SEM Observations of Korean Haloptype Varroa destructor (Acari: Varroidae) Collected from Apis mellifera Colonies
Abstract
Scanning electron microscopic observations on the Korean haplotype of Varroa destructor collected from Apis mellifera was indentifed by mtDNA analysis to describe external morphology K-type V. destructor. SEM micrographs showed that the K-type V. destructor is more oval than Japan/Thai-Vietnam type and has three kinds of setae viz, serrated, spiny, and smooth marginal setae. The marginal setae are somewhat flexible. The K-type V. destructor is larger than V. jacobsoni and V. rindereri. The peritreme of K-type V. destructor is smaller than V. jacobsoni and V. rindereri. No sternal pores were observed in the K-type V. destructor, which is present in V. jacobsoni and V. rindereri. The K-type V. destructor has a more metapodal setae than V. jacobsoni and V. rindereri.
Keywords:
SEM micrographs, K-type Varroa destructor, KoreaINTRODUCTION
The parasitic mite, Varroa destructorAnderson and Trueman (2000), is the most important mite in apiculture (De Jong, 1997) due to its destructive nature of honeybees (Rinderer et al., 2001). Two types of haplotypes V. destructor mites; the Korean haplotype Varroa destructor (K-type) from Korea and the Japanese haplotype (J-type) from Japan, cause brood damage to the European honey bee, Apis mellifera (Anderson and Trueman, 2000; Garrido et al., 2003; Solignac et al., 2005). The K-type Varroa destructor is more oval than the J-type (de Gunzman et al., 1997; Anderson and Trueman, 2000; Garrido et al., 2003).
The K-type V. destructor have successfully infested A. mellifera brood (Anderson and Trueman, 2000). The K-type V. destructor also shifted from A. cerana to A. mellifera near Vladivostok (north Korean Penninsula) from Ukraine during late 1950s (de Guzman et al., 1997; Oldroyd, 1999), whereas the J-type V. destructor first shifted from A. cerana to A. mellifera in Japan during the previous century after introduction of A. mellifera (Sakai and Okada, 1973) and then spread to A. mellifera in Thailand and Brazil (Anderson and Trueman, 2000) and then in North America (de Guzman et al., 1997). Varroa mites reproduce in the cells with developing honeybee larvae of eastern honeybee, Apis cerana and the European honeybee, Apis mellifera. They feed on the haemolymph of developing larvae and adult bees, resulting in the transmittion of viruses and bacteria (Rosenkranz et al., 2010). Infestation rates of the K-type V. destructor are high that were found in all major beekeeping countries except Australia (USDA, 1987; Bradbear, 1988; Matheson, 1995). This ectoparasite feeds on the hemolymph of adult bees and the larvae and is subjected to microbial invasion (De Jong and De Jong, 1983), bacteria and viruses (Koch and Ritter, 1989; Shimanuki et al., 1992), especially acute paralysis virus (Ball, 1985; Allen et al., 1986), causing reduced life (De Jong and De Jong, 1983) and collapse of the infested colonies within one to three years (Chen and Siede, 2007; Cox-Foster et al., 2007; Johnson et al., 2009). V. destructor is a highly destructive pest which is responsible for reduced honey and brood production (Delfinado-Baker, 1988).
Previous studies on V. destructor have concentrated on distribution (Munoz, et al., 2008), host-parasite relations (de Gunzman et al., 1997; Garrido et al., 2003; Piccirillo and De Jong, 2003), genetic variation (Anderson and Fuchs, 1998; de Guzman and Rinderer, 1999; Anderson, 2000; Anderson and Trueman, 2000; Solignac et al., 2005), mite infestation (de Guzman et al., 1997; Anderson and Fuchs, 1998), and hygienic behavior of bees against V. destructor (Harbo and Harris, 2005; Spivak and Reuter, 2001). Previous descriptions of V. destructor have been brief and most of the available information on V. destructor exists at genetic level (Anderson and Trueman, 2000; Solignac et al., 2005).
The body size, body setae, marginal setae, chelicerae, palps, peritreme, and legs have not yet been described for the K-type V. destructor. Although mtDNA can be used to identify the K-type V. destructor (Solignac et al. at 2005), a simple technical method is desirable. Identification of V. destructor by using the mtDNA analysis is far beyond the capabilities of extension agents and beekeepers. Our knowledge on V. destructor morphology and behavior is still too fragmentary to lead to a satisfying controlling method. Here, we describe the morphology of the K-type V. destructor using SEM in combination with mtDNA analysis and compare morphological features of K-type V. destructor with published studies to clearly understand the K-type V. destructor mites. SEM micrographs of the K-type V. destructor will be helpful for accurate identification required for eradication.
MATERIALS AND METHODS
Sampling
Adult K-type V. destructor mites were collected by opening worker sealed brood of A. mellifera at the Rural Development Administration (RDA), Suwon, Republic of Korea in November 2012 and October 2013, and the apiary of the College of Agriculture and Life Science (CALS), Seoul National University (SNU), Seoul November to March 2015. All specimens were preserved in 70% ethanol and studied in the Department of Agricultural Biology, National Academy of Agriculture Science (NAAS), RDA, and CALS, SNU, Republic of Korea.
