
Protective Effect of Korean Native Bee-honey against Influenza A virus in Raw 264.7 Cells
Abstract
Influenza A virus (IAV) remains a major public health concern due to its high infectivity, potential for rapid mutation, and significant morbidity and mortality rates. Developing alternative and natural therapeutic agents has become critical in combating this virus, especially given rising antiviral resistance. Honey, particularly from Korean Native bees, is known for its medicinal properties, including its antioxidant, anti-inflammatory, and immune-modulatory effects. This study investigates the protective effects of Korean Native bee-honey on IAV-infected Raw 264.7 murine macrophage cells, focusing on viral replication, and modulation of immune responses. Our findings indicate that treatment with Korean Native bee-honey enhances the survival of infected Raw 264.7 cells, reduces viral replication, and promotes immune responses via upregulation of innate immune cytokines. These results suggest that Korean Native bee-honey could be a valuable natural intervention for managing influenza infections, warranting further exploration for its therapeutic potential in antiviral defense.
Keywords:
Influenza A virus, Korean native bee-honey, Immune responses, Interferon-βINTRODUCTION
Influenza A virus (IAV) is a highly contagious pathogen responsible for seasonal epidemics and occasional pandemics, posing a persistent threat to global health. Its ability to undergo rapid genetic shifts and drifts allows it to evade host immune responses and develop resistance to antiviral drugs (Ramos and Fernandez-Sesma, 2015; Hussain et al., 2017; Attaway et al., 2021). Despite the availability of vaccines and antiviral treatments, these options have limitations, including strain mismatches and adverse side effects, which emphasize the need for novel preventive and therapeutic strategies. Natural products, particularly honey, have garnered interest for their potential antiviral properties due to their rich content of bioactive compounds, including phenolics, flavonoids, and enzymes that exhibit antioxidative and immunomodulatory effects.
The innate immune response is the body’s initial defense against viral infections, relying on pattern recognition receptors (PRRs) like Toll-like receptors and RIG-Ilike receptors to detect viral components and trigger cytokine and interferon production. Interferons, particularly IFN-β, play a central role by activating interferon-stimulated genes (ISGs) in neighboring cells, creating an antiviral environment that curtails viral replication and mobilizes adaptive immunity (Ohman et al., 2009; Downey et al., 2017; Malik and Zhou, 2020). However, viruses have evolved mechanisms to evade these responses by inhibiting PRR signaling, blocking IFN production, or disrupting ISG function, which enhances their survival (Bonjardim et al., 2009; Ivashkiv and Donilin, 2014; Stanifer et al., 2019). Studying these interactions between host defenses and viral evasion tactics informs antiviral therapy development by highlighting potential intervention targets.
Bee-honey is a naturally sweet substance created by bees from flower nectar and has been valued for centuries in cooking and medicine. It primarily consists of carbohydrates, especially glucose and fructose, providing a rapid energy source. Additionally, honey contains small amounts of vitamins and minerals like vitamin C, calcium, and iron, along with flavonoids and phenolic compounds (Kamal et al., 2019; Chirsanova et al., 2021). It may also include antioxidants that protect cells from free radical damage, as well as natural antibacterial and anti-inflammatory properties that aid in healing (Arawwawala and Hewageegana, 2017).
