A Study on the Implementation of HACCP System Categories in the Honeybee Industry with Case Studies of Australian B-QUAL and Canadian CBISQT
Abstract
With the recent amendment of the Livestock Industry Act (LIA), honeybees have been classified as livestock, and rearing, disease control, and hygiene standards for honeybees are now applied similarly to other livestock. However, honeybees are not yet included as livestock under the Livestock Products Sanitation Control Act (LPSCA), and thus HACCP has not been applied to honeybee farms. This study investigated hygiene management systems currently applied to honeybee farms abroad and explored ways to adapt domestic HACCP standards for application to honeybee farms in Korea. Australia’s B-QUAL and Canada’s CBISQT certifications are internationally recognized farm food safety programs based on HACCP principles. These programs establish detailed hygiene standards for beekeeping facilities, processing equipment, veterinary drugs, and packaging materials, as well as monitoring and corrective actions for effective management. In Korea, HACCP is currently applied to livestock farms raising animals such as cattle, pigs, and chickens. We compared and evaluated existing farm HACCP criteria with domestic regulations and international standards to propose applicable criteria for honeybee industry. These findings indicate that applying HACCP to honeybee farms in Korea is feasible. The results of this study can serve as baseline data for establishing hygiene practices in apiaries. Further scientific hazard analysis on apicultural products will support the implementation of HACCP in beekeeping operations.
Keywords:
Beekeeping, Hazard analysis, HACCP, B-QUAL, CBISQTINTRODUCTION
In recent years, Colony Collapse Disorder (CCD) has been observed in countries such as the United States and across Europe. This phenomenon is reported to result from the complex interaction of various factors, including climate change, pests and diseases, nutritional deficiencies, and pesticide exposure (vanEngelsdorp et al., 2009; Jung and Lee, 2018). In Korea, honeybee mortality and disappearance during the winter have also been reported (Jung and Bae, 2022), and potential causes are suggested to include Varroa mites and abnormal weather patterns. Notably, it has been found that some farms have excessively used various pesticides to control Varroa mites (RDA, 2022), which may leave residues in apicultural products such as honey and royal jelly, underscoring the need for strict management of pesticide use.
Unlike livestock products such as milk and meat, honey is well known to be safe and free from microbes due to its high osmotic pressure and low pH, both of which result from its high sugar concentration (Weston et al., 2000). These properties create an environment that inhibits the growth of most microorganisms and helps to ensure the microbial safety of honey. Along with these characteristics, honey also has antibacterial effects due to its content of methylglyoxal (MGO), phenolic acids, hydrogen peroxide, and other unidentified compounds with bactericidal activity (Ali, 2005; Lee et al., 2008; Choi et al., 2010; Sultanbawa et al., 2015). However, some studies have reported the detection of certain microbes in honey, including molds, yeasts, and bacterial spores (Klutse et al., 2021). The primary sources of these contaminants are pollution from dust, air, soil, and nectar around the apiary, followed by cross-contamination occurring during post-harvest handling by people. Therefore, to produce safe honey products, it is essential to establish and follow safe honey handling procedures with proper hygiene management.
Livestock HACCP is a scientific hygiene management system designed to prevent the contamination or introduction of biological (B), chemical (C), and physical (P) hazards into livestock products (MFDS, 2024). It is applied at all stages, from farms to feed factories, slaughterhouses, processing plants and retail. For livestock farms, HACCP has been implemented for various species, beginning with pig farms in 2006, followed by cattle (2007), chickens (2008), ducks (2009), and deer (2013) (KAHAS, 2017).
With the recent amendment of LIA (MAFRA, 2020), honeybees have been classified as livestock, and systematic management for their rearing, disease control, and hygiene standards has been established under the Act on the Prevention of Contagious Animal Diseases (APCAD; MAFRA, 2024). However, HACCP standards for beekeeping have yet to be established. To effectively manage veterinary drug use and hygiene practices in beekeeping, the application of HACCP is considered necessary.
In response, this study was conducted to investigate the hygiene and quality management systems applied to honeybee farms abroad, including in Australia and Canada, to enable the application of HACCP to domestic honeybee farms. Furthermore, application strategies were proposed by comparing domestic HACCP evaluation criteria with standards used in these countries to identify suitable approaches for domestic implementation.
HYGIENE AND QUALITY MANAGEMENT SYSTEMS FOR APIARIES OVERSEAS
1. B-QUAL in Australia
The Australian beekeeping industry is supported by the Australian Department of Agriculture, Fisheries, and Forestry with the implementation of the National Residue Survey (NRS) program for the production of clean and environmentally friendly products (Mckee, 2003). The NRS program conducts tests for environmental contaminants and chemical residues in both domestically produced and imported honey. Samples are collected from producers or packaging facilities, and if any residues are detected, a thorough traceback investigation is conducted to identify the source of the issue. In cases of confirmed misuse, non-compliance penalties and/or commercial sanctions are applied. To ensure compliance with these government regulations, the Australian Honeybee Industry Council (AHBIC) developed the B-QUAL food safety program (AHBIC, 2002). This quality assurance program is based on the Food Standards Code of Food Standards Australia New Zealand (FSANZ) and HACCP principles, and it is implemented across the entire supply chain from apiaries to packaging and retail facilities.