Scanning Electron Microscopy
Fifteen adult female K-type V. destructor mites were fixed overnight at 4°C in Karnovsky’s fixative (25% glutaraldehyde and 8% paraformaldehyde in 0.2 M cacodylate buffer pH 7.2). Samples were then washed three times in distilled water and fixed in 1% of osmium tetroxide for 2 hours at 4°C All specimens were washed again in distilled water and dehydrated in a graded ethanol series (50, 75, 90, 95, and 100%) for 20 minutes. After dehydration, the samples were dried in a critical point dryer, mounted on SEM stubs, and coated with gold. The specimens were examined with a SEM (Hitachi S2460N, Japan) at 20 kV accelerating voltages.
Morphological measurements
Dorsal and ventral body lengths were measured from the anterior margin to the posterior end of the body. Dorsal and ventral body widths were measured in the middle of the body from one margin to the other. Length and width of sternal, genital, endopodal, metapodal, anal shields, and pedipals were measured. Peritreme length was measured from the distal to proximal ends of the tube. Chelicerae teeth were measured from the base to the tip. Gnathosoma length was measured from the tip of the palps to the basal margin of the subcapitulum. Gnathosoma width was measured at the widest section of the subcapitulum and the length of the legs was measured from their outer margin.
Amplification of mitochondrial COI gene
DNA of K-type V. destructor was extracted from the whole body of one mite by the standard proteinase K method (Kocher et al., 1989). A partial COI gene (472 base pairs) was amplified by PCR using primer CI-J-1751 ('5-GGAGCTCCTGACATAGCATTCCC-3') and CI-N-2191 ('5-CCCGGTAAAATTAAAATATAAACTTC-3') (Simon et al., 1994). PCR conditions were as follows: an initial denaturation at 94°C for 5 minutes, 40 cycles of 94°C for 30 seconds, 50°C for 40 seconds, and 72°C for 45 seconds, and a final extension at 72°C for 7 minutes. To confirm the successful DNA amplification, electrophoresis was carried out using 0.5 X TAE buffer in 1% of agarose gel. The PCR product was then purified using a PCR purification Kit (Qiagen, Germany). DNA sequencing was performed using an ABI 310 Genetic Analyzer (PE Applied Biosystems, USA). Each strand was sequenced twice for accuracy. The sequence alignment 472 nucleotides of the COI fragment was aligned with COI genes of three references V. destructor (GenBank accession number. GQ379060, GQ379059, and GQ379063) and V. jacobsoni (GenBank accession number GQ387679).
Data Analysis
The means of dorsal body setae lengths, hypostomal setae, C1, C2, and C3, lengths and anal setae, an1, an2 and an3, lengths were compared by covariance analysis (oneway ANOVA) to determine any significant differences in lengths. The mean of dorsal and ventral body lengths and widths, lengths of chelicerae teeth (t1 and t2), and apotele claws (ap.c1 and ap.c2) were compared using independent t-tests.
RESULTS
Body sizes
The dorsal shield of female K-type V. destructor is ellipsoid, orange-red (Fig. 1A), and the marginal area densely sclerioted. The dorsal shield is 1193.1±35.3μm long (n=18) and 1601.2±73.4μmwide (n=18), the ventral shield is 1233.6±9.0μm long (n=20) and 1233.7±9.2μm wide (n=18) (Fig. 1B). An independent t-test revealed that there were significant differences between the means of dorsal and ventral body lengths (t=4.966, df=36, P<0.0001) and widths (t=2.737, df=36, P<0.01).
Body setae
There are five types of body setae, four with different sizes on the dorsum and one the same size on the venter which project backwards (Fig. 2A). The dorso-anterior setae is short, 51.8±8.4μm long (range 36.7-71.6μm, n=45) (Fig. 2B), the dorso-posterior setae are 112.6± 8.0μmlong (n=10), i.e. longer than the dorso-anterior ones (Fig. 2C), but shorter than the lateral setae. The dorsolateral setae are 143.7±25.1μm long (range 103.1-194.6μm) (Fig. 2D). A one-way ANOVA test revealed that there are significant differences in dorsal seta sizes (ANOVA F(2,75) = 287.5, p=.000). The ventral body setae are 79.2±5.6μm long (range 71.7-87.3μm, n=17). All dorsal setae are serrate (Fig. 2E), whereas the ventral setae are entire, the same size, and barbate (Fig. 2F).
