Amino Acid Dynamics in Bee Feed: Comparative Account of Amino Acid Composition among Pollen, Bee-pollen and Bee Bread
Abstract
The comprehensive understanding of amino acid dynamics from fresh pollen to bee-collected pollen and ultimately to bee bread remains an enduring inquiry in apicultural research. In this study, we investigate the alterations in amino acid composition across three pivotal stages: fresh pollen, bee-collected pollen, and bee bread. Notably, pollen demonstrated significantly higher protein levels (31.7 g/100 g) compared to both bee pollen (21.1 g/100 g) and bee bread (20.4 g/100 g). Although protein content between bee pollen and bee bread showed no substantial difference, a detailed analysis of amino acids unveiled distinct patterns. Notably, ten amino acids were significantly more abundant in bee pollen, indicating a decrease during the transition to bee bread. Interestingly, proline levels increased significantly in bee bread, consistent with previous findings. This study highlights dynamic fluctuations in amino acid composition influenced by honey bee behavior and symbiont interactions, tailored to their nutritional needs. These observations are specific to rapeseed pollen but may offer insights into other pollen types in future studies.
Keywords:
Apis mellifera, Bee keeping, Colony nutrition, Honey bee, Nutrition, Pollen transformationINTRODUCTION
Honey bees, along with other animal pollinators, are crucial for pollinating nearly 75% of the world's crops, making them pivotal to global agriculture (IPBES, 2016). However, their populations are facing a signifi- cant decline due to a combination of factors. Habitat degradation has reduced the availability of natural environments where bees can forage and nest (Naug, 2009). Intensive agricultural practices, especially mono- cropping, create large areas with minimal floral diversity, limiting the bees’ food sources. The extensive use of chemical insecticides and herbicides further contaminates their habitats and food supply (Tosi et al., 2017; AASSA, 2022). Additionally, the unpredictable consequences of climate change detrimentally impact the environment, leading to inadequate nutrition for honey bees (Ziska et al., 2016; Descamps et al., 2021). These stressors collectively undermine the health and sustainability of honey bee populations. Therefore, it is imperative to focus on both the nutritional ecology and the feeding behavior of honey bees (Ghosh et al., 2023; Quinlan and Grozinger, 2023). By understanding the complex dynamics of nutrient transfer from ecosystems to honey bee physiology and health, we can develop strategies to better support and preserve these vital polli- nators. Ensuring the well-being of honey bees is essential for maintaining the pollination services they provide to agriculture worldwide.
Honey bees (Apis mellifera) are highly organized and eusocial insects known for their crucial role in pollination. Within the complex world of a bee colony, their feeding behavior plays a vital role in sustaining the health and productivity of the entire hive (Brodschneider and Crailsheim, 2010). This behavior is finely tuned and adaptive, guided by a combination of innate instincts and environmental cues. Understanding the intricacies of honey bee feeding behavior provides valuable insights into their biology, ecology, and the essential services they provide to ecosystems and agriculture.
Honey bees gather both nectar and pollen during their foraging activities. Nectar provides carbohydrates, while pollen serves as the primary source of protein, fat, and micronutrients such as vitamins and minerals. The foraging behaviour of honey bees, particularly for pollen, can be attributed to the nutritional reward they derive from flower resources, notably influenced by factors such as protein content or the ratio of macronutrients, particularly the protein-to-lipid ratio, which guides their foraging decisions (Ghosh et al., 2020; Vaudo et al., 2020). Protein, comprising essential amino acids, is of particular interest, as honeybees lack the ability to synthesize around 10 essential amino acids and thus must acquire them from their dietary sources (De Groot, 1953). During the scarcity of forage in winter, beekeepers commonly provide sugar solution to their hives. As spring begins, they introduce pollen patties to stimulate honey bee activity. In a series of experiments, we examined how the nutritional content of bee pollen changes as it transforms into pollen patties and then into bee bread (Ghosh and Jung, 2020, 2022). Comparing bee pollen to pollen patties, we observed a general reduction in total protein and individual amino acids, likely due to the addition of various ingredients, particularly sugar, in the production of pollen patties (Ghosh and Jung, 2020). Although no significant differences were found between pollen patties and bee bread within the first seven days, except for a notable increase in proline, we noted a temporal rise in individual amino acids and overall protein levels over 14 days (Ghosh and Jung, 2022). This increase in proline is likely attributed to the addition of nectar, which is abundant in proline, during the transformation of bee pollen or supplied pollen patties into bee bread. However, understanding the complete amino acid dynamics from fresh pollen to bee-collected pollen and eventually to bee bread remains a longstanding question. Thus, our present study aims to investigate the changes in amino acid composition across these three stages: fresh pollen, bee-collected pollen, and bee bread.