This study aims to explore the protective effects of Korean Native bee-honey on IAV-infected Raw 264.7 cells. We hypothesize that Korean Native bee-honey will inhibit viral infection by modulating immune responses. Our findings will contribute to understanding the therapeutic potential of Korean Native bee-honey as a natural remedy for influenza, supporting its use in antiviral applications.
MATERIALS AND METHODS
1. Cells and virus
The Raw 264.7 mouse macrophage cell line, obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA), was maintained in DMEM containing 10% fetal bovine serum (FBS, Gibco, Grand Island, NY, USA) and 1% penicillin-streptomycin. The cells were cultured at 37℃ with 5% CO2. Influenza virus strain A/PR/8/34 (H1N1), engineered to express green fluorescent protein (GFP), was utilized in the study (Kwon et al., 2023).
2. Korean native bee-honey
Samples of Korean native bee-honey, harvested in 2021-2024 from various locations across Korea, were supplied by the Korea Beekeeping Cooperative and the Rural Development Administration (Table 1).
3. Antiviral assay
Raw 264.7 cells were plated in 24-well plates at a density of 1×105 cells/well and incubated for 24 h in a CO2 incubator. In the pretreatment assay, cells were treated with different samples of 5 mg/mL Korean native bee-honey (1-48) for 24 h before being infected with IAV at a multiplicity of infection (MOI) of 10 for 2 h. Mouse interferon-beta (IFN-β, 1000 units) served as a positive control. Following treatment, GFP expression of the virus was visualized using fluorescence microscopy (Nikon, Tokyo, Japan), and fluorescence intensity was quantitatively analyzed by flow cytometry (CytoFLEX; Beckman Coulter Inc., Pasadena, CA, USA).
4. Detection of IFN-β
The IFN-β assay was conducted following the manufacturer’s protocol. In summary, Raw 264.7 cells were incubated with Korean native bee-honey at concentrations of 1.25, 2.5, and 5 mg/mL for 24 h, after which the supernatants were harvested. The supernatants were transferred to antibody-coated plates and incubated at room temperature for 2 h as per the manufacturer’s instructions, and the absorbance at 450 nm was measured using an Epoch Microplate Reader.
5. Statistical analysis
The results are presented as the mean±SEM. Statistical significance between treatment and control groups was assessed using the chi-squared test with Bonferroni’s correction. Differences among three or more groups were analyzed by one-way ANOVA followed by Tukey’s post hoc test.
RESULTS AND DISCUSSION
1. Antiviral effect of Korean native bee-honey against IAV
To evaluate the anti-IAV effects of Korean native bee-honey under pretreatment conditions, we used GFP-expressing viruses as previously described. Raw 264.7 cells were infected with IAV-GFP for 2 h after a 24-h exposure to 5 mg/mL Korean native bee-honey (1-48). As a result, it was confirmed that some types of Korean traditional honey suppressed influenza virus infection. The selection criterion was a reduction in virus-GFP expression by more than 50% compared to the virus infection group. Accordingly, Korean native bee-honeys numbered 6, 7, 13, 16, 25, 28, 29, and 35 were found to suppress influenza virus infection when used as a pretreatment (Fig. 1).
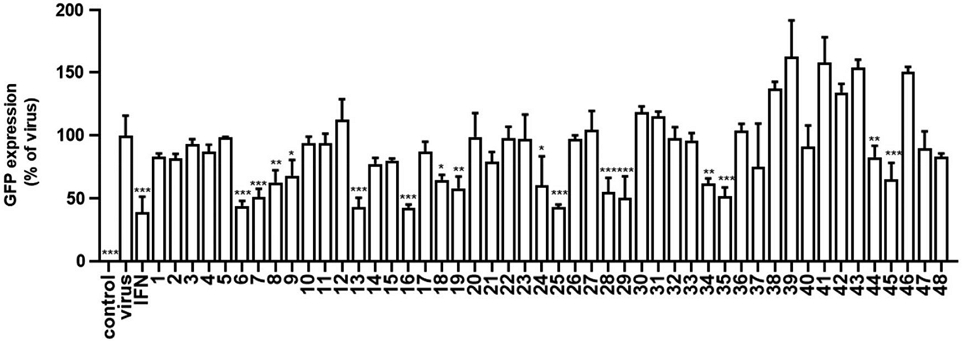
Antiviral effect of Korean native bee-honey (1-48) against IAV. The cells were pre-treated with Korean native bee-honeys (1-47) at 5 mg/mL be-fore infection with IAV-GFP. The experiment was performed three times independently. Bar graph (mean±SEM) statistics were determined using a one-way ANOVA with Tukey’s post hoc test, ***P<0.001, **P<0.01, and *P<0.05 compared with the virus infection group.
