Evaluation of the Attractive Effects on Insect Pheromones for Varroa destructor
Abstract
Honey bees play important roles in the maintenance and preservation of agricultural ecosystems in that they contribute to fruit and seed production by pollinating crops. However, ectoparasitic mites, Varroa destructor, cause considerable damage to honey bee colonies. V. destructor is a major problem for apiculture, and the search for novel control methods is an essential task for researchers. Therefore, we carried out an experiment with twenty insect pheromones, which are well known for their function and structure, to develop an attractant for effectively controlling V. destructor during the phoretic phase. For the study, we investigated the attractive effects of 40 different pheromones. From the preliminary screening (Z)-8-dodecenyl acetate ((Z)-8-12:Ac), (E)-8-(E)-10-dodecenyl acetate (E)-8, (E)-10-12:Ac), (E)-11-tetradecenyl acetate ((E)-11-14:Ac) and (Z)-11-tetradecenyl acetate ((Z)-11-14Ac) showed relatively good attraction effect for V. destructor. For the four selected pheromones, the attraction effect was tested at 50 mg, 25 mg, 12.5 mg, and 6.25 mg concentrations to check the concentration-dependent responsiveness. [Z]-8-12:Ac, [E]8, [E]10-12:Ac were found to have a higher attraction effect than other pheromones. After then, the two selected pheromones were tested the attraction effect of pheromone exposure time, and the attraction effect of both pheromones was lasted up to 3 hours. Although this study was also considered the effect of pheromone chemical structure on V. destructor, there was no chemical structure-dependent attraction. However, (Z)-8-12:Ac and (E)-8, (E)-10-12:Ac were found to have relatively high attraction effects depending on pheromone concentration and exposure time. Therefore, these pheromones should be further investigated as potential alternative attractant for V. destructor control. In addition, further studies are warranted to determine the synergistic effects among different components, and establish appropriate delivery methods to develop safer and more effective attractant.
Keywords:
Honey bee, Insect pheromone, Attractant, Varroa destructorINTRODUCTION
The honey bee is not only a producer of sweeteners, but also plays a crucial role in the transmission of pollens of flowers, which is essential for fruit production in horticulture industry (Ellis et al., 2020). The economic value of honey bees through pollen mediation is estimated to be 14.6 billion United States dollars (Morse and Calderone, 2000), and the amount paid for pollination services in the United States was 319 million dollars to beekeepers for pollination in 2017 (Bond et al., 2021). In addition to honey, the apiculture industry also produces various secondary and tertiary products such as cosmetics, medical supplies, basic substances, health and food additives, increasing the overall value of the industry.
Varroa destructor is known as the greatest threats to western honey bee (Apis mellifera L.) health worldwide (Nazzi and Le Conte, 2016). V. destructor, originally from oriental honey bees, now parasitizes European honey bee strains, and in Korea, the predominant parasite affecting bee is V. destructor. The overall effects of V. destructor infestation can include a shortened lifespan and weight loss in worker bees by directly feeding on the honey bee’s fat body and transmitting viruses to each other (Ramsey et al., 2019; Vilarem et al., 2021). V. destructor infestation can also weaken the colonies, reducing honey production and even leading to colony death.