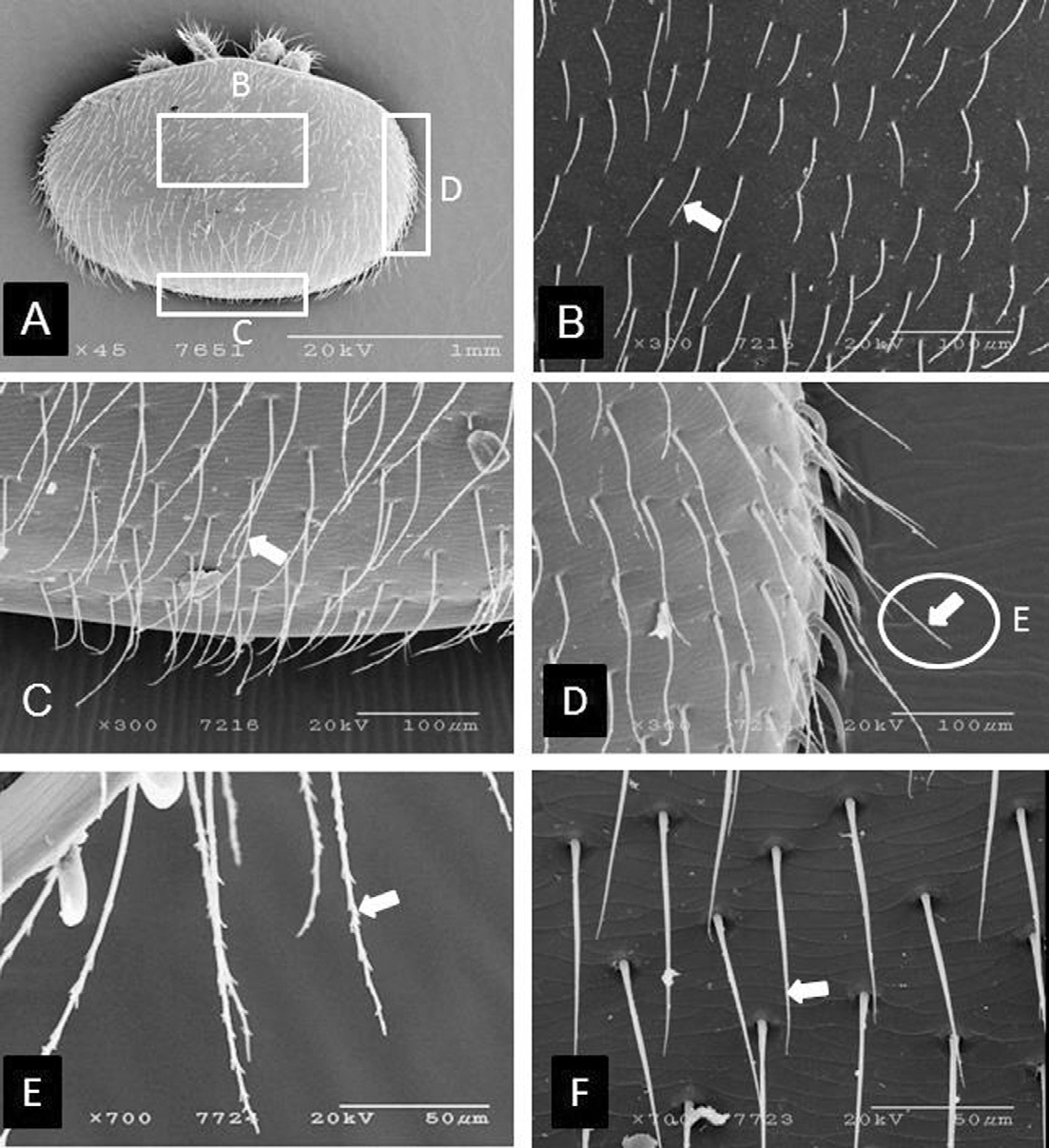
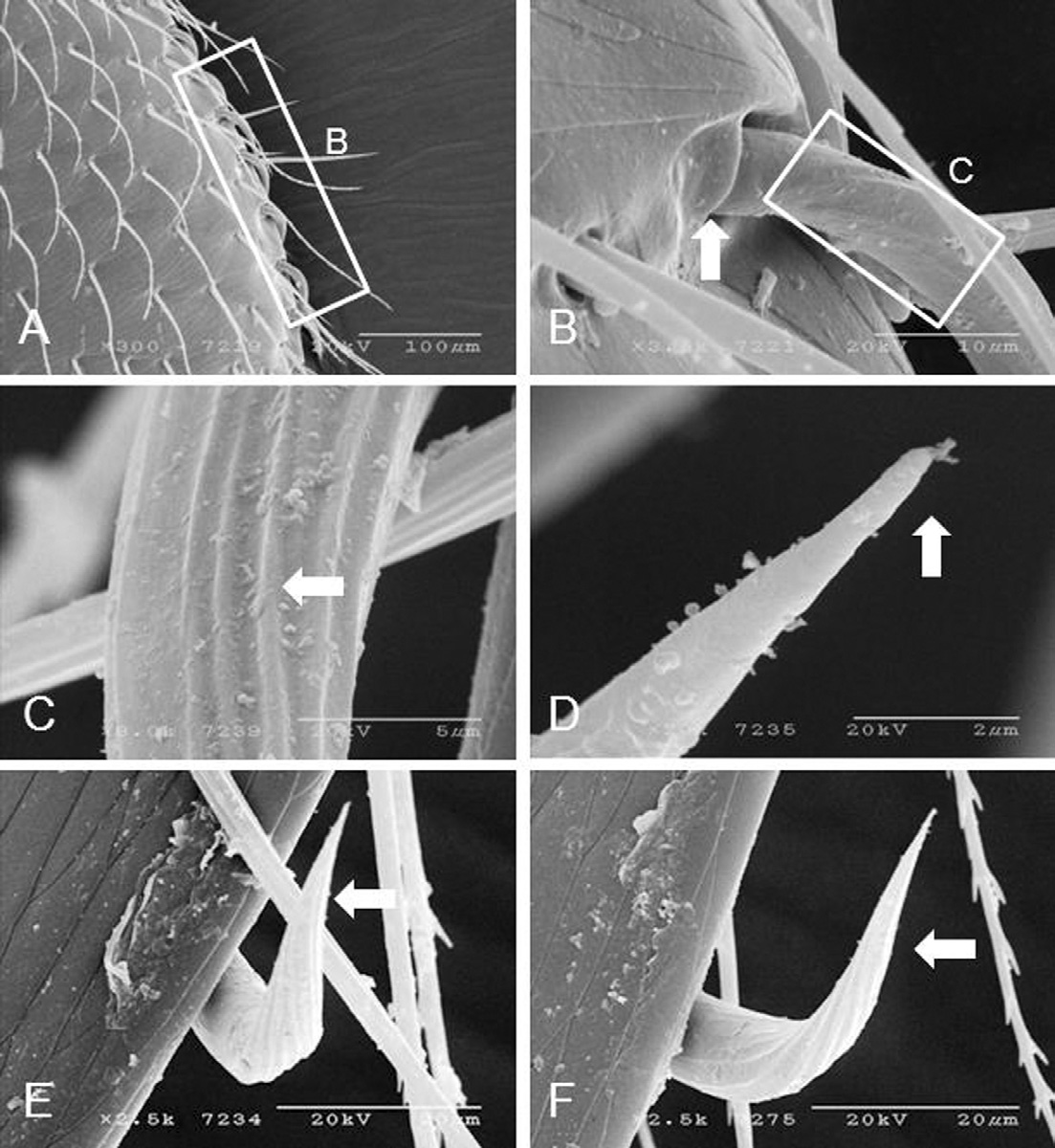
Scanning electron micrographs of different kinds of body setae of K-type Varroa destructor. (A) dorsal setae in different dorsal body parts, (B) higher magnification images of mid-dorsal setae, (C) higher magnification image of posterior setae, (D) lateral setae, (E) higher magnification image of serrated body setae, and (F) ventral setae (simple and barbate). Arrows indicate types of setae.
Marginal setae
Marginal setae on both sides of the body originate from the dorsal side of the body (Fig. 3A), which has an individual socket (Fig. 3B). The marginal setae is basally ribbed (Fig. 3C), and half way to the proximal end are smooth and pointed (Fig. 3D) and flexible (Fig. 3E and F). The number of marginal setae is 20.5±1.9 (range 17-24, n=36). The marginal setae is 36.9±2.7μm long (range 31.9-42.0μm, n=19) and 3.8±0.4μm wide (range 2.1-4.5μm, n=19). The distance between each marginal setum is 33.1±10.1μm(range 21.6-52.4μm, n=19).
Ventral shields
Ventral shields are light brown, surrounded by longitudinal muscle, and includes five shields (Fig. 4A). The sternal shield is 487.3±70.9μm long (range 430.1-578.5μm, n=6) and 189.7±7.8μm wide (range 179.4-200.1μm, n=6), densely sclerotized, lack a sternal pore and have 6 pairs of setae with an average length of 101.2± 15.2μm (n=23) (Fig. 4B). The genital shield is 672.5± 14.9μm long (range 657.6-687.5μm, n=8), and 747.6± 8.9μmwide (range 