In additional, we treated Raw 264.7 cells with various concentrations of selected Korean native bee-honey and infected them with viruses to assess viral inhibition. As a result, we observed that Korean native bee-honey was most effective at a high concentration of 5 mg/mL (Fig. 2). This data indicated the antiviral efficacy of Korean native bee-honey by region, showing that the antiviral effects varied depending on the region.
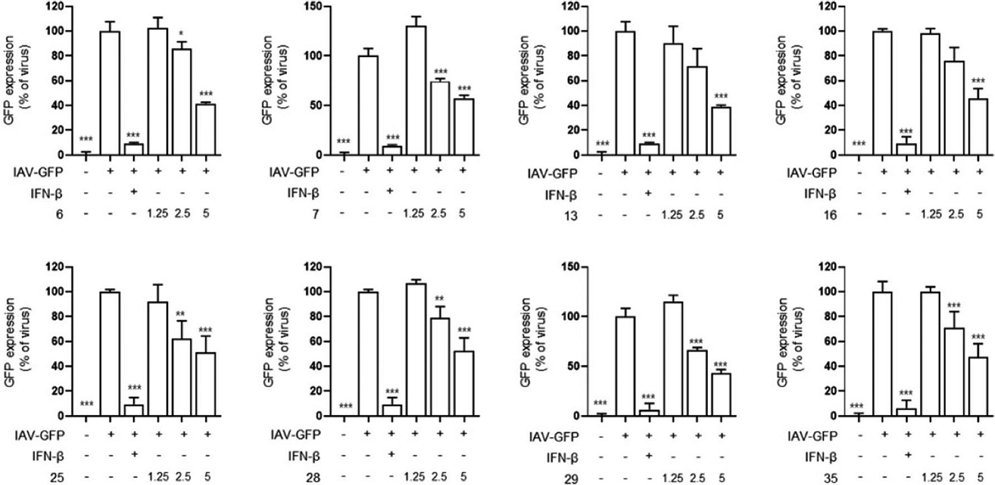
Protective effect of Korean native bee-honey against IAV. The cells were pre-treated with Korean native bee-honey at concentrations of 1.25, 2.5, and 5 mg/mL be-fore infection with IAV-GFP. The experiment was performed three times independently. Bar graph (mean±SEM) statistics were determined using a one-way ANOVA with Tukey’s post hoc test, ***P<0.001, **P<0.01, and *P<0.05 compared with the virus infection group.
We suggest that variations in the antiviral potency of native honey may result from regional differences in the types and availability of floral nectar, warranting further investigation through additional experiments.
2. Korean native bee-honey increases the secretion of IFN-β
We measured the secretion of IFN-β to determine whether the inhibitory effect of the eight selected Korean native bee-honeys on influenza virus infection was related to the increase in the secretion of IFN-β, an innate immune factor. Raw 264.7 cells were treated with various concentrations of Korean native bee-honey and secreted IFN-β was measured using a detection kit. As shown Fig. 3, eight selected Korean native bee-honeys were found to increase the secretion of interferon beta at 2.5 and 5 mg/mL. Therefore, we propose that Korean native bee-honey inhibits viral infection by increasing the secretion of interferon, an innate immune factor.
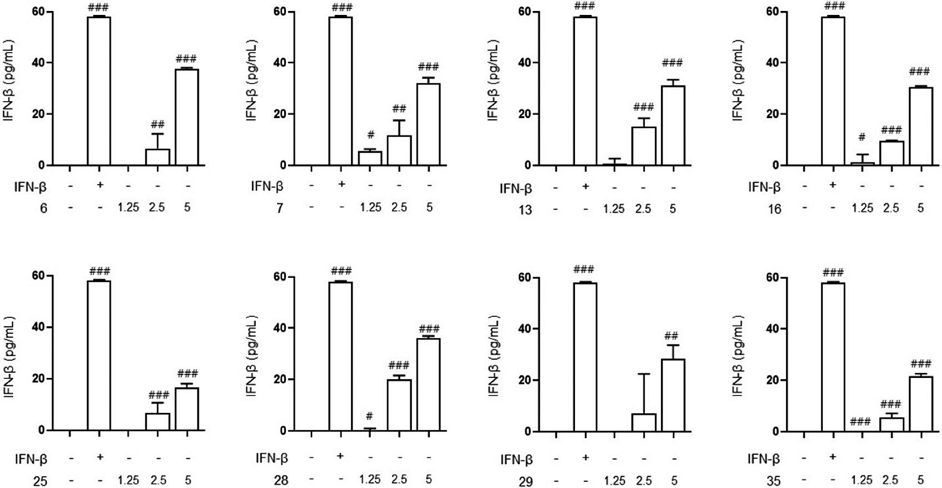
Korean native bee-honey increases the secretion of IFN-β. The Raw 264.7 were treated with Korean native bee-honey at concentrations of 1.25, 2.5, and 5 mg/mL for 24 h. The IFN-β secretion was measured using an ELISA Kit. The experiment was performed three times independently. Bar graph (mean±SEM) statistics were determined using a one-way ANOVA with Tukey’s post hoc test, ###P<0.001, ##P<0.01, and #P<0.05 compared with the un-treatment group.
Given the findings that native honey enhances interferon, a key innate immune factor, we propose that future studies should explore its effects on a broader range of innate immune factors, including interferon, and investigate the underlying mechanisms of action.
CONCLUSION
Our study demonstrates that Korean native bee-honey exhibits antiviral effects against influenza A virus (IAV) when used as a pretreatment in Raw 264.7 cells. Furthermore, the research suggests that the antiviral activity of Korean native bee-honey is likely mediated through an innate immune response, as evidenced by the increased secretion of interferon-beta (IFN-β). This enhanced IFN-β secretion indicates that Korean native bee-honey activates immune pathways that contribute to viral inhibition, positioning it as a potential natural antiviral agent.
Acknowledgments
This work was carried out with the support of the “Cooperative Research Program for Agriculture Science & Technology Development (NO. RS-2023-00228817).”
References
-
Arawwawala, M. and S. Hewageegana. 2017. Health benefits and traditional uses of honey: A review. J. Apith. 2(1): 9-14.
[https://doi.org/10.5455/ja.20170208043727]