Currently, various methods are used to control V. destructor, but chemical control has been recognized as a quick and effective approach among beekeepers. However, this method comes with the risk of honey contamination, the accumulation of residues within the hive and toxic effects to the bee. Recently, V. destructor has shown resistance to synthetic chemicals such as pyrethroids and coumaphos, which are commonly used in beekeeping to control them (Pettis et al., 2004). Although miticides and Integrated Pest Management Control (IPMC) have been suggested and tested for V. destructor control (Jack and Ellis, 2021; Vilarem et al., 2021), their toxicity to honey bees and the natural environment remains a concern. Therefore, there have been many trials to find natural chemicals that are effective against V. destructor (Rbee and Zedan, 2018; Bakar et al., 2019). Therefore, alternative control measures that are relatively safe for the environment, humane health, and bees are being sought. However, V. destructor spend a lot of time in the brood cell cause they reproduction occurs in the brood cell, it is important to effectively attract in order to control.
Insect pheromones are chemicals compounds utilized by insect species in nature to communicate with each other between species. Insect pheromones can interfere with the basic behavioral functions of certain arthropods. There are various types of insect pheromones, and they have evolved in many different ways. Some substances exhibit aggregation, while others may act as repellents. Also, the concentration of pheromones plays a critical role as attractant or arrestant in insect behavior (Dethier et al., 1960; Shorey, 1973). Insect pheromones have beneficial aspects. Their physio-chemical properties make them much less harmful to the environment than synthetic chemical pesticides, and resistance occurs more slowly due to the complexed mixture of constituents (Koul et al., 2008). Therefore, insect pheromones have been developed to control insect, and it is also used as potential alternative measure to chemical control (Gupta and Milatovic, 2014).
However, the effect of insect pheromones on V. destructor has been little studied, and their commercial utilization has not yet been applied. Although V. destructor, as parasites of honey bees, do not belong to Insecta, they would be respond to insect pheromones as attractants or repellents. In this context, this study was carried out that insect pheromones would offer a good alternative to control V. destructor in honey bee colonies. Therefore, this study aimed to explore the possibility of new attractants for the control of V. destructor based on insect pheromone variety, and showed the effects of insect pheromone to control V. destructor.
MATERIALS AND METHODS
The experiment was conducted at the Korea National University of Agriculture and Fisheries in the middle of Korean peninsula, Jeonju, during 2022-2023.
1. Insect pheromones
In this study, twenty various insect pheromones based on their structure were applied to determine the attractiveness to V. destructor (Table 1). All of the insect pheromones were above 95% of purity and provided by National Academy of Agricultural Science (NAAS).
2. Preparation of V. destructor
For the collection and maintenance of V. destructor, six langstroth bee hives were provided by local beekeepers located in Cheongju from June through August 2022 and 2023, and the honey bee hives were placed in the apiary of the Korean National University of Agriculture and Fisheries during the experiment.
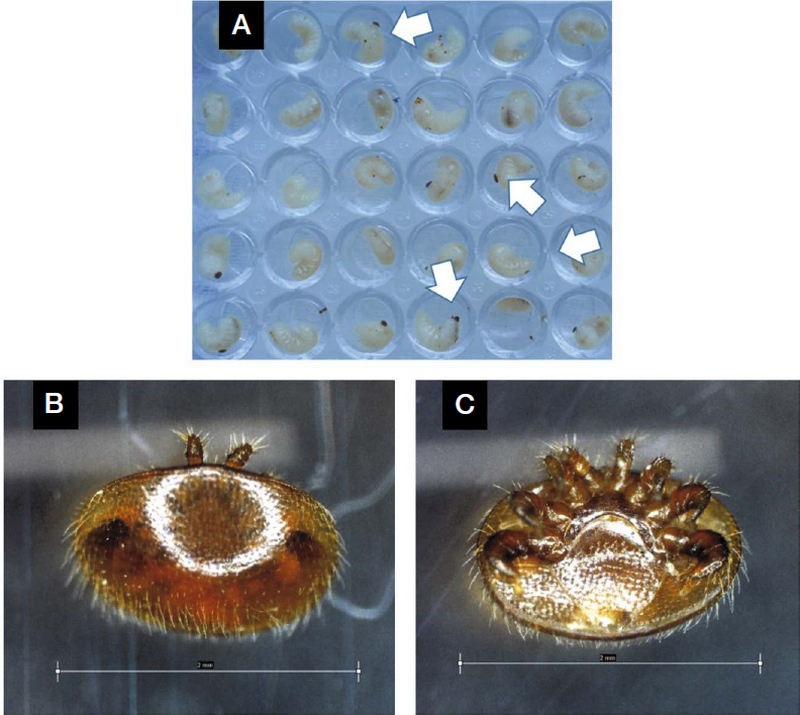
V. destructor were collected from honey bee hives by the sugar powder method (Milbrath, 2016). The method is to use powdered sugar to collect mites from adult bees. First, about 20 g of powdered sugar was poured into a jar containing 300 live bees. Then, a lid made of screen mesh was placed on the container and gently shaked so that the powdered sugar was applied evenly to all the bees. The jar was placed on a hard surface under the shade for 2 minutes to allow the mites dislodged from the bees, and then the jar was shaken upside-down lightly over a white tray for 1 minute. The collected mites were applied to honeybee larvae being capped before brood stage and transferred to a controlled environmental incubator (32°C and 65% relative humidity with no light; 1750 (W)×810 (D)×1760 (H), VS-1203PFC, Korea) in the laboratory. At the following day, the larvae were naturally fallen to the bottom, and they were carefully transferred to 96 well plates to coexist with V. destructor for 2 or 3 days (Fig. 1).