737.3-761.6μm, n=8). The anterior and posterior margins are almost rounded with 105.25±1.3 genital setae. The genital setae is 34.65±3.2μm long (range 29.7-40.6μm, n=13) (Fig. 4C). The endopodal shield is 210.01+0.0μm long, 21.0±0.1μm wide, adjacent to coxa-IV, and with 7 setae. The average length of the endopodal setae is 73.8±14.2μm long (Fig. 4D). The metapodal shield is 355.5±14.3μm long, 144.3±10.9μm wide, broadly triangular, not fused with the genital, endopodal, or anal shields; and with an average of 80.6± 6.12 metapodal setae. The metapodal setae is of two types, short and long. The marginal metapodal setae is longer (100.0±10.5μm) than middle metapodal setae (64.7± 6.1μm) (Fig. 4E). The anal shield is 153.8±8.5μm long (range 144.5-161.4μm, n=5), 259.1±16.9μm wide (range 239.2-279.9μm, n=5), separated from the genital plate and metapodal shield, centrally located, triangular, surrounded by three different sizes of anal setae: an1 (69.1±0.2μm long), an2 (54.4±2.4μm long) and an3 (45.6±7.0μm long). A one-way ANOVA analysis revealed that there is a significant difference in the length of these three kinds of anal setae (F =15.247, df=2, p=.027). A Turkey’s post hoc tests revealed that an3 is shorter (Sig.=.024) than an1 and an2 (Sig=.083). The para-anal setae (an1 and an2) are close to the anus, whereas the post-anal seta (an3) is posterior to the anus (Fig. 4F). The anal opening extends outside through the anal plate, which is bounded laterally by tiny hinged plates.