Table 1 describes the certification process and post-certification maintenance of the Australian B-QUAL certification system. The managing authority for the B-QUAL certification process is B-QUAL Australia Pty Ltd. The certification process involves completion of the B-QUAL training program, followed by an application for certification, which includes payment of the assessment fee and annual membership fee. This is followed by a certification assessment conducted by an auditor. If all standard requirements are met, a certificate is issued.
For post-certification maintenance (annual audit), annual or biennial on-site visits are conducted to monitor compliance with the approved QA system. Biennial visits are applicable only for beekeepers with a flawless audit result in the previous year who sell honey directly to packers (excluding consumer sales). Audits are performed by auditors contracted through B-QUAL Australia Pty Ltd. and national service providers. In cases of non-compliance, continued visits are made to provide necessary guidance, and additional fees are applied as required.
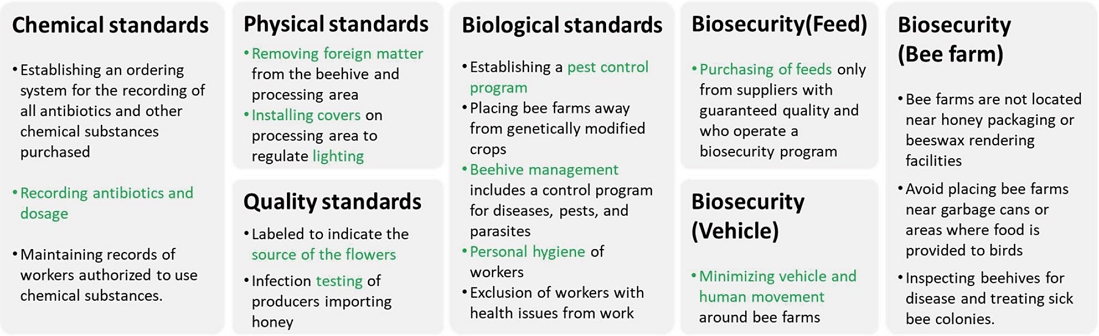
As shown in Fig. 1, the B-QUAL certification system encompasses key quality assurance standards aimed at ensuring the safety and quality of honey production.
Chemical Standards require maintaining records for all antibiotics and chemicals used, along with tracking and recording treated colonies. Biological Standards focus on worker hygiene, locating sites away from genetically modified crops, implementing pest control programs, and ensuring safe waste disposal to reduce contamination risks. Physical Standards make sure that hives and equipment are properly maintained to prevent contamination and guarantee safe honey extraction. Under Quality Standards, honey products must meet specific requirements for floral source and purity, and authenticity testing is required for imported honey. Management Standards mandate a HACCP-based food safety program, employee training, facility compliance, equipment maintenance, and careful handling of honey during storage and transportation, while also ensuring inventory control, proper labeling, and traceability. Biosecurity Standards emphasize sourcing bees from certified suppliers, conducting regular inspections, implementing disease control measures, and regulating bee movement. Finally, Professional Activity Standards provide guidelines for pollination, queen bee production, pollen, propolis, and royal jelly production to maintain quality with hygienic practices, proper storage, and careful handling.
2. CBISQT in Canada
The Canadian On-Farm Food Safety (COFFS) Program, a farm-level food safety management system, was developed based on the HACCP principles outlined by CODEX to enhance food safety and build consumer confidence (Rajic et al., 2007). The program is mandatory for livestock farms and is implemented across various livestock sectors: the Verified Beef Production (VBP) program for beef cattle, the Safe, Safer, Safest (SSS) program for broiler chickens, the Start Clean-Stay Clean (SCSC) program for laying hens, the Canadian Quality Milk (CQM) program for dairy cows, and the Canadian Pork Excellence (CPE) program for swine. For beekeeping, this program is called the Canadian Bee Industry Safety Quality Traceability (CBISQT) (CHC, 2014).
This program categorizes potential biological, chemical, and physical hazards associated with the apiary environment, primary processing facilities, and honey houses as follows:
- • Biological hazards: Toxin-producing spores from Clostridium botulinum and Bacillus spp. are common soil- and dust-borne bacteria that can contaminate honey products (especially honey) during harvesting and extraction.
- • Chemical hazards: Residues of medications (e.g., antibiotics), on-hive pesticide treatments (such as miticides), petrochemical-based products, and farm chemicals used off-hive (including pest management products, water treatment aids, and maintenance and sanitation products) can contaminate raw honey if misused during the preseason (before the active season of foraging, production, and extraction) or postseason.
- • Physical hazards: Potential sources include unhygienic personnel, faulty equipment, pest activity, improper waste disposal, or external contaminants (e.g., unfiltered glass, plastic, or metal fragments) introduced during processing or packaging due to breakage or other forms of contamination.