3. Experiment design of ring test to evaluate attractive effect of insect pheromones on V. destructor
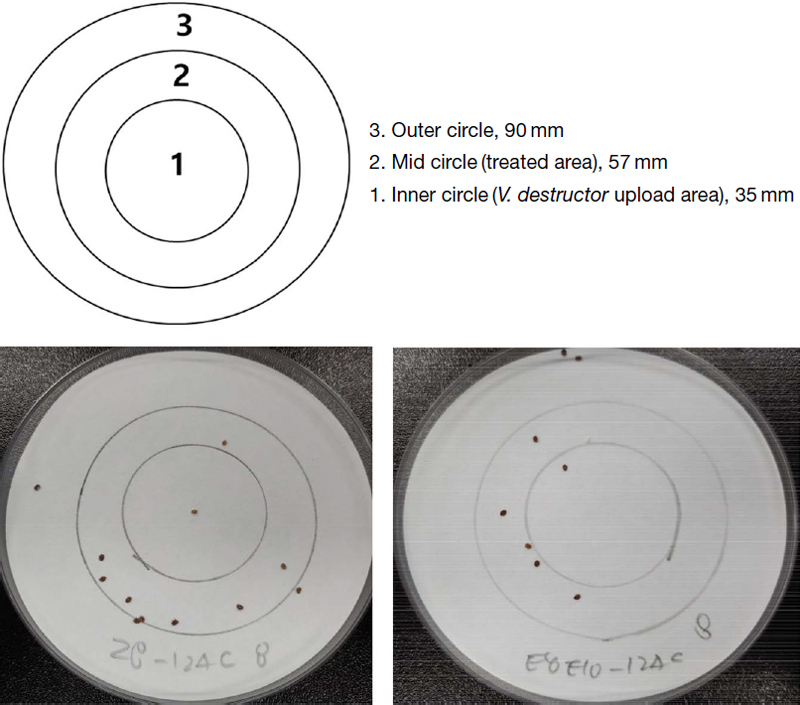
To determine the attractant properties of insect pheromones, the ring test method (Rickli et al., 1994; Donzé et al., 1998) was adapted as illustrated in Fig. 2. For the ring test, a petri dish (90 mm×15 mm, SPL Life Sciences, Korea) was equipped with filter paper (Whatman no. 2) with two concentric circles of 35 and 57 mm in diameter. Then, 50 mg of each pheromone, was diluted into 10 μL of ethanol (Sigma, St. Louis, MO, USA), and was applied on the middle circle. Subsequently, 10 V. destructor were placed at the inner circle, allowing them to move freely on the field. Afterward, the petri dish was covered with a lid and was exposed at room temperature (25-28℃) and 70% RH. And then the distribution of the V. destructor on the filter paper was calculated to evaluate the attractive rate. The attractive rate (%) was calculated as [number of mites in Mid Circle/total number of mites applied]×100%. The observations over 4 hours after mite placement were not counted, because the mites might be starved and thirsty which could affect their attracting and repelling behavior (Gashout and Guzmán-Novoa, 2009). Each experiment in this study was repeated in three times with different mites, and ethanol was used as the control treatment in each experiment.
4. Experiment 1: Attractive effects by the different insect pheromones for V. destructor
To select right insect pheromones of the attractive effect for V. destructor, twenty different insect pheromones were applied to V. destructor at the same concertation as ratios of 50 mg/10 μL for 3 hours at 30℃ and 70% RH under room condition. The attractive effects of twenty different insect pheromones were determined by observing the V. destructor position on the mid circle of the filter paper through visual inspection after 3 hours of the insect pheromone treatments.
5. Experiment 2: Attractive effects by the concentration of insect pheromones for V. destructor
A second experiment was conducted to figure out right concentration for the four pheromones, (Z)-8-12:Ac, (E)-8, (E)-10-12:Ac, (E)-11-14Ac and (Z)-11-14Ac, that were highly effective in the first experiment. The concentrations of pheromones were serially prepared with 50 mg, 25 mg, 12.5 mg and 6.25 mg of 10 μL of ethanol, respectively, and then treated for 3 hours at 30℃ and 70% RH under room condition. V. destructor located in the mid circle was counted to determine attractive effects by different concentration of the four insect pheromones.
6. Experiment 3: Attractive effects by the concentration and exposed time of insect pheromones for V. destructor
In the second experiment, two pheromones, (Z)-8-12 : Ac and (E)-8, (E)-10-12:Ac, were highly effective to V. destructor. Therefore, third experiment was conducted the ring test at different time. The two pheromones were applied as the concentration of 50 mg/10 μL with ethanol, and then treated for 1, 2 and 3 hours at 30℃ and 70% RH. V. destructor located in the mid circle at the different time was observed and determined attractive effects by different time of the two insect pheromones.
7. Statistical analysis
The attractive effect of insect pheromones for V. destructor was calculated through the mean of three replicated measurements. All experimental data for each treatment were analyzed by ANOVA via SAS (Enterprise Guide 7.1, SAS Institute Inc., Cary, NC, USA), and represented significance differences at P<0.05. Also, Duncan’s multiple range and LSD tests (P<0.05) were conducted to compare any significant difference among various treatments.
RESULTS AND DISCUSSION