-
Attaway, A. H., R. G. Scheraga, A. Bhimraj, M. Biehl and U. Hatipoğlu. 2021. Severe covid-19 pneumonia: pathogenesis and clinical management. BMJ. 372.
[https://doi.org/10.1136/bmj.n436]

-
Bonjardim, C. A., P. C. Ferreira and E. G. Kroon. 2009. Interferons: signaling, antiviral and viral evasion. Immunol. Lett. 122(1): 1-11.
[https://doi.org/10.1016/j.imlet.2008.11.002]

-
Chirsanova, C. A., T. Capcanari, A. Boiştean and E. M. I. Khanchel. 2021. Bee honey: History, characteristics, properties, benefits and adulteration in the beekeeping sector. J. Soc. Sci. 4(3): 98-114.
[https://doi.org/10.52326/jss.utm.2021.4(3).11]

-
Downey, J., E. Pernet, F. Coulombe, B. Allard, I. Meunier, J. Jaworska, S. Qureshi, D. C Vinh, J. G Martin, P. Joubert and M. Divangahi. 2017. RIPK3 interacts with MAVS to regulate type I IFN-mediated immunity to Influenza A virus infection. PLoS Pathog. 13(4): e1006326.
[https://doi.org/10.1371/journal.ppat.1006326]

-
Hussain, M., H. D. Galvin, T. Y. Haw, A. N. Nutsford and M. Husain. 2017. Drug resistance in influenza A virus: the epidemiology and management. Infect. Drug Resist. 10: 121-134.
[https://doi.org/10.2147/IDR.S105473]

-
Ivashkiv, L. B. and L. T. Donlin. 2014. Regulation of type I interferon responses. Nat. Rev. Immunol. 14(1): 36-49.
[https://doi.org/10.1038/nri3581]

-
Kamal, M. M., M. H. U. Rashid, S. C. Mondal, H. F. El Taj and C. Jung. 2019. Physicochemical and microbiological characteristics of honey obtained through sugar feeding of bees. J. Food Sci. Technol. 56: 2267-2277.
[https://doi.org/10.1007/s13197-019-03714-9]

-
Kwon, E. B., S. G. Kim, Y. S. Kim, B. Kim, S. M. Han, H. J. Lee, H. M. Choi and J. G. Choi. 2023. Castanea crenata honey reduces influenza infection by activating the innate immune response. Front. Immunol. 14: 1157506.
[https://doi.org/10.3389/fimmu.2023.1157506]

-
Malik, G. and Y. Zhou. 2020. Innate immune sensing of influenza A virus. Viruses 12(7): 755.
[https://doi.org/10.3390/v12070755]

-
Ohman, T., J. Rintahaka, N. Kalkkinen, S. Matikainen and T. A. Nyman. 2009. Actin and RIG-I/MAVS signaling components translocate to mitochondria upon influenza A virus infection of human primary macrophages. J. Immun. 182(9): 5682-5692.
[https://doi.org/10.4049/jimmunol.0803093]

-
Ramos, I. and A. Fernandez-Sesma. 2015. Modulating the innate immune response to influenza A virus: potential therapeutic use of anti-inflammatory drugs. Front. Immunol. 6: 361.
[https://doi.org/10.3389/fimmu.2015.00361]

-
Stanifer, M. L., K. Pervolaraki and S. Boulant. 2019. Differential regulation of type I and type III interferon signaling. Int. J. Mol. Sci. 20(6): 1445.
[https://doi.org/10.3390/ijms20061445]