To control these hazards effectively, the CBISQT Producer Manual systematically applies Good Production Practice (GPP) as a guideline for managing food safety risks throughout the production and packaging processes. The GPP framework consists of 12 practices (Table 2), each structured according to HACCP principles and encompassing food safety hazards, acceptable limits for control, control procedures, monitoring procedures, corrective action procedures, training protocols, and recordkeeping practices. The main points outlined are as follows:
The CBISQT Program’s GPPs provide essential guidelines for all stages of honey production and addresses areas from apiary management to finished product packaging, with a strong emphasis on preventing contamination and ensuring safety.
Apiary management guidelines (GPP1) focus on selecting suitable locations, implementing off-hive pest control, and maintaining hive equipment. These practices aim to reduce contamination risks linked to the environment and activities in the apiary. Managing the condition and suitability of inputs arriving at the farm (GPP2) can prevent potential hazards early when receiving feed supplements, hive equipment, farm chemicals, medications, packaging materials, and processing equipment. Visual inspection of inputs at the time of receipt is essential, particularly to ensure correct identification, food-grade specifications, and good condition. Storage practices (GPP3) are crucial in preventing cross-contamination by isolating hive equipment, processing tools, and feed supplements in designated areas. Regular pest control and maintenance of storage conditions are also required to maintain safety. Honeybee health management (GPP4) emphasizes the safe handling and use of feed supplements, medications, and treatments to support hive health while ensuring compliance with food safety standards. Special care is taken in cases where antibiotics or treatments are used. Honey harvesting practices (GPP5) detail safe handling and transportation methods for honey supers so that honey remains uncontaminated from the field to the processing facility. The design and construction of processing facilities (GPP6) should incorporate contamination control measures to address potential sources of biological and chemical hazards in the processing environment. This includes attention to cleanliness and the prevention of airborne contaminants. Guidelines for handling raw honey (GPP7) recommend sanitary methods for all contact surfaces and equipment used in the honey house or processing areas to minimize contamination hazards. Proper management of the environment and procedures in packaging, storage, and shipping ensures that finished honey (GPP8) remains free from contamination. Practices such as filling, sealing, and labeling are carefully managed to prevent risks during farm-based packaging processes. Facility management (GPP9) encompasses maintenance, cleaning, pest control, and waste disposal in the processing environment to mitigate potential contamination risks, with an emphasis on the upkeep of all equipment and areas in contact with honey. Potable water management (GPP10) is also addressed, recognizing that although water is not directly added to raw honey, it plays an indirect role in cleaning and sanitizing containers, tools, and processing surfaces. Safe handling and treatment of water prevent contamination from pathogens and harmful chemicals. Finally, the role of personnel (GPP11) is critical in preventing hazards throughout production. Adequate skills and continuous training in good hygienic practices, such as hand washing, sanitary footwear, and regular equipment cleaning, are emphasized, as these practices help maintain product safety and reduce contamination risks from pathogenic organisms.
COMPARISON OF DOMESTIC HACCP STANDARDS WITH CANADIAN AND AUSTRALIAN STANDARDS
The domestic HACCP evaluation is conducted based on the farm assessment form in accordance with the Ministry of Food and Drug Safety (MFDS) notification (MFDS, 2023). The farm implementation assessment form consists of 38 prerequisite management items and 15 HACCP management items. Specifically, the prerequisite management section includes 11 items for Farm Management, 10 for Sanitation Management, 12 for Feeding Management, and 5 for Incoming and Outgoing Management. The HACCP management section includes 16 items in total, such as 2 items for the HACCP Team, 2 for Livestock Manuals and Layouts, 1 for Hazard Analysis, 2 for CCP Determination and Critical Limit Setting, 5 for CCP Monitoring and Corrective Actions, 2 for HACCP Verification, and 2 for Education and Training.
In this study, we assessed the feasibility of applying the prerequisite management evaluation items to honeybee farms by comparing the regulatory basis in domestic laws with standards from Australia (B-QUAL) and Canada (CBISQT), as shown in Table 3.
1. Comparison of domestic and international regulations by HACCP evaluation criteria
(1) Evaluation criteria
- • A barrier facility for access control should be installed at the farm entrance, and information and biosecurity warning signs should be posted on the entry gate.
- • Facilities for disinfecting entrants, vehicles, and supplies should be installed.
- • Visitors to the farm should wear dedicated clothing and footwear, and access to the inside of livestock housing should be restricted to external personnel.
- • An access logbook should be placed at the farm entrance to record entries.
- • Livestock rearing facilities should be installed in accordance with livestock industry licensing standards, and ventilation should be provided to expel harmful gases.
- • Lighting and temperature/humidity monitoring devices should be installed in livestock facilities, and a shoe disinfectant mat should be placed at the entrance.
- • Measures should be in place to prevent animals other than livestock from entering the livestock housing facilities.