1. Attractive effects for V. destructor by the diverse insect pheromones
The attractive effects of the twenty insect pheromones on V. destructor were observed, and they showed various rates of attractive effects for V. destructor ranged from 10 to 100% (Fig. 3). Among them, the most potent attractive effects were observed with (Z)-8-12:Ac (100%), followed by (E)-8, (E)-10-12:Ac (60%), E11-14 : Ac (57%) and (Z)-11-14 : Ac (57%).
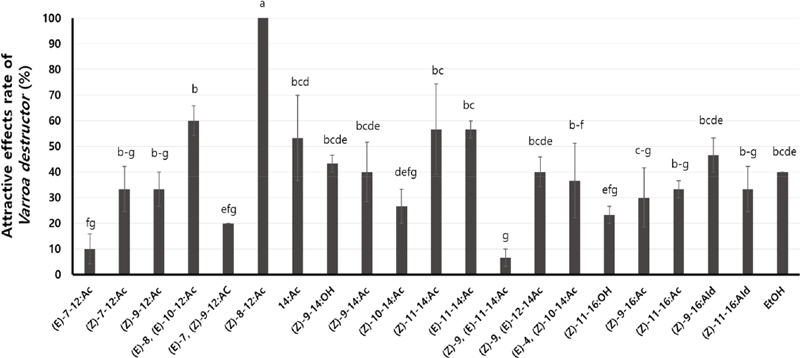
Rate (%) of attractive effects from the insect pheromones for V. destructor. Means within each column followed by the same letters are not significantly different according to Duncan’s multiple range test at P<0.05 (n=10, R=3).
In this study, we hypothesized that different pheromone structures would cause different attractant effect on V. destructor. Indeed, 12-carbon pheromones such as (Z)-8-12:Ac and (E)-8, (E)-10-12:Ac showed higher effects compared to 14-carbon or 16-carbon pheromones. However, some of 12-carbon pheromones showed relatively low attractiveness, this suggested that not all 12-carbon pheromones were equally effective at luring V. destructor. Moreover, there were no discernible differences between the sis and trans forms of the pheromones.
2. Attractive effects for V. destructor by the concentration of insect pheromones
In the first experiment, we selected four pheromones: (Z)-8-12:Ac (E)-8, (E)-10-12:Ac, (E)-11-14Ac and (Z)-11-14Ac, and subsequently assessed their distinct attractive effects at various serially diluted concentration after 3 hours of exposure. Among these pheromones, attraction rates of (Z)-8-12:Ac were demonstrated as 86.7%, 70.0%, 80.0% and 30.0% when applied at concentration of 50 mg, 25 mg, 12.5 mg and 6.25 mg, respectively. Conversely, (E)-8, (E)-10-12:Ac showed attraction rates as 50.0%, 50.0%, 60.0% and 63.3% at the same respective concentration. Additionally, Z11-14Ac displayed attraction rates of 53.3%, 40.0%, 43.3% and 53.3% and E11-14Ac showed attraction rates of 36.7%, 30.0%, 33.3% and 46.7% at the same respective concentration (Fig. 4).
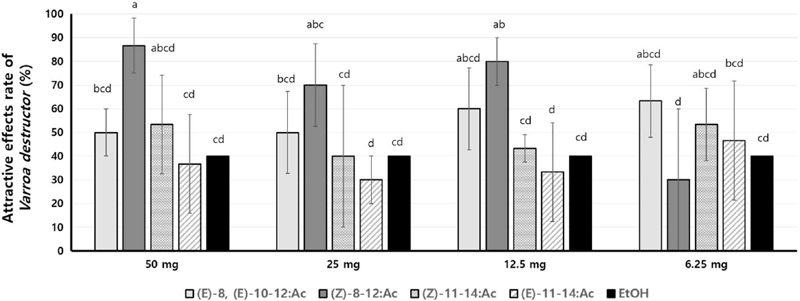
Attractive effects rate (%) by the concentration of the insect pheromones for Varroa destructor. Means within each column followed by the same letters are not significantly different according to Duncan’s multiple range test at P<0.05 (n=10, R=3).
These results suggest that (Z)-8-12Ac exhibited a high attractive effect at concentrations of 50 mg, 25 mg and 12.5 mg; however, its attractiveness decreased at 6.25 mg. On the other hand, (E)-8, (E)-10-12:Ac maintain a relatively high attractive effect even at low concentrations, such as a 6.25 mg. In contrast, both (Z)-8-12:Ac and (E)-11-14Ac showed lower effectiveness compared to the other two pheromones. Therefore, we selected two pheromones, (Z)-8-12:Ac and (E)-8, (E)-10-12:Ac which showed relatively high attractive effect on V. destructor.
Generally, insect pheromones can be influenced by the concentration and many pheromones may not be effective at lower concentrations. However, (E)-8, (E)-10-12:Ac was effective at lower concentrations, and showed higher effect than (Z)-8-12:Ac at 6.25 mg of concentration.
3. Attractive effects of insect pheromones on V. destructor by the exposed time
Selected 2 pheromones, (Z)-8-12:Ac and (E)-8, (E)-10-12:Ac, were assessed for their distinct attractive effects at various exposed time at concentration of 50 mg (Fig. 5). As the result, (Z)-8-12:Ac demonstrated attraction rates of 60.8%, 65.8% and 68.3% when applied at exposed time of 1, 2 and 3 hours, respectively. On the other hand, attraction rates of (E)-8, (E)-10-12:Ac were 45.0%, 51.7% and 55.8% at the same exposed time, respectively. This study showed that both (Z)-8-12:Ac and (E)-8, (E)-10-12:Ac remained attractiveness effect on V. destructor, as the exposed time increased.
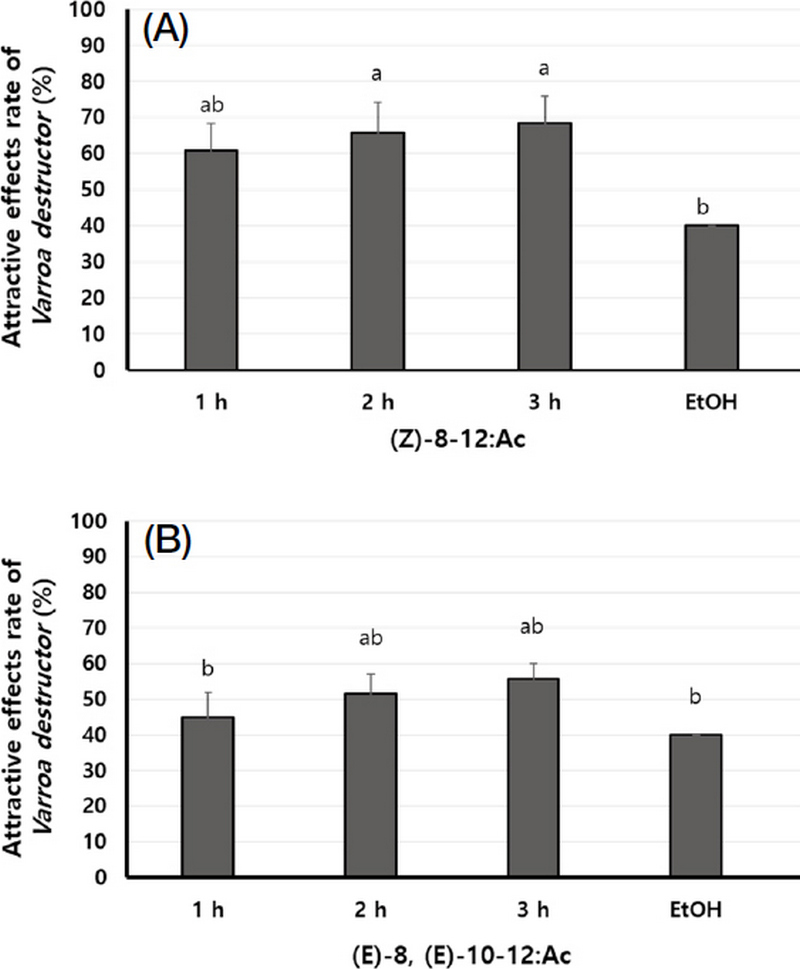