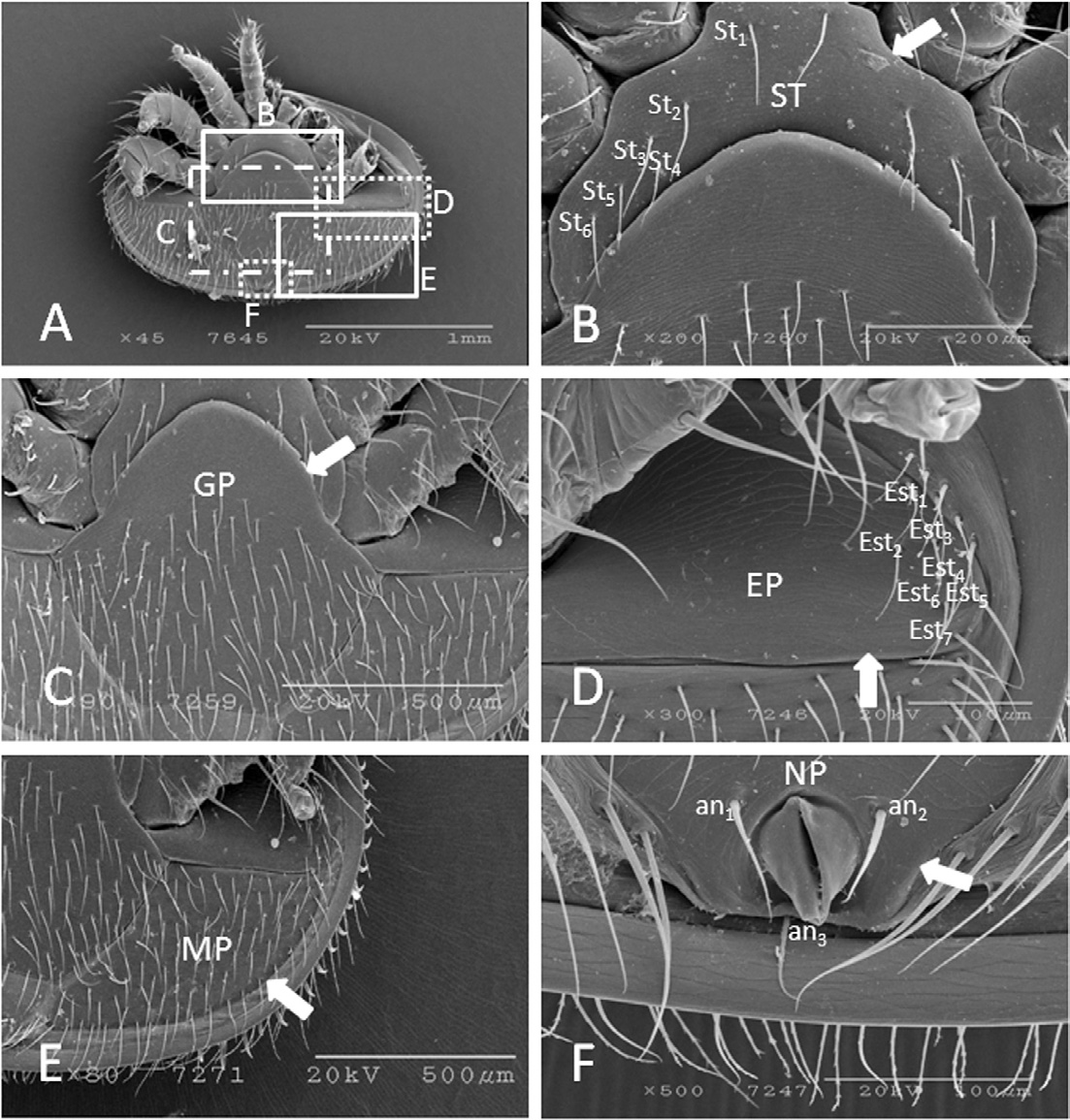
Scanning electron micrographs of ventral shields of K-type Varroa destructor. (A) ventral shields, (B) sternal shield enlarged, (C) genital shield, (D) endopodal shield, (E) metapodal shield, and (F) anal shield. Arrows indicate specific areas of the shields. SP=sternal plate, GP=genital plate, P=endopodal plate, MP=metapodal plate, NP=anal plate, St=sternal setae, Est=endopodal setae, an1-3=anal setae.
Stigma and peritreme
The peritreme is 195.7±8.9μm long (range 184.7-208.8μm, n=5), 7.4±1.6μmwide (range 5.6-9.4μm, n=5), inverted J-shape, covered by the peritremal shield, and extends up to leg-IV (Fig. 5A and B). The tip of the peritreme is bulbous (Fig. 5C). The stigmata 767.0±78.1μm2(range 698.8-852.2μm2, n=3) is adjacent to coxae-III and IV, and is surrounded by the stigmal shield (Fig. 5D).