(2) References
- • (Domestic Laws) APCAD, Article 17; ER of APCAD, Article 20; BIPSA, Article 5; LIA, Articles 22 and 26; ED of LIA, Article 14-2
- • (CBISQT) GPP 6, Processing Facilities; GPP 11, Personnel
- • (B-Qual) 5.1.3 Facilities; 6.7 Wild Areas
(3) Rationale for implementation
- • To minimize the spread of disease in livestock farms, epidemiological investigations are conducted along with quarantine measures in areas surrounding the affected farms. These investigations include general farm status, biosecurity measures, vehicle access, and visitor logs. In particular, honeybee diseases such as foulbrood can easily spread to entire colonies when infection occurs, causing extensive larval mortality and significant damage to beekeepers (Berenyi et al., 2006). Therefore, biosecurity measures are essential in preventing disease transmission in honeybee farms. In accordance with the APCAD and the BIPSA, entry barriers should be installed at farm entrances, and fencing should be established around the farm perimeter to prevent access by outsiders. Additionally, biosecurity warning signs indicating restricted access and notices outlining entry procedures should be displayed. Disinfection facilities must be installed for people, vehicles, and external materials entering the farm, and a logbook should be kept recording and maintain details of all visitor and vehicle entries. Farms are also required to establish rearing facilities according to the registration standards under the LIA, and lighting as well as temperature and humidity meters must be installed in honey processing facilities.
- • In CBISQT’s GPP 6, honey processing facilities provide guidelines related to the design and construction of typical farm honey processing facilities to prevent potential contamination of raw honey before, during, and after processing seasons. These guidelines address the sources and control procedures for potential biological and chemical hazards in the processing facility environment. Doors and fences should display “No Access” signs to control unauthorized visitors, and all lighting fixtures must be fitted with shatterproof covers. Inspection areas (zones for visual hive checks, box disassembly, manual lid removal, and extraction inspection points) must maintain lighting of at least 540 lux.
- • According to B-QUAL 5.1.3 Facility, proper ventilation, natural light, and artificial lighting should be utilized in processing areas. In 6.7 Wild Area, it is advised to take preventive measures if an area is suspected of disease outbreaks.
(1) Evaluation criteria
- • Facilities and equipment for farm disinfection must be available, and regular disinfection should be carried out. Tools and equipment used on the farm must also be cleaned and disinfected.
- • The farm should conduct regular pest control and ensure proper drainage around the farm.
- • Salmonella spp. testing should be conducted for livestock and rearing facilities.
- • Waste should be separated, stored in designated areas, and regularly processed and removed.
- • Regular hygiene and biosecurity training should be provided to farm workers.
- • Hazardous chemicals should be stored separately in designated areas.
(2) References
- • (Domestic Laws) APCAD, Articles 6, 17, and 51; ER of LIA, Article 30
- • (CBISQT) GPP 1. Beekeeping Yard Management; GPP 6. Processing Facilities; GPP 9. Facility Management; GPP 11. Personnel
- • (B-Qual) 2. Biological Standards; 6.9. Disease Spread Within and Between Beekeeping Yards
(3) Rationale for implementation
- • According to the guidelines for cleaning and disinfecting livestock farms (Animal and Plant Quarantine Agency, 2016), high-pressure sprayers should be used to disinfect livestock buildings, and disinfectants should be diluted just before use. While regular Salmonella spp. testing is conducted for cattle, pigs, and poultry, it is challenging to apply the same to bee farms. Therefore, it is necessary to select indicator substances related to hygiene and disease for regular testing.
- • In CBISQT GPP1 Beekeeping Yard Management, contamination from pathogenic bacteria (e.g., spores of Clostridium spp. and Bacillus spp.) can occur due to improper cleaning or sanitation practices with hive equipment. Contamination from chemical residues (e.g., maintenance products, medications) in used hive equipment (including contaminated honey/comb) due to improper disposal is also possible. Therefore, it is necessary to make sure that the equipment used in honey production is properly cleaned and disinfected. Compliance should be confirmed with regular monitoring and visual inspections by trained personnel. GPP 9 Facility Management addresses the proper maintenance and cleaning of processing equipment and facilities, as well as the safe disposal procedures for farm chemicals and waste honey within the facility. GPP 11 covers the implementation of training programs for ongoing education to control or prevent risks related to honey products.
- • In B-QUAL 2. Biological Standards, hive tools should be cleaned before starting work in a new apiary and after use on suspected hives. Honey containers must be cleaned and dried both inside and out. Wastewater, sewage, and trash between buildings should be handled safely and hygienically, with wastewater processed through septic systems or other appropriate methods.
(1) Evaluation criteria
- • The feed must be sourced exclusively from HACCP-certified factories, and all incoming feed materials should undergo rigorous quality inspections, including sensory evaluations, immediately upon receipt to ensure compliance with safety standards. The storage locations and transport tools for the feed should be regularly cleaned and disinfected. If self-manufactured feed is used, specific management standards must be established and strictly followed.
- • Drinking water for animals should come from either a municipal water supply or groundwater that meets the required water quality standards. The watering facilities must be kept clean and disinfected regularly to maintain hygiene and safety.
- • A plan to prevent residues of veterinary medicines and related substances must be developed and implemented. Records of all incoming and outgoing veterinary products should be maintained.
