of emerged queens and pupal mortalities under the different rearing conditions were recorded (Table 1).
Pupal mortality was higher in the incubator (10%) than in the QR (2%) and QL (0%) colonies, with emergence rates of 90%, 98% and 100%, respectively (Table 1). The high pupal mortality in the incubator can be attributed to rapid temperature changes. The larvae were initially reared under lower temperatures (above 5℃) than those in the incubator. The results of our study are consistent with those of other studies that have reported the sensitivity of insects to changes in temperature, which affects their growth and development (Li et al., 2017; Cui et al., 2018). Zero pupal mortality in the QL colony might be due to the absence of the queen, which leads to worker bees focusing on raising new queens. Other studies on larval acceptance rate showed a higher percentage of acceptance in queenless colonies than queenright colonies (Cengiz et al., 2009).
2. Effects of rearing condition on queen weight at emergence and rate of emergence
The length of the sealed queen cells and queen weights at emergence were measured based on different rearing conditions (Table 2).
The mean lengths of sealed queen cells were higher in QL (27.04±0.42 mm) than in QR (26.24±0.29 mm) colonies although no significant difference was observed. In our study, the difference in the length of sealed queen cells between the QL and QR colonies was not significant compared with that in similar studies (Cengiz et al., 2009). The relationship between the lengths of sealed queen cells and queen weight at emergence from QR and QL colonies was investigated using Pearson correlation.
The results showed no correlations in QR (R2=0.111) and QL (R2=0.263) colonies. The length of sealed queen cells did not significantly affect the weight of queen bees at emergence. In other studies, queen-worker differentiation was affected by queen cell size (Shi et al., 2011) whereas Wu et al. (2018) reported that queen weight at emergence increased with larger diameter queen cells and had better performance.
The weights of queen bees at emergence from QR and QL colonies differed significantly when reared in hives (P<0.001) and in the incubator (P<0.01). However, it is important to understand the variation in queen weight between QR and QL colonies, to better select starter colonies. The emergence weight of 198.20±8.74 mg obtained in QR colonies (Cengiz et al., 2009) and 178.42±2.05 mg obtained in QL colonies (Dodologlu et al., 2004) were similar to our findings. However, our value of 182.94±3.35 mg in QL colony was lower than 199.07±7.55 mg reported by Cengiz et al. (2009). Additionally, a significant difference was recorded in queen weight at emergence among the three pupa rearing groups (df=2, M.S=7936.875, F=13.21, P<0.0001). Queen weight at emergence was highest in the QR and QL colonies of the incubator, followed by QR and QL colonies (Table 2). The temperature of the incubator, which was maintained at a consistently 34.7±0.42ºC could influence brood rearing condition and consequently queen weight at emergence because good brood rearing demands an optimum temperature of 32-35℃. The increased body weight of emerged queens in an incubator initially reared from both QR and QL colonies could be associated with pupa rearing temperature, as Kobori and Hanboosong (2017) reported that growth and development in insects is highly affected by temperature. Worker bees spend considerable energy and time in regulating brood rearing temperature (Kronenberg and Heller, 1982). Consequently, optimal brood rearing temperature may not be attained at all periods as workers have other duties to perform in the colony. Many aspects of insect biology, including body size, are affected by changes in temperature (Atkinson, 1994). Cao et al. (2012) reported that insect molting and temperature are closely related. Pupae transferred from the QR colony to the incubator emerged to queen bees with higher body weight (217.36±5.5 mg) (Table 2). Some studies on queen quality have concluded that it is advantageous to raise new queens from queenright colonies, although queens emerging from queenless colonies could develop higher body weights than queenright colonies (Dodologlu et al., 2004; Cengiz et al., 2009). Thermoregulation in maintaining optimal temperature in brood-rearing honey bee colonies may not be stable, while a stable temperature is needed for survival and good development to increase adult quality (Jones et al., 2005). This could be attributed to the energy required for thermoregulation (Stabentheiner et al., 2010) as workers also spend some energy to forage to help the colonies meet its requirements. The present study suggests that QR colonies can be used as initial rearing colonies, while pupae were transferred to an incubator set at an optimum temperature.
The number of queen bees that emerged was recorded based on the time of emergence (Fig. 2). The emergence rate of queen bees differed significantly (df=2, M.S=1089.0, F=75.977, P<0.0001). However, the emergence rate on days 1 and 3 did not show any significant variation (Fig. 2). More queen bees emerged in the incubator (nine bees) on the third day than in QR (three bees) and QL (three bees) colonies reared in hives. The life cycle of the queen bees from the egg stage to adulthood at emergence was 16 days. The deviations in the emergence periods under different rearing conditions were insignificant, and the queen survival rate within 32 days after emergence was high in QL, incubator, and QR colonies. The low survival rate in QR could be due to the presence of the old queen, which discouraged workers to pay attention to new queens. This is consistent with the results reported by Al-Fattah et al. (2016), who found that queen bee survival rate was significantly higher in queenless colonies (77.4%) than in queenright colonies (68.0%). They further discussed that the reversal effect on the survival rate of stored queens was due to the presence of originally mated free-laying queens around the caged virgin queens in the colony. In other studies, increasing the degree of queenlessness led to a decrease in the number of rejected queens (Szabo, 1977; Shawer, 1981).
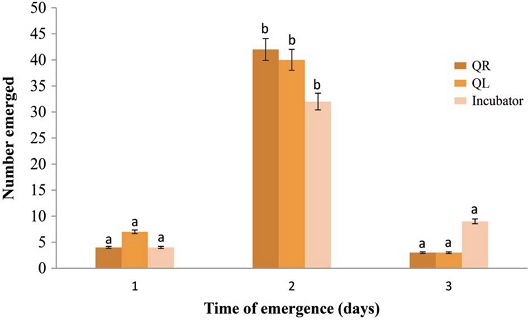
Effects of rearing conditions on emergence rate. Time of emergence was categorized as: day 1 (late hours of day 15); day 2 (early and late hours of day 16); day 3 (early hours of day 17). Means with different letters are significantly different at P<0.0001, α=0.05. QR, queenright colony; QL, queenless colony.
CONCLUSION
Queen weight at emergence has been documented as an essential factor for improving queen quality, and is affected by both environmental and human-induced factors. In this study, queen weight at emergence was the highest in the queenright and queenless colonies in an incubator compared to the queenright and queenless colonies in the hive. Therefore, rearing queen bees initially from queenright colonies and pupae in an incubator at an optimum and stable temperature could aid in improving queen weight at emergence. However, further studies are needed to investigate their acceptance, mating, and reproductive success rates.
Acknowledgments
We are grateful to the bee breeding laboratory, National Institute of Agricultural Science (NIAS), Rural Development Administration (RDA), and the Institute of Agricultural Research for Development (IRAD) for their collaboration with this study. This research was funded by a grant from the Research Program for Agricultural Science & Technology Development (Project No. PJ01575503), National Institute of Agricultural Sciences, Rural Development Administration, Republic of Korea. We would like to thank Editage (www.editage.co.kr) for English language editing.
References
- Al-Fattah, M. A., Y. Y. Ibrahim and H. A. Sharaf El-Din. 2016. Effect of Virgin Queens Storage on Their Survival Rate, Attractiveness and Acceptance by the Honey Bee Colonies. Egypt. Acad. J. Biolog. Sci. 9: 63-69.
-
Atkinson, D. 1994. Temperature and organism size: a biological law for ectotherms? Adv. Ecol. Res. 25: 1-58.
[https://doi.org/10.1016/S0065-2504(08)60212-3]