Attractive effects rate (%) by the exposed time of the insect pheromones, A for (Z)-8-12:Ac and B for (E)-8, (E)-10-12:Ac for V. destructor. Means within each column followed by the same letters are not significantly different according to LSD test at P<0.05 (n=10, R=3).
According to the results of the above experiments, insect pheromones (Z)-8-12:Ac and (E)-8, (E)-10-12:Ac showed attractive effect on V. destructor in laboratory conditions. Notably, (Z)-8-12:Ac demonstrates higher effect than (E)-8, (E)-10-12:Ac. Although (Z)-8-12:Ac was a small effect at low concentration (6.25 mg), the optimal concentration for attractiveness was determined as 12.5 mg, which remained the effectiveness for three hours.
In conclusion, this study suggested two potential insect pheromones as effective attractants of V. destructor, (Z)-7-12:Ac (87%) and (E)-8, (E)-10-12:Ac. These pheromones are relatively small carbon pheromones with 12 carbons and are thought to have a higher attractant effect due to this structural characteristic. Therefore, these pheromones should be further investigated as potential alternative attractant for V. destructor control. In addition, other studies are warranted to determine the synergistic effects among different components, and establish appropriate delivery methods to develop safer and more effective attractant.
In general, insect pheromones are used to attract and kill insects such as moths by utilizing sex pheromones. In particular, (Z)-8-12:Ac is a component of moth sex pheromones used to attract and kill moths (Frerot et al., 1979; Varela et al., 2011). Varela et al. (2011) used (Z)-8-12:Ac to attract and kill the oriental fruit moth and Grapholita molesta (Busck) (Lepidoptera: Tortricidae). Hofmeyer and Calitz (1991) utilized (Z)-8-12:Ac as false codling moth (FCM), Cryptophlebia leucotreta (Meyr.) attractant mixed with (E)-8, (E)-10-12:Ac [trans-8-dodecenyl acetate] as its isomer. Although the attractive effect of the isomer, (E)-8, (E)-10-12:Ac was not investigated in this study, future experiments will be required to analyze the effects of structural differences between these isomers.
(E)-8, (E)-10-12:Ac is also a component of the sex pheromone of moths, and has been studied for its use in attractants (Greenway, 1984; Witzgall et al., 1993). Bengtsson et al. (1994) showed the attraction of the pea moth, Cydia nigricana, to (E)-8, (E)-10-12:Ac and 100 μg of (E)-8, (E)-10-12:Ac (99.8% isomeric purity by GC) was used to attract C. nigricana. This was very low concentration compared to the 6.25-50 mg applied in this study. This was considered as V. destructor does not have the antennae that insects have, so its ability to receive olfactory input is very low, making pheromones less effective in attracting them.
The chemosensory appendages of V. destructor include the forelegs and gnathosoma, which are known to detect odorants using lipid carrier proteins in the organelles (Mani et al., 2022). There have been no studies using pheromones to control V. destructor. The difference arises from V. destructor’s unique olfactory sensing mechanism, which reacts differently from that of insects. First of all, the feature of V. destructor is not able to detect odorants form a long distance would be a big limitation to use insect pheromones to attract them. However, this is the first study to test the effectiveness of using insect pheromones to attract V. destructor, and we believe that it may provide a clue to study the relationship between V. destructor and insect pheromones in the future.
Acknowledgments
This work was carried out with the support of the “Cooperative Research Program for Agriculture Science and Technology Development (project no. RS-2021-RD009908)”, Rural Development Administration, republic of Korea.
References
-
Bakar, M. A., M. A. Aqueel, A. B. M. Raza, R. Mahmood, Z. A. Qadir, M. Arshad and M. Sohail. 2019. Evaluation of Essential Oils for the Management of Parasitic Bee Mites, Varroa destructor (Acari: Varroidae) In Vitro. Pak. J. Agric. Res. 32: 566-571.
[https://doi.org/10.17582/journal.pjar/2019/32.4.566.571]