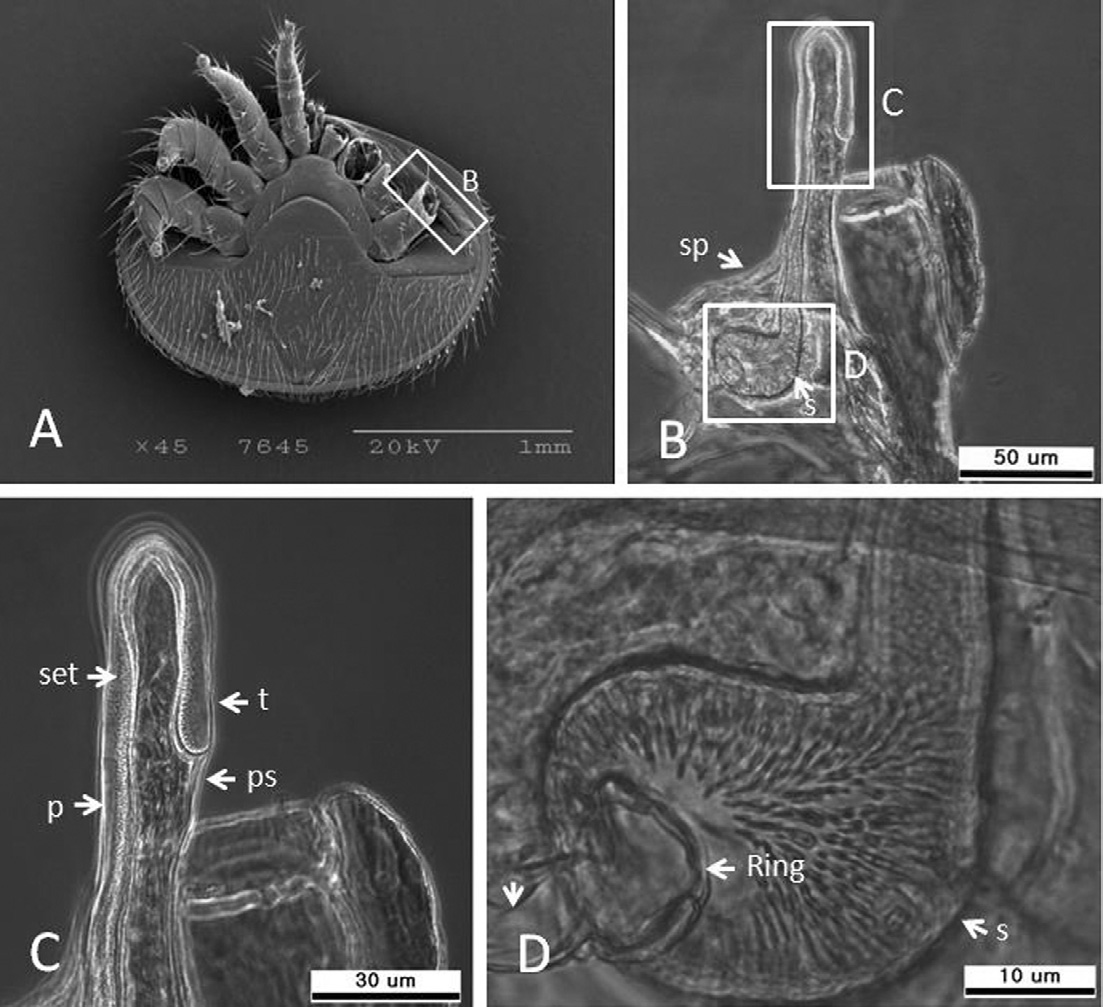
Peritreme and stigma of K-type Varroa destructor. (A) SEM image of the peritreme, (B) peritreme and stigma under the compound microscope (200X), (C) magnification of peritreme bulb (400X), and (D) higher magnification of the stigma (1000X). per=peritreme, ps=peritremal shield, stg=stigma, sp=stigma plate, set=setules. S=stigma.
Gnathosoma
The gnathosoma is 312.6±14.5μm long (range 297.1± 329.4μm, n=4), 138.3±5.1μm wide (range 134.2-145.8μm, n=4), located antero-ventrally between coxa-I, and semi-concealed under the dorsal shield. The hypostome has 3 pairs of hypostomal setae viz, C1, C2, and C3, which are smooth and spiniform (Figs. 6A and B). C1 and C3 are longer than C2, while C1 and C2 are close together, but C3 is separated. C3 is located near coxa-I. The length of C1 is 20.5±4.9μm, C2 13.1±2.2μmand C3 27.2 ±0.5μm. A one-way ANOVA analysis revealed that there are statistically significant differences in the lengths of these hypostomal setae (F(2,11)=20.606, p=.001). The tritosternum is 128.7±0.4μm long (range 128.4-129.1μm, n=4), well-developed, basally broad, with 6 pairs of barbs on the lateral side (Fig. 6C) and 11 barbs basally (Fig. 6D). The laciniae are barbed and bifid (Fig. 6E). The chelicerae are three-segmented and bear two teeth, t1 (mean=1.32± 0.1μm long, n=4) and t2 (mean=1.31±0.2μm long, n=4) (Fig. 6F). An independent t-test failed to reveal any significant differences between the sizes of t1 and t2 (t=.045, df=6, p=.965). The distance between t1 and t2 is 2.6±0.2μm, (range 2.45-2.98μm, n=4), and the tip of fixed digital is slightly curved and pointed.