-
Amiri, E., M. K. Strand, O. Rueppell and D. R. Tarpy. 2017. Queen Quality and the Impact of Honey Bee Diseases on Queen Health: Potential for Interactions between Two Major Threats to Colony Health. Insects 8: 22-26.
[https://doi.org/10.3390/insects8020048]

- Cao, M. L., B. Tao, S. Liu, J. G. Dong and Y. Z. He. 2012. Influence of temperature on an experimental population of Athetis lepigone (Möschler). Acta. Phytophy. Sin. 39: 531-535.
- Cengiz, M., B. Emsen and A. Dodologlu. 2009. Some Characteristics of Queenbees (Apis mellifera L.) Rearing in Queenright and Queenless Colonies. J. Anim. Vet. Adv. 8: 1083-1085.
-
Cui, J., S. Zhu, R. Bi, W. Xu, Y. Gao and S. Shi. 2018. Effect of Temperature on the Development, Survival, and Fecundity of Heliothis viriplaca (Lepidoptera: Noctuidae). J. Econ. Entomol. 111: 1940-1946.
[https://doi.org/10.1093/jee/toy151]

-
Dedej, S., K. Hartfelder, P. Aumeier, P. Rosenkranz and W. Engels. 1998. Caste determination is a sequential process: Effect of larval age at grafting on ovariole number, hind leg size and cephalic volatiles in the honey bee (Apis mellifera carnica). J. Apic. Res. 37: 183-190.
[https://doi.org/10.1080/00218839.1998.11100970]

-
DeGrandi-Hoffman, G. M., M. Spivak and J. H. Martin. 1993. Role of thermoregulation by nestmates on the development time of honey bee (Hymenoptera: Apidae) queens. Ann. Entomol. Soc. Am. 86: 165-172.
[https://doi.org/10.1093/aesa/86.2.165]