-
Bengtsson, M., G. Karg, P. A. Kirsch, J. Löfqvist, A. Sauer and P. Witzgall. 1994. Mating disruption of pea moth Cydia nigricana F. (Lepidoptera: Tortricidae) by a repellent blend of sex pheromone and attraction inhibitors. J. Chem. Ecol. 20: 871-887.
[https://doi.org/10.1007/BF02059584]

- Bond, J. K., C. Hitaj, D. Smith, K. Hunt, A. Perez and G. Ferreira. 2021. Honey Bees on the Move: From Pollination to Honey Production and Back. USDA-ERS report. June. Washington DC, USA.
- Boo, K. S. and C. H. Jung. 1998. Field tests of synthetic sex pheromone of the apple leafminer moth, Phyllonorycter ringoniella. J. Chem. Ecol. 24: 1939-1947.
-
Dethier, V. G., B. L. Browne and C. N. Smith. 1960. The designation of chemicals in terms of the responses they elicit from insects. J. Econ. Entomol. 5: 134-136.
[https://doi.org/10.1093/jee/53.1.134]

-
Donzé, G., S. Schnyder-Candrian, S. Bogdanov, P. A. Diehl, P. M. Guerin, V. Kilchenman and F. Monachon. 1998. Aliphatic alcohols and aldehydes of the honey bee cocoon induce arrestment behavior in Varroa jacobsoni (Acari: Mesostigmata), an ectoparasite of Apis mellifera. Arch. Insect Biochem. Physiol. 37(2): 129-145.
[https://doi.org/10.1002/(SICI)1520-6327(1998)37:2<129::AID-ARCH2>3.0.CO;2-P]