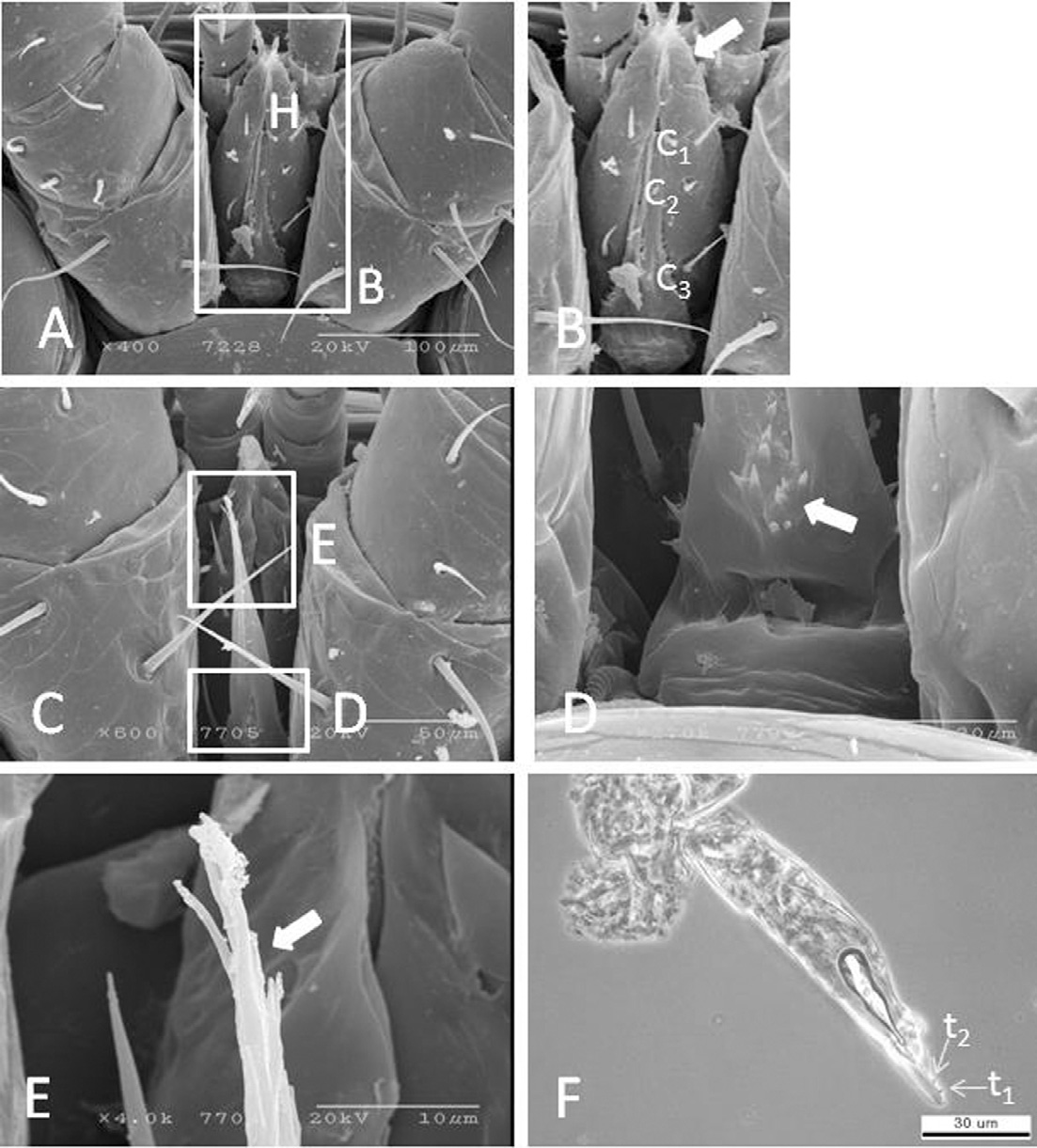
Scanning electron micrographs of gnathosoma of K-type Varroa destructor. (A) hypostome, (B) hypostomal setae, (C) tritosternum, (D) basal of tritosternum showing teeth, (E) tip of tritosternum under the compound microscope, and (F) cheliceral digit with teeth under the compound microscope (400X). H=hypostoma, h=hypostomal setae, T=tritosternum, C1-3=hypostomal setae.