-
Delaney, D. A., J. J. Keller, J. R. Caren and D. R. Tarpy. 2011. The Physical, Insemination, and Reproductive Quality of Honey Bee Queens (Apis mellifera L.). Apidologie 42: 1-13.
[https://doi.org/10.1051/apido/2010027]

-
De Souza, D. A., M. A. F. Bezzera-Laure, T. M. Francoy and L. S. Gonçalves. 2013. Experimental evaluation of the reproductive quality of Africanized queen bees (Apis mellifera ) on the basis of body weight at emergence. Gen. Mol. Res. 12: 5382-5391.
[https://doi.org/10.4238/2013.November.7.13]

-
Dodologlu, A., B. Emsen and F. Genc. 2004. Comparison of some characteristics of queen honey bees (Apis mellifera L.) reared by using Doolittle method and natural queen cells. J. Appl. Anim. Res. 26: 113-115.
[https://doi.org/10.1080/09712119.2004.9706518]

- Doolittle, G. M. 1915. Scientific queen-rearing as practically applied; being a method by which the best of queen-bees are reared in perfect accord with nature’s ways: for the amateur and veteran in bee-keeping. 6th ed. Hamilton, Illinois: American Bee Journal. 126 p.
-
Gilley, D. C., D. R. Tarpy and B. B. Land. 2003. The effect of queen quality on the interactions of workers and dueling queen honey bees (Apis mellifera L.). Behav. Ecol. Sociobiol. 55: 190-196.
[https://doi.org/10.1007/s00265-003-0708-y]

-
Groh, C., J. Tautz and W. Rossler. 2004. Synaptic organization in the adult honey bee brain is influenced by brood temperature control during pupal development. Proc. Natl. Acad. Sci. 101: 4268-4273.
[https://doi.org/10.1073/pnas.0400773101]

-
Hartfelder, K., K. R. Guidugli-Lazzarini, M. S. Cervoni, D. E. Santos and F. C. Humann. 2015. Old threads make new tapestry - Rewiring of signalling pathways underlies caste phenotypic plasticity in the honey bee, Apis mellifera L. Adv. Insect. Physiol. 48: 1-36.
[https://doi.org/10.1016/bs.aiip.2014.12.001]

-
Heinrich, B. 1993. The Hot-Blooded Insects: Mechanisms and Evolution of Thermoregulation. Cambridge: Harvard University Press, 600 pp.
[https://doi.org/10.4159/harvard.9780674418516]

-
Jones, J. C., P. Helliwell, M. Beekman, R. Maleszka and B. P. Oldroyd. 2005. The effects of rearing temperature on developmental stability and learning and memory in the honey bee, Apis mellifera. J. Comp. Physiol. A. 191: 1121-1129.
[https://doi.org/10.1007/s00359-005-0035-z]

-
Kahya, Y., H. V. Gençer and J. Woyke. 2008. Weight at emergence of honey bee (Apis mellifera caucasica) queens and its effect on live weights at the pre and post mating periods. J. Apic. Res. 47: 118-125.
[https://doi.org/10.1080/00218839.2008.11101437]

-
Kairo, G., B. Provost, S. Tchamitchian, F. B. Abdelkader, M. Bonnet, M. Cousin, J. Sénéchal, P. Benet, A. Kretzschmar and L. P. Belzunces. 2016. Drone exposure to the systemic insecticide Fipronil indirectly impairs queen reproductive potential. Sci. Rep. 6: 31904.
[https://doi.org/10.1038/srep31904]

-
Kairo, G., Y. Poquet, H. Haji, S. Tchamitchian, M. Cousin, M. Bonnet, M. Pelissier, A. Kretzschmar, L. P. Belzunces, and J. Brunet. 2017. Assessment of the toxic effect of pesticides on honey bee drone fertility using laboratory and semifield approaches: A case study of fipronil. Environ. Toxicol. Chem. 36: 2345-2351.
[https://doi.org/10.1002/etc.3773]

-
Kobori, Y. and Y. Hanboosong. 2017. Effect of temperature on the development and reproduction of the sugarcane white leaf insect vector, Matsumuratettix hiroglyphicus (Matsumura) (Hemiptera: Cicadellidae). J. Asia-Pac. Entomol. 20: 281-284.
[https://doi.org/10.1016/j.aspen.2017.01.011]

-
Kronenberg, F. and H. C. Heller. 1982. Colonial Thermoregulation in Honey Bees (Apis mellifera). J Comp. Physiol. 148: 65-76.
[https://doi.org/10.1007/BF00688889]