-
El-Sayed, A., P. Witzgall and H. Arn. 1998. Location of the pheromone producing gland in the European grapevine moth, Lobesia botrana (Lepidoptera: Tortricidae). Appl. Entomol. Zool. 33(4): 507-511.
[https://doi.org/10.1303/aez.33.507]

-
Ellis, R. A., T. Weis, S. Suryanarayanan and K. Beilin. 2020. From a free gift of nature to a precarious commodity: Bees, pollination services, and industrial agriculture. J. Agrarian Change 20: 437-459.
[https://doi.org/10.1111/joac.12360]

-
Frerot, B., E. Priesner and M. Gallois. 1979. A sex attractant for the green budworm moth, Hedya nubiferana. Z. Naturforsch. C 34 (12): 1248-1252.
[https://doi.org/10.1515/znc-1979-1229]

-
Gashout, H. A. and E. Guzmán-Novoa. 2009. Acute toxicity of essential oils and other natural compounds to the parasitic mite, Varroa destructor, and to larval and adult worker honey bees (Apis mellifera L.). J. Apic. Res. 48: 263-269.
[https://doi.org/10.3896/IBRA.1.48.4.06]

-
Greenway, A. R. 1984. Sex pheromone of the pea moth, Cydia nigricana (F.) (Lepidoptera: Olethreutidae). J. Chem. Ecol. 10: 973-982.
[https://doi.org/10.1007/BF00987506]

-
Gupta, R. C. and D. Milatovic. 2014. Insecticides (Chp.23) in Biomarkers in Toxicology. pp. 389-407. Academic Press: London, Wall U. K.
[https://doi.org/10.1016/B978-0-12-404630-6.00023-3]

-
Hattori, M., S. Wakamura, K. Igita, K. Yasuda and Tridjaka. 2001. Comparison of the characteristics and sex pheromone of Etiella behrii (Zellwr), a newly identified pod borer of soybean in Indonesia, with E. zinckenella (Treit.) JARQ 35(1): 19-24.
[https://doi.org/10.6090/jarq.35.19]

-
Hendry, L. B., S. H. Korzeniowski, D. M. Hindenlang, Z. Kosarych, R. O. Mumma and J. Jugovich. 1975. An economical synthesis of the major sex attractant of the oak leaf roller - cis-10-tetradecenyl acetate. J. Chem. Ecol. 1: 317-322.
[https://doi.org/10.1007/BF00988833]

- Hofmeyer, J. H. and F. J. Calitz. 1991. Disruption of male orientation to female false codling moth, Cryptophlebia leucotreta (Lepidoptera: Tortricidae), using synthetic sex pheromone. Phytophylactica 23(2): 157-166.
-
Jack, C. J. and J. D. Ellis. 2021. Integrated pest management control of Varroa destructor (Acari: Varroidae), the most damaging pest of (Apis mellifera L. (Hymenoptera: Apidae)) colonies. J. Insect Sci. 21: 6.
[https://doi.org/10.1093/jisesa/ieab058]

-
Jung, C. R., Y. J. Park and K. S. Boo. 2003. Optimal sex pheromone composition for monitoring Spodoptera exigua (Lepidoptera: Noctuidae) in Korea. J. Asia Pac. Entomol. 6(2): 175-182.
[https://doi.org/10.1016/S1226-8615(08)60183-1]

-
Kakizaki, M. and H. Sugie. 2003. Sex pheromone of the flax budworm, Heliothis maritima adaucta Butler (Lepidoptera: Noctuidae). Appl. Entomol. Zool. 38(1): 73-78.
[https://doi.org/10.1303/aez.2003.73]

-
Kehat, M., S. Greenberg and Y. Tamaki. 1976. Field evaluation of the synthetic sex pheromone, as an attractant for males of the cotton leafworm, Spodoptera littoralis (Boisd.), in Israel. Appl. Entomol. Zool. 11(1): 45-52.
[https://doi.org/10.1303/aez.11.45]

- Koul, O., S. Walia and G. S. Dhaliwal. 2008. Essential oils as green pesticides: potential and constraints. Biopestic. Int. 4(1): 63-84.
-
Mani, K., B. T. Nganso, P. Rodin, A. Otmy, A. Rafaeli and V. Soroker. 2022. Effects of Niemann-Pick type C2 (NPC2) gene transcripts silencing on behavior of Varroa destructor and molecular changes in the putative olfactory gene networks. Insect Biochem. Mol. Biol. 148: 103817.
[https://doi.org/10.1016/j.ibmb.2022.103817]