- • Livestock disease prevention measures should be actively carried out. Standards for controlling internal and external parasites must be established and implemented according to a set schedule to maintain the health of the animals.
(2) References
- • (Domestic Laws) ER of LIA, Article 30; GA, Article 20
- • (CBISQT) GPP 2. Receiving Inputs; GPP 4. Livestock Health Management; GPP 10. Potable Water Management
- • (B-Qual) 1. Chemical Standards; 2. Biological Standards; 6.4. Feeding; 6.9. Disease Spread Within and Between Beekeeping Yards
(3) Rationale for implementation
- • Antibiotic residues have been found in all foods intended for human consumption, whether of animal or non-animal origin, raising concerns about the seriousness of antibiotic residues in food and feed (Ghimpețeanu et al., 2022). In the early 2000s, incidents such as the BSE (Bovine Spongiform Encephalopathy) and dioxin crises brought food safety concerns to the forefront in Europe and highlighted the need to strengthen quality programs in livestock feed. As a result, safety management in feed factories, which had previously been operated under GMP standards, was integrated with HACCP to establish a comprehensive quality assurance program that encompasses all feed ingredient suppliers (Johan, 2003). In Korea, the HACCP certification system for assorted feed factories was implemented in 2005 (Choe, 2011), and it is also applied to feed used in honeybee farms.
- • When groundwater is used for making self-produced feed or cleaning equipment in honeybee farms, testing must be conducted every three years for 20 parameters, including pH, coliform counts, and heavy metals, to meet potable water standards.
- • Kang et al. (2010) reported that monitoring results for veterinary drug residues in honey indicated levels below the allowable limit of 0.1 mg/kg. Additionally, recent monitoring by the MFDS on imported honey in 2024 (Food Safety Korea, accessed on October 21, 2024) reported no non-compliance cases related to veterinary drugs. Currently, Korea has established MRL standards for 10 veterinary drugs in honey, including neomycin, streptomycin, and others, and since 2024, the Positive List System (PLS) has been applied to all livestock products. Under this system, a uniform limit of 0.01 ppm is applied to veterinary drugs without specific residue limits. In honeybee farms, the external parasite Varroa destructor has been identified as a major cause of honeybee mortality (Schäfer et al., 2010; Van Dooremalen et al., 2012), and thus, standards for the use of veterinary drugs should be established and implemented for regular pest control.
- • In CBISQT, the purpose of GPP 2 is to provide guidelines to prevent or reduce the risk of contamination of raw honey due to incorrect materials and/or input conditions. This involves blocking potential sources of biological and chemical hazards with a thorough visual inspection of all incoming items and verification of approved veterinary drugs. GPP 4 addresses the risk of contamination of honey, sucrose, high-fructose corn syrup (HFCS), and/or pollen due to unsanitary conditions (e.g., contaminated containers or tools) and contaminated drums, top feeders, and feed containers, which may lead to pathogenic bacterial contamination (e.g., spores of Clostridium spp. and Bacillus spp.). It emphasizes mixing only under hygienic conditions with clean containers and tools and stipulates that approved/registered veterinary drugs and pest control products (e.g., for Varroa spp.) should be used in appropriate doses according to label instructions before and after the season as part of the production cycle. GPP 10 addresses good management practices for potential hazards that may arise when receiving, treating, and distributing potable water in farm operations. While water is not directly added during the processing of raw honey on the farm, potable water is indirectly used for cleaning and sanitizing bulk honey containers, processing tools, equipment, and surfaces in extraction, processing/packing, and storage areas. Untreated or improperly treated water may contain pathogenic microorganisms such as bacteria (e.g., C. botulinum, Bacillus cereus, Escherichia coli), viruses (e.g., Norovirus), and protozoa (e.g., Cryptosporidium parvum), which require careful management.
- • According to 6.4. Feeding in B-QUAL, feed should be purchased only from quality-assured suppliers that operate a biosecurity program, and bee-derived products that have not undergone irradiation should be used. Appropriate measures for disease management should be taken, and treatment details must be recorded. Additionally, in accordance with 2. Chemical Standards section, the purchase of all antibiotics and other chemicals should be documented, and chemical treatment procedures, including records of recommended antibiotics and dosages, must be followed.
(1) Evaluation criteria
- • When introducing livestock, the inspection results for livestock diseases and the usage history of veterinary drugs must be verified, and clinical symptoms should be observed for a designated period of time.
- • Management standards for outgoing livestock (e.g., pre-shipment requirements, compliance at shipment, and handling of non-compliant cases) should be established and followed.
- • After shipment, facilities used for outgoing livestock should be cleaned and disinfected.
- • Traceability management for livestock products must be implemented.