MATERIALS AND METHODOLOGY
1. Sample collection
Honey bee colonies were positioned in the rapeseed field in Andong during the months of May and June. We retrieved rapeseed flowers from the field and transported them to the laboratory in a chilling box. Using forceps, we carefully extracted the anthers from the stamen. The anthers in distilled water were then placed in an Eppendorf tube and submerged in a sonication bath (Ultrasonic Cleaning system, Sae Han Ultrasonic Co., Seoul, South Korea) for approximately 10 minutes at 100 power/gain to facilitate the release of pollen grains from the anthers. Following this, the supernatant i.e. pollen grains in water was collected and the empty anthers that had precipitated to the bottom of the Eppendorf tube were discarded. The supernatant underwent centrifugation (CF-10, Daihan Scientific, Daegu, South Korea) at 10,000 rpm for 10 minutes. The pollen grains then settled at the bottom of the Eppendorf tube. We discarded the supernatant, and the pollen grains were then precipitated and dried using a freeze dryer. The dried pollen was utilized for further analysis.
To harvest bee pollen, we installed a pollen trap at the entrance of a beehive for a certain period of time. This trap typically consists of a grid or mesh through which the bees must pass to enter the hive but some of the pollen attached to their bodies is gently collected as they pass through the trap. With the help of the pollen trap, bee pollen was collected from returning forager bees as they entered the hive. We allowed honey bees to enter the hive with their foraged bee-pollen, except during certain periods, so that they could prepare bee bread from the pollen. The bee bread was then harvested from the beehives. Both bee-pollen and bee bread samples also freeze-dried and ground into powder before analysis.
Composite sampling was utilized, meaning that several individual samples were combined to form a single representative sample for analysis. Pollen sampling involved composite collection from 1 gram, while for bee-pollen and bee bread, composite sampling required at least 50 grams from each. This approach was applied to collect samples of pollen, bee-pollen, and bee bread. For each type of sample-pollen, bee-pollen, and bee bread-two composite samples (n=2) were prepared. Following this, amino acid analysis was performed separately on each of the two composite samples for pollen, bee-pollen, and bee bread to determine their respective amino acid profiles.
2. Amino acid estimation
Amino acid was estimated following the standard procedure of AOAC (1990). Twenty mg of the sample underwent acid hydrolysis using 6N hydrochloric acid at a temperature of 110 degrees Celsius for duration of 24 hours. The resultant hydrolyzed product was subjected to rotary evaporation, followed by reconstitution using the provided company buffer. Subsequently, the solution was filtered using a 20-micrometer filter, and the resulting filtrate was introduced into the amino acid analyzer system (Sykam Amino acid analyze S433, Sykam GmbH, Eresing, Germany) for analysis.
3. Statistics
The experiment was carried out in duplicate and results were reported as the mean and standard deviation. To assess the significance of amino acid content differences among pollen, bee-pollen, and bee bread, ANOVA was conducted, followed by a post hoc test using SPSS 16.0 (IBM).
RESULTS
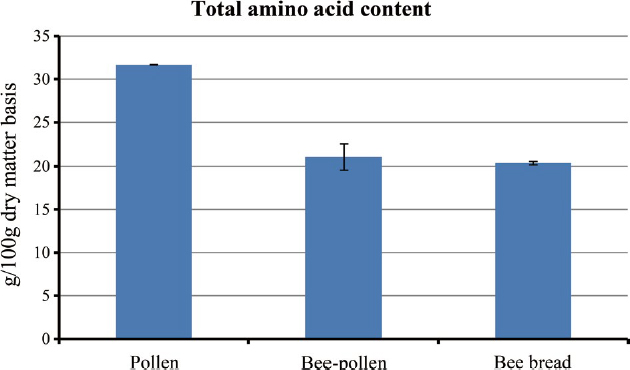
Table 1 illustrates the comprehensive view of the total amino acid content found in pollen, bee pollen, and bee bread samples. Pollen exhibited significantly higher protein levels (31.7 g/100 g) compared to bee pollen (21.1 g/100 g) and bee bread (20.4 g/100 g), as depicted in Fig. 1. While there were no noteworthy disparities in protein content between bee pollen and bee bread, a detailed analysis of amino acid composition unveiled distinct patterns.
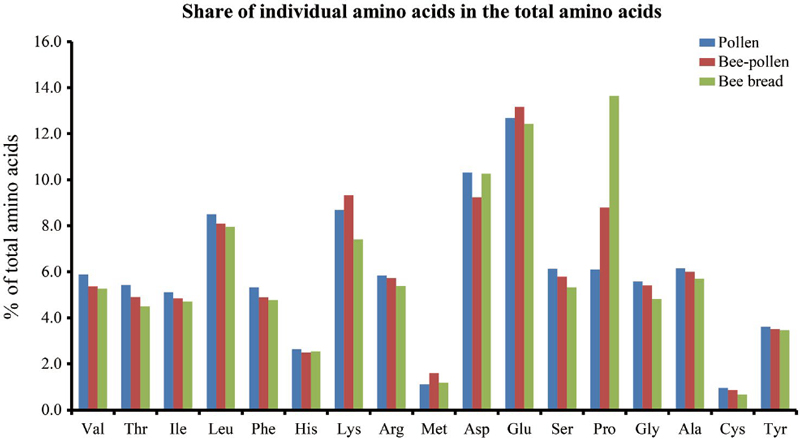
Upon closer examination, as depicted in Table 1 and Fig. 2, although the overall amino acid content of bee pollen and bee bread displayed no substantial divergence, the individual amino acids exhibited varying trends. Notably, ten amino acids-valine, isoleucine, lysine, threonine, arginine, glutamic acid, serine, glycine, alanine, and cysteine-were found to be significantly more abundant in bee pollen in comparison to bee bread, indicating the decrement in the levels of these amino acids in the bee bread. Interestingly the level of proline increased significantly in the bee bread compared to bee pollen. This is in agreement with our previous findings where proline content increased significantly from pollen patty to bee bread.
DISCUSSION