- Mankin, R. W. 1991. Evolution of pheromonal specificity in insect chemoreceptors. Chemical Senses 3: 61-77.
-
Marex, J., K. Frantisek and H. Ivan. 2000. (E,Z)-7,9-Dodecadien-1-yl acetate acts as attractant for males of the Genus Idaea (Lepidoptera: Geometridae: Sterrhinae). Plant. Porct. Sci. 36(3): 95-100.
[https://doi.org/10.17221/9631-PPS]

- Milbrath, M. 2016. Varroa mite monitoring using a sugar roll to identify populations of Varroa destructor in honey bee colonies. Am. Bee J. 156: 1119-1122.
- Morse, R. A. and N. W. Calderone. 2000. The value of honey bees as pollinators of U.S. crops in 2000. Bee Culture 128: 1-15.
-
Murakami, Y., H. Sugie, T. Fukumoto and F. Mochizuki. 2005. Sex pheromone of Grapholita dimorpha Komai (Lepidoptera: Tortricidae), and its utilization for monitoring. Appl. Entomol. Zool. 40(3): 521-527.
[https://doi.org/10.1303/aez.2005.521]

-
Nazzi, F. and Y. Le Conte. 2016. Ecology of Varroa destructor, the major ectoparasite of the western honey bee, Apis mellifera. Annu. Rev. Entomol. 61: 417-432.
[https://doi.org/10.1146/annurev-ento-010715-023731]

-
Pettis, J. S., A. M. Collins, R. Wilbanks and M. F. Feldlaufer. 2004. Effects of coumaphos on queen rearing in the honey bee, Apis mellifera. Apidologie 35(6): 605-610.
[https://doi.org/10.1051/apido:2004056]

-
Raina, A. K., J. A. Klun, J. D. Lopez and B. A. Leonhardt. 1986. Female sex pheromone of Heliothis phloxiphaga (Lepidoptera: Noctuidae): chemical identification, male behavioral response in the flight tunnel, and field tests. Environ. Entomol. 15(4): 931-935.
[https://doi.org/10.1093/ee/15.4.931]

-
Ramsey, S. D., R. Ochoa, G. Bauchan, C. Gulbronson, J. D. Mowery, A. Cohen, D. Lim, J. Joklik, J. M. Cicero, J. D. Ellis, D. Hawthorne and D. V. Engelsdorp. 2019. Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci. U.S.A. 116: 1792-1801.
[https://doi.org/10.1073/pnas.1818371116]

- Rbee, A. E. A. and O. A. A. Zedan. 2018. Essential oils and Formic acid as good elements in the integrated management of parasite varroa mite Varroa destructor (Anderson and Trueman) in honey bee colonies. Ann. Agric. Sci. 56: 779-784.
-
Rickli, M., P. A. Diehl and P. M. Guerin. 1994. Cuticle alkanes of honeybee larvae mediate arrestment of bee parasite Varroa jacobsoni. J. Chem. Ecol. 24: 2437-2453.
[https://doi.org/10.1007/BF02033212]

-
Shorey, H. H. 1973. Behavioral responses to insect pheromones. Annu. Rev. Entomol. 18: 349-380.
[https://doi.org/10.1146/annurev.en.18.010173.002025]

-
Varela, N., J. Avilla, S. Anton and C. Gemeno. 2011. Synergism of pheromone and host-plant volatile blends in the attraction of Grapholita molesta males. Entomol. Exp. Appl. 141(2): 114-122.
[https://doi.org/10.1111/j.1570-7458.2011.01171.x]

-
Vilarem, C., V. Piou, F. Vogelweith and A. Vétillard. 2021. Varroa destructor from the laboratory to the field: control, biocontrol and IPM perspectives-A Review. Insects 12: 800.
[https://doi.org/10.3390/insects12090800]

-
Witzgall, P., M. Bengtsson, C. R. Unelius and J. Löfqvist. 1993. Attraction of pea moth Cydia nigricana F. (Lepidoptera: Tortricidae) to female sex pheromone (E, E)-8, 10-dodecadien-1-yl acetate, is inhibited by geometric isomers E, Z, Z, E, and Z, Z. J. Chem. Ecol. 19: 1917-1928.
[https://doi.org/10.1007/BF00983796]

-
Zhang, Y. N., L. X. Du, J. W. Xu, B. Wang, X. Q. Zhang, Q. Yan and G. Wang. 2019. Functional characterization of four sex pheromone receptors in the newly discovered maize pest Athetis lepigone. J. Insect Physiol. 113: 59-66.
[https://doi.org/10.1016/j.jinsphys.2018.08.009]