(2) References
- • (Domestic Laws) LPSCA, Article 12-2; LIA, Article 35; ER of LIA, Article 30
- • (CBISQT) GPP 8. Finished Honey; CPP 12. Record control, Traceability and product recall
- • (B-Qual) 5.1.7 Labeling; 6.2 Introduction
(3) Rationale for implementation
- • Before introducing honeybee colonies, their health status should be inspected by a veterinarian or apiculture expert, and the colonies should be quarantined for a certain period to monitor for infectious diseases. Also, it is necessary to review diagnostic reports from the Animal and Plant Quarantine Agency or provincial laboratories, as well as verify the origin of the colonies. When purchasing individual queen bees, only new queen cages and related beekeeping equipment should be used for shipping. The queens should be introduced after evaluating their quality, including effective egg-laying capacity, colony size, brood formation rate, reproductive potential, and productivity of various products such as honey and royal jelly. Additionally, before honey products are released, standards for release (e.g., maturation period, food specifications, residue levels) should be established and adhered to. In cases where products do not meet the release standards, methods such as traceability management are required to determine the cause. Honey is graded in accordance with LIA Article 35 (Grading of Livestock Products), and information such as the producer, nectar source, harvesting region, and harvesting period is tracked with traceability management.
- • According to the GPP 8 section in CBISQT, honey must be handled and transported only under safe conditions to prevent contamination by pathogenic bacteria (e.g., spores of Clostridium spp. and Bacillus spp., as well as E. coli and Salmonella spp.). In the GPP 12 section, all relevant records established by the CBISQT program must be maintained and managed for at least 8 years to guarantee safe honey products, facilitate traceability, and enable product recalls if necessary.
- • In B-QUAL 6.2. Introduction, queen bees and hives must be purchased only from B-QUAL-certified suppliers in Australia, and the health status should be evaluated both prior to purchase and upon arrival. Additionally, Australian Quarantine and Inspection Service (AQIS) entry and exit requirements must be followed for imported queen bees. All outgoing products are clearly identified and documented for ownership traceability. According to 5.1.7. Labeling, labels must be affixed according to legal requirements, and appropriate records must be maintained.
2. Application of HACCP standards to honeybee farms
To enhance safety management in domestic honeybee farms with the application of HACCP standards, this study investigated certification systems for hygiene and quality management in overseas honeybee farms, specifically B-QUAL in Australia and CBISQT in Canada. Additionally, the operational standards of each certification system and the basis in domestic law were compared and analyzed according to the HACCP evaluation items for domestic farms.
As shown in Table 3, in the Farm Management section, it was determined that entry barriers and perimeter fencing at the farm entrance are challenging to implement for migratory honeybee farms but could be applicable for stationary honeybee farms and during the winter season, suggesting a need to improve the evaluation criteria. Additionally, the facility and operational standards for livestock manure treatment facilities among the farm HACCP evaluation items were deemed unnecessary for honeybee farms and were classified as “Not applicable.” The installation of informational and biosecurity warning signs at the farm entrance, recording entry details of farm visitors, installing lighting and ventilation in honey processing facilities, and installing disinfection facilities at the farm entrance are not only mandatory under domestic regulations but also required by certification standards in Australia and Canada. Therefore, these evaluation items were classified as “Applicable.”
In the Sanitation Management section, while farm HACCP requires testing for Salmonella, honeybee farms would benefit from testing for bacteria such as Clostridium spp. and Bacillus spp., suggesting a need to modify the evaluation items. Additionally, specific standards for hive management that are similar to those used in livestock facilities may be necessary. According to domestic regulations and international standards, evaluation items related to cleaning and disinfecting tools and equipment used on farms, regularly controlling pests and parasites, and conducting hygiene and biosecurity training for farm workers were classified as “Applicable” and should be maintained as they are.
In the Feeding Management section, the farm HACCP evaluation items require the use of HACCP-certified compound feed. However, since there are currently few HACCP-certified honeybee feed factories domestically, this evaluation item was classified as “Improvement” with a temporary deferment as a possible approach. Additionally, honeybee farms producing sugar-fed honey using HACCP-certified compound feed must strictly adhere to labeling standards to ensure consumers are not misled into believing it is ‘HACCP-certified honey’. Evaluation items such as using groundwater that meets water quality standards when cleaning facilities for packaging and concentration in apiaries, maintaining the cleanliness of watering facilities, establishing measures to prevent veterinary drug residues, and recording intake and discharge history were deemed applicable to honeybee farms and classified as “Applicable”.
In the Incoming and Outgoing Management section, evaluation items such as verifying veterinary drug usage records and observing clinical symptoms for incoming honeybee colonies, establishing and operating shipment standards, and managing the traceability of honey were classified as “Applicable” since they align with both domestic laws and international standards.
CONCLUSION
This study evaluated the applicability of international hygiene and quality management systems to domestic honeybee farms and provided essential guidance and recommendations for implementing HACCP standards in farm operations.