The results from this study on rapeseed pollen align with previous reports, showing that fresh pollen has a higher protein content compared to bee pollen. Human and Nicolson (2006) similarly discovered that the protein content of fresh pollen from Aloe greatheadii var. davyana was significantly greater than that of bee pollen. However, the protein content (50.8%) of fresh Aloe pollen exceeded that of fresh rapeseed pollen obtained in our current investigation. This difference may arise from the methodological approach employed in estimating protein content. Specifically, crude protein was assessed for Aloe pollen, while for rapeseed, we calculated the total proteinogenic amino acids as the protein content, which inherently tends to be lower than the total crude protein content. Consistently, the amino acid composition revealed leucine as the primary essential amino acid, followed by lysine, while glutamic acid was notably abundant among non-essential amino acids, aligning with earlier research findings (Human and Nicolson, 2006; Yang et al., 2013; Ghosh and Jung, 2017; Taha et al., 2019). The increase of proline may be possibly due to the addition of nectar to make the corbicular bee-pollen and the subsequent fermentation to make bee-bread. The most abundant amino acid in the total amino acid content of nectar is proline and therefore mixing the pollen with nectar might increase the level of proline (Nepi et al., 2012). The elevated concentration of proline found in bee-bread compared to the corresponding levels in bee-pollen aligns with the findings of a previous investigation conducted by Bayram et al. (2021). Proline is a crucial source of energy for insects. It can be metabolized very rapidly. Oxidative proline degradation provides an efficient, short burst of energy that is utilized during the initial lift phase of insect flight (Carter et al., 2006). Thus, bees may utilize two “fuels” available in nectar: proline for the initial phase of flight (take-off from flowers), and sugars to extend their energy requirements during prolonged flight (Carter et al., 2006).
Bee-bread formation involves a series of biological and environmental processes that impact the composition of amino acids. First, bees initiate the creation of bee-bread through the fermentation of bee-pollen within the hive. During fermentation, certain amino acids might undergo breakdown or modification, consequently reducing their individual concentrations in the resulting bee-bread. This phenomenon could explain the decrease in some amino acid levels observed in both this study and earlier investigation by Aylanc et al. (2023). However, it is worth noting that the outcomes regarding amino acid reduction are not consistently uniform. Bayram et al. (2021) demonstrated contrasting results, showing that in one instance, the conversion of bee-pollen into bee-bread led to a depletion in the levels of most amino acids compared to bee-pollen, while in another case, the results were reversed. This fermentation process relies on the activity of various microorganisms present in the hive environment and bee-pollen (Ghosh et al., 2022), which metabolize specific amino acids, possibly contributing to their depletion in bee-bread. Top of Form Additionally, bees themselves play a role in modifying amino acids through enzymatic activity in their saliva or gut during the conversion of pollen into bee-bread. Moreover, selective consumption of pollen components by bees during processing can lead to variations in the concentration of individual amino acids between bee-bread and bee-pollen as honey bee can perceive concentration differences in amino acid (Ruedenauer et al., 2021). Furthermore, the storage conditions within the hive, including factors such as temperature, humidity, and exposure to other substances, may affect the stability of amino acids in bee-bread over extended periods, potentially leading to their degradation or modification.
Hence, there exists a fluctuation in the amino acid makeup over the course of the process, with honey bees likely influencing this composition through their feeding behavior and the influence of symbionts, tailored to their needs. However, this observation pertains specifically to rapeseed pollen and could potentially extend to other pollen types in future investigations.
Acknowledgments
This research was funded by the BSRP through the National Research Foundation of Korea (NRF), Ministry of Education, grant number NRF-2018R1A6A1A03024862, and RDA agenda on Smart beekeeping (RS-2023-00232847).
References
- A.O.A.C. 1990. Official Methods of Analysis, 15th ed.; Associ- ation of Official Analytical Chemists: Washington, DC, USA.
- AASSA (The Association of Academies and Societies of Sciences in Asia). 2022. Risk Assessment of Neonicotinoids in the Asia-Pacific Region, AASSA Secretariat, The Korean Academy of Science and Technology (KAST), Seongnam-si, Republic ok Korea, 101 pages.
-
Aylanc, V., S. I. Falcão and M. Vilas-Boas. 2023. Bee pollen and bee bread nutritional potential: chemical composition and macronutrients digestibility under in vitro gastroin- testinal system. Food Chem. 413: 135597.
[https://doi.org/10.1016/j.foodchem.2023.135597]