- Kuang, X. J., X. H. Sun, F. Huang and F. S. Xue. 2010. Effect of temperature on mating behavior of Colaphellus bowringi Baly. J. Environ. Entomol. 32: 307-311.
-
Li, G. P., H. Q. Feng, B. Huang, J. Zhong, C. H. Tian, F. Qiu, and J. R. Huang. 2017. Effects of short-term heat stress on survival and fecundity of two plant bugs: Apolygus lucorumm (Meyer-Dür) and Adelphocoris suturalis Jakovlev (Hemiptera: Miridae). Acta. Ecol. Sin. 37: 3939-3945.
[https://doi.org/10.5846/stxb201603250533]

- Qian, X., Y. Y. Wang, H. H. Xie, J. Dou, Z. W. Li, R. Jashenko, and R. Ji. 2017. Effects of temperature on the activities of key enzymes related to respiratory metabolism in adults of Gomphocerus sibiricus (Orthoptera: Acrididae). Acta. Entomol. Sin. 60: 499-504.
-
Rangel, J., J. J. Keller and D. R. Tarpy. 2013. The effects of honey bee (Apis mellifera L.) queen reproductive potential oncolony growth. Insect. Soc. 60: 65-73.
[https://doi.org/10.1007/s00040-012-0267-1]

-
Rangel, J., K. Böröczky, C. Schal and D. R. Tarpy. 2016. Honey bee (Apis mellifera) queen reproductive potential affects queen mandibular gland pheromone composition and worker retinue response. PLoS ONE 11: e0156027.
[https://doi.org/10.1371/journal.pone.0156027]

- Seeley, T. D. and B. Heinrich. 1981. Regulation of temperature in the nests ofsocial insects. pp. 159-234. in Insect Thermoregulation, eds. by B. Heinrich. Wiley, NewYork.
- Shawer, M. B. 1981. Factors affecting the behaviour of honeybee workers towards introduced queens. J. Agric. Res. 7: 244-254.
-
Shi, Y. Y., Z. Y. Huang, Z. J. Zeng, Z. L. Wang, X. B. Wu, W. Y. Yan. 2011. Diet and cell size both affect queen-worker differentiation through DNA methylation in honey bees (Apis mellifera, Apidae). PLoS ONE 6: e18808.
[https://doi.org/10.1371/journal.pone.0018808]

-
Shi, S. S., J. Cui and L. S. Zang. 2014. Development, survival, and reproduction of Megacopta cribraria (Heteroptera: Plataspidae) at different constant temperatures. J. Econ. Entomol. 107: 2061-2066.
[https://doi.org/10.1603/EC14287]

-
Stabentheiner, A., H. Kovac and R. Brodschneider. 2010. Honeybee colony thermoregulation-regulatory mechanisms and contribution of individuals in dependence on age, location and thermal stress. PLoS ONE 5: e8967.
[https://doi.org/10.1371/journal.pone.0008967]

-
Szabo, T. I. 1977. Behavioura1 studies of queen introduction in the honeybee: Multiple queen introduction. J. Apic. Res. 16: 65-83.
[https://doi.org/10.1080/00218839.1977.11099865]

-
Tarpy, D. R., S. Hatch and D. J. C. Fletcher. 2000. The influence of queen age and quality during queen replacement in honeybee colonies. Anim. Behav. 59: 97-101.
[https://doi.org/10.1006/anbe.1999.1311]

-
Tarpy, D. R., J. J. Keller, J. R. Caren and D. A. Delaney. 2011. Experimentally induced variation in the physical reproductive potential and mating success in honey bee queens. Insect. Soc. 58: 569-574.
[https://doi.org/10.1007/s00040-011-0180-z]

-
Vanengelsdorp, D., J. J. Hayes, R. M. Underwood and J. A. Pettis. 2009. Survey of honey bee colony losses in the U.S., Fall 2007 to Spring 2008. PLoS ONE 3: e4071.
[https://doi.org/10.1371/journal.pone.0004071]

-
Vanengelsdorp, D., D. R. Tarpy, E. J. Lengerich and J. S. Pettis. 2013. Idiopathic brood disease syndrome and queen events as precursors of colony mortality in migratory beekeeping operations in the eastern United States. Prev. Vet. Med. 108: 225-233.
[https://doi.org/10.1016/j.prevetmed.2012.08.004]

- Wang, J., B. L. Li, J. X. Wu and X. L. Xu. 2016. Effects of fluctuating temperature on the reproduction and metabolism of primary energy substances in Mythimna separata (Lepidoptera: Noctuidae). Acta. Entomol. Sin. 59: 917-924.
-
Wu, X., L. Zhou, C. Zou and Z. Zang. 2018. Effects of queen cell size and caging days of mother queenon rearing young honey bee queens Apis mellifera L, J. Apic. Sci. 62: 215-222.
[https://doi.org/10.2478/jas-2018-0025]