However, to apply HACCP to domestic beekeeping farms, several improvements are necessary. Honey production in Korea is primarily concentrated during the blooming season of black locust (Robinia pseudoacacia), leading to the widespread practice of migratory beekeeping, where honeybee colonies are moved from southern to northern regions (Kim, 2012). However, during migratory beekeeping, approximately 90% of nectar sources are located on privately owned land, and more than half of these cases involve unauthorized entry and honey harvesting without prior agreements (Kang et al., 2017). This situation results in issues such as residual pesticide contamination in nectar sources and insufficient biosecurity facilities. To address these challenges, the mandatory implementation of the Livestock Vehicle Registration System, as stipulated by APCAD (MAFRA, 2024), should be extended to migratory beekeeping operations. Currently, livestock transport and feed vehicles are required to have GPS installed, with operational status recorded in farm visitor logbook. This system should be adapted for migratory beekeeping farms by introducing GPS-based beekeeping logs to systematically monitor and manage the movement of honeybee colonies. RFID-based access control systems should also be installed at key nectar sites to automatically record beekeepers’ access information on a web server and manage it in a database. This would enable immediate verification of access records in cases of pesticide contamination or infectious disease outbreaks, facilitating the swift identification of contamination sources and transmission pathways.
In addition, the application of HACCP to honeybee farms should be implemented gradually based on the type of beekeeping practice. In Canada and Australia, safety management standards focus on applying HACCP to facilities that package honey, using GMP-based infrastructure. Considering these practical aspects, HACCP in Korea should also target beekeeping farms equipped with facilities for honey bottling, concentration, and refinement. In 2017, the pesticide-contaminated egg incident in Korea led to the establishment of the Edible-Egg Sorting and Packaging Business to regulate the distribution process of eggs (MFDS, 2018a). According to the revised legislation, edible eggs must be distributed through edible-egg sorting and packaging facilities where HACCP is mandatorily applied. Operators in this industry are required to comply with strict standards under LPSCA (MFDS, 2018b), including building and facility standards, equipment standards such as egg graders and crack detectors, and mandatory application of HACCP. However, the mandatory implementation of HACCP imposed socio-economic costs and increased the burden on livestock farms due to strengthened regulatory enforcement by the government (An, 2021). Considering such cases, to improve hygiene management levels in beekeeping industry, it is essential for the government to develop and distribute hygiene management manuals for honeybee farms. Furthermore, HACCP standards based on scientific evidence should be developed and gradually expanded for application to honeybee farms. This will contribute to enhancing food safety, improving productivity, and fostering consumer trust in bee products.
Acknowledgments
This research was supported by a grant from the National Institute of Agricultural Sciences, Rural Development Administration, Republic of Korea (Project No.: PJ016177).
References
- Ali, A. A. 2005. Honey, milk and antibiotics. Afr. J. Biotechnol. 4(13): 1580-1587.
- An, D. Y. 2021. Implementation and Alternatives for On-Farm Edible Egg Grading and Packaging business. Korean Poult. J. 53(8): 150-153.
- Animal and Plant Quarantine Agency. 2016. Guidelines for Cleaning and Disinfecting Livestock Farms.
- Australian Honeybee Industry Council (AHBIC). 2002. B-Qual Approved Supplier Program 2.2., B-QUAL Australia Pty. Ltd.
-
Berenyi, O., T. Bakonyi, I. Derakhshifar, H. Koglberger and N. Nowotny. 2006. Occurrence of six honeybee viruses in diseased Austrian apiaries. Appl. Environ. Microbiol. 72: 2414-2420.
[https://doi.org/10.1128/AEM.72.4.2414-2420.2006]

- Canadian Honey Council (CHC). 2014. Producer Manual - Good Production Practices. Version 1.0.
- Choe, C. G. 2011. FIRI Research Trends: Implementation of HACCP System in Feed Factories and Salmonella Detection Trends. Korea Feed Association. 50.
- Choi, Y. S., M. Y. Lee, I. P. Hong, N. S. Kim, H. K. Kim, K. H. Byeon, M. L. Lee and K. G. Lee. 2010. cDNA Structure Analysis of the Antimicrobial Peptides (Royalisin and Hymenoptaecin) Gene from the Asiatic Honeybee Apis cerana. J. Apic. 25(1): 17-19.
- Food Safety Korea. Accessed on: October 21, 2024. Available from: http://www.foodsafetykorea.go.kr, .
-
Ghimpețeanu, O. M., E. N. Pogurschi, D. C. Popa, N. Dragomir, T. Drăgotoiu, O. D. Mihai and C. D. Petcu. 2022. Antibiotic Use in Livestock and Residues in Food - A Public Health Threat: A Review. Foods 11: e1430.
[https://doi.org/10.3390/foods11101430]

-
Johan, D. H. 2003. Feed for Food: HACCP in the animal feed industry. Food Control 14: 95-99.
[https://doi.org/10.1016/S0956-7135(02)00111-1]

-
Jung, C. E. and M. L. Lee. 2018. Beekeeping in Korea: Past, present, and future challenges. Asian beekeeping in the 21st century. pp. 175-197.
[https://doi.org/10.1007/978-981-10-8222-1_8]

-
Jung, C. E. and Y. H. Bae. 2022. Production and characteristics of winter generation honeybees, Apis mellifera: Discussion with overwintering failure. J. Apic. 37(3): 265-274.