-
Bayram, N. E., Y. C. Gercek, S. Çelik, N. Mayda, A. Ž. Kostić, A. M. Dramićanin and A. Özkök. 2021. Phenolic and free amino acid prifiles of bee bread and bee pollen with the same botanical origin - similarities and differences. Arabian J. Chem. 14: 103004.
[https://doi.org/10.1016/j.arabjc.2021.103004]

-
Brodschneider, R. and K. Crailsheim. 2010. Nutrition and health in honey bees. Apidologie 41: 278-294.
[https://doi.org/10.1051/apido/2010012]

-
Carter, C., S. Shafir, L. Yehonatan, R. G. Palmer and R. Thorn- burg. 2006. A novel role for proline in plant floral nec- tars. Naturwissenschaften 93: 72-79.
[https://doi.org/10.1007/s00114-005-0062-1]

- De Groot, A. P. 1953. Protein and amino acid requirements of the honey bee (Apis mellifera L.). Physiol. Comp. Oecol. 3: 197-285.
-
Descamps, C., M. Quinet and A.-L. Jacquemart. 2021. Climate change-induced stress reduce quantity and alter com- position of nectar and pollen from a bee-pollinated spe- cies (Borago officinalis, Boraginaceae). Front. Plant Sci. 12: 755843.
[https://doi.org/10.3389/fpls.2021.755843]

-
Ghosh, S. and C. Jung. 2017. Nutritional value of bee-collected pollens of hardy kiwi, Actinidia arguta (Actinidiaceae) and oak, Quercus sp. (Fagaceae). J. Asia-Pac. Entomol. 20: 245-251.
[https://doi.org/10.1016/j.aspen.2017.01.009]

-
Ghosh, S. and C. Jung. 2020. Changes in nutritional composi- tion from bee pollen to pollen patty used in bumblebee rearing. J. Asia-Pac. Entomol. 23: 701-708.
[https://doi.org/10.1016/j.aspen.2020.04.008]

-
Ghosh, S. and C. Jung. 2022. Temporal changes of nutrient composition from pollen patty to bee bread with special emphasis on amino and fatty acids composition. J. Asia-Pac. Entomol. 25: 101873.
[https://doi.org/10.1016/j.aspen.2022.101873]

-
Ghosh, S., H. Jeon and C. Jung. 2020. Foraging behaviour and preference of pollen sources by honey bee (Apis melli- fera) relative to protein content. J. Ecol. Environ. 44: 4.
[https://doi.org/10.1186/s41610-020-0149-9]

-
Ghosh, S., S. M. Namin and C. Jung. 2022. Differential bacterial community of bee bread and bee pollen revealed by 16S rRNA high-throughput sequencing. Insects 13: 863.
[https://doi.org/10.3390/insects13100863]