[https://doi.org/10.17519/apiculture.2022.09.37.3.265]

-
Kang, D. Y., A. Seol, J. C. Oh, K. Jung, H. Han and J. S. Chung. 2017. Analyzing the Management Characteristics of Beekeeping Households According to Their Beekeeping Types. J. Apic. 32(1): 1-9.
[https://doi.org/10.17519/apiculture.2017.04.32.1.1]

- Kang, E. G., Y. H. Jung, J. H. Jung, M. R. Kim, K. J. Lee, J. Jung, J. S. Park, K. N. Bahn, Y. M. Jang and C. S. Kang. 2010. Monitoring of Veterinary Medicine Residues in Honey. Korean J. Food Sci. Technol. 42(6): 643-647.
- Kim, S. H. 2012. Exploration and Prospects of Nectar Plants in Korea, The 28th Conference of the Apicultural Society of Korea and Symposium.
-
Klutse, C. K., D. A. Larbi, D. K. Adotey and Y. Serfor-Armah. 2021. Assessment of effect of post-harvest treatment on microbial quality of honey from parts of Ghana. Radiat. Phys. Chem. 182: e109368.
[https://doi.org/10.1016/j.radphyschem.2021.109368]

- Korea Agency of HACCP Accreditation and Services (KAHAS). 2017. HACCP Standard Manual.
-
Lee, H. J., J. J. Churey and R. W. Worobo. 2008. Antimicrobial activity of bacterial isolates from different floral sources of honey. Int. J. Food Microbiol. 126: 240-244.
[https://doi.org/10.1016/j.ijfoodmicro.2008.04.030]

- Mckee, B. 2003. Prevention of Residues in Honey: A Future Perspective. Apiacta 38: 173-177.
- Ministry of Agriculture, Food and Rural Affairs (MAFRA). 2020. Enforcement Decree of the Livestock Industry Act (Presidential Decree No. 30286, Dec. 31, 2019).
- Ministry of Agriculture, Food and Rural Affairs (MAFRA). 2024. Act on the Prevention of Contagious Animal Diseases (Act No. 19706, September 14, 2023).
- Ministry of Food and Drug Safety (MFDS). 2018a. Enforcement Decree of Livestock Products Sanitary Control Act (Presidential Decree No. 28835, Apr. 24, 2018).
- Ministry of Food and Drug Safety (MFDS). 2018b. Enforcement Rules of Livestock Products Sanitary Control Act (Prime Ministerial Decree No. 1456, Apr. 25, 2018).
- Ministry of Food and Drug Safety (MFDS). 2023. Food and Livestock Safety Management Certification Standards (Notification No. 2023-26, April 6, 2023).
- Ministry of Food and Drug Safety (MFDS). 2024. Livestock Products Sanitary Control Act (Act No. 20532, Oct. 22, 2024).
-
Rajic, A., L. A. Waddell, J. M. Sargeant, S. Read, J. Farber, M. J. Firth and A. Chambers. 2007. An Overview of Microbial Food Safety Programs in Beef, Pork, and Poultry from Farm to Processing in Canada. J. Food Prot. 70(5): 1286-1294.
[https://doi.org/10.4315/0362-028X-70.5.1286]

- Rural Development Administration (RDA). 2022. (March 13, 2022). Results of a joint public-private investigation on winter honeybee losses across beekeeping farms nationwide [Press release].
-
Schäfer, M. O., W. Ritter, J. S. Pettis and P. Neumann. 2010. Winter Losses of Honeybee Colonies (Hymenoptera: Apidae): The Role of Infestations with Aethina tumida (Coleoptera: Nitidulidae) and Varroa destructor (Parasitiformes: Varroidae). J. Econ. Entomol. 103: 10-16.
[https://doi.org/10.1603/EC09233]

-
Sultanbawa, Y., D. Cozzolino, S. Fuller, A. Cusack, M. Currie and H. Smyth. 2015. Infrared spectroscopy as a rapid tool to detect methylglyoxal and antibacterial activity in Australian honeys. Food Chem. 172: 207-212.
[https://doi.org/10.1016/j.foodchem.2014.09.067]

-
Van Dooremalen, C., L. Gerritsen, B. Cornelissen, J. J. van der Steen, F. van Langevelde and T. Blacquiere. 2012. Winter survival of individual honeybees and honeybee colonies depends on level of Varroa destructor infestation. PLoS One 7: e36285.
[https://doi.org/10.1371/journal.pone.0036285]

-
vanEngelsdorp, D., J. D. Evans, C. Saegerman, C. Mullin, E. Haubruge, B. K. Nguyen, M. Frazier, J. Frazier, D. Cox-Foster, Y. Chen, R. Underwood, D. R. Tarpy and J. S. Pettis. 2009. Colony collapse disorder: A descriptive study. PLoS One 4: e6481.
[https://doi.org/10.1371/journal.pone.0006481]

-
Weston, R. J., L. K. Brocklebank and Y. R. Lu. 2000. Identification and quantitative levels of antibacterial components of some New Zealand honeys. Food Chem. 70: 427-435.
[https://doi.org/10.1016/S0308-8146(00)00127-8]