-
Ghosh, S., Y. Wang and C. Jung. 2023. Editorial: Nutritional ecology of wild and managed bees. Front. Ecol. Evol. 11:1223769.
[https://doi.org/10.3389/fevo.2023.1223769]

-
Human, H. and S. W. Nicolson. 2006. Nutritional content of fresh, bee-collected and stored pollen of Aloe greathea-dii var. davyana (Asphodelaceae). Phytochemistry 67: 1486-1492.
[https://doi.org/10.1016/j.phytochem.2006.05.023]

- IPBES. 2016. Summary for policymakers of the assessment report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services on pollina- tors, pollination and food production. S. G. Potts, V. L. Imperatriz-Fonseca, H. T. Ngo, J. C. Biesmeijer, T. D. Breeze, L. V. Dicks, L. A. Garibaldi, R. Hill, J. Settele, A. J. Vanbergen, M. A. Aizen, S. A. Cunningham, C. Eardley, B. M. Freitas, N. Gallai, P. G. Kevan, A. Kovács-Hostyánszki, P. K. Kwapong, J. Li, X. Li, D. J. Martins, G. Nates-Parra, J. S. Pettis, R. Rader and B. F. Viana (eds.). Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services, Bonn, Germany. 36 pages.
-
Naug, D. 2009. Nutritional stress due to habitat loss may explain recent honeybee colony collapses. Biol. Conserv. 142: 2369-2372.
[https://doi.org/10.1016/j.biocon.2009.04.007]

-
Nepi, M., C. Soligo, D. Nocentini, M. Abate, M. Guarnieri, G. Cai, L. Bini, M. Puglia, L. Bianchi and E. Pacini. 2012. Amino acids and protein profile in floral nectar: much more than a simple reward. Flora - Morphol. Distrib. Func. Ecol. Plants 207: 475-481.
[https://doi.org/10.1016/j.flora.2012.06.002]

-
Quinlan, G. M. and C. M. Grozinger. 2023. Chapter Five- Honey bee nutritional ecology: From physiology to landscapes. Adv. Insect Physiol. 64: 289-345.
[https://doi.org/10.1016/bs.aiip.2023.01.003]

-
Ruedenauer, F. A., N. W. Biewer, C. A. Nebauer, M. Scheiner, J. Spaethe and S. D. Leonhardt. 2021. Honey bees can taste amino and fatty acids in pollen, but not sterols. Front. Ecol. Evol. 9: 684175.
[https://doi.org/10.3389/fevo.2021.684175]

-
Taha, E. A., S. Al-Kahtani and R. Taha. 2019. Protein content and amino acids composition of bee-pollens from major floral sources in Al-Ahsa, eastern Saudi Arabia. Saudi J. Biol. Sci. 26: 232-237.
[https://doi.org/10.1016/j.sjbs.2017.06.003]

-
Tosi, S., J. C. Nieh, F. Sgolastra, R. Cabbri and P. Medrzycki. 2017. Neonicotinoid pesticides and nutritional stress synergistically reduce survival in honey bees. Proc. R. Soc. B 284: 20171711.
[https://doi.org/10.1098/rspb.2017.1711]

-
Vaudo, A. D., J. F. Tooker, H. M. Patch, D. J. Biddinger, M. Coccia, M. K. Crone, M. Fiely, J. S. Francis, H. M. Hines, M. Hodges, S. W. Jackson, D. Michez, J. Mu, L. Russo, M. Safari, E. D. Treanore, M. Vanderplanck, E. Yip, A. S. Leonard and C. M. Grozinger. 2020. Pollen protein: lipid macronutrient ratios may guide broad pat- terns of bee species floral preferences. Insects 11: 132.
[https://doi.org/10.3390/insects11020132]

-
Yang, K., D. Wu, X. Ye, D. Liu, J. Chen and P. Sun. 2013. Char- acterization of chemical composition of bee pollen in China. J. Agric. Food Chem. 61: 708-718.
[https://doi.org/10.1021/jf304056b]

-
Ziska, L. H., J. S. Pettis, J. Edwards, J. E. Hancock, M. B. Tomecek, A. Clark, J. S. Dukes, I. Loladze and H. W. Polley. 2016. Rising atmospheric CO2 is reducing the protein concentration of afloral pollen source essen- tial for North American bees. Proc. R. Soc. B 283: 20160414.
[https://doi.org/10.1098/rspb.2016.0414]