Palptarsi
The pedipalps are 179.4±9.7μm long (range 167.3-192.2μm, n=5) and five-segmented (Fig. 7A). The pedipaltarsi have long and short apotele claws (ap.c1 and ap.c2). The ap.c1 is 37.6±3.1μm long and ap.c2 is 10.1±1.9μm. (Figs. 7B and C). An independent t-test revealed that there is a significant difference in the lengths of ap.c1 and ap.c2 (t=22.690, df=17, P=.001). The ap.c2 is inserted on the base of ap.c1. The pedipal-tarsi have 16 sensory setae (Fig. 7D). Pedipal setation: trochanter 1, femur 1, genu 1, tibia 5, and tarsi 16 setae. The palp-femural has only one seta 70.71μm long, which is longer than the gena seta (54.45μm).
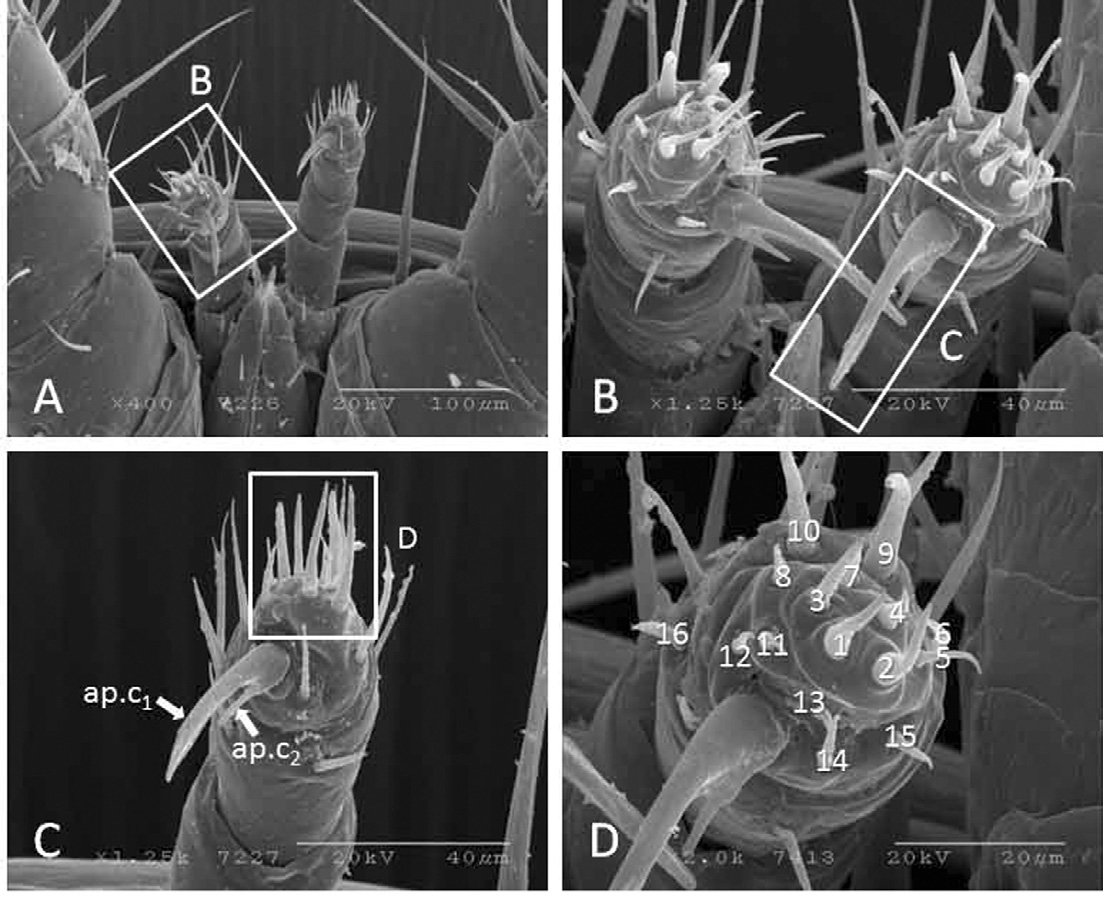
Scanning electron micrographs of palptarsi of K-type Varroa destructor. (A) pedipals, (B) palpal apotele claws, (C) higher magnification of claws, and (D) higher magnification of palpal-tarsus showing palpal setae arrangement. Note: Roman numbers indicate sensory setae on palptarsi. ap.c1=long claw, ap.c2=short claw.
Leg
The legs have varied lengths. Leg-I is 624.4μm long, somewhat rounded, and the condylophore is 78.94μmlong (Fig. 8A). Leg-II is 584.3μm long and dorso-ventrally flat with a short condylophore (Fig. 8B). Leg-III is 812.8μm long, completely flattened, with short tarsi and the condylophore is 100.68μm long (Fig. 8C). Leg-IV is 970.3μm long, completely flattened, and the condylophore is 86.07μm long (Fig. 8D). Leg setae are smooth, simple, and spiniform. The tarsi of leg-I-IV have ambulacral sucker-like structures which are used for walking and attachment. Leg setation: coxae 2-2-2-2, tronchanters 5-6-6-6, femur 6-9-8-6, genu 4-8-11-10, tibia 8-6-8-8, and tarsus 29-18-20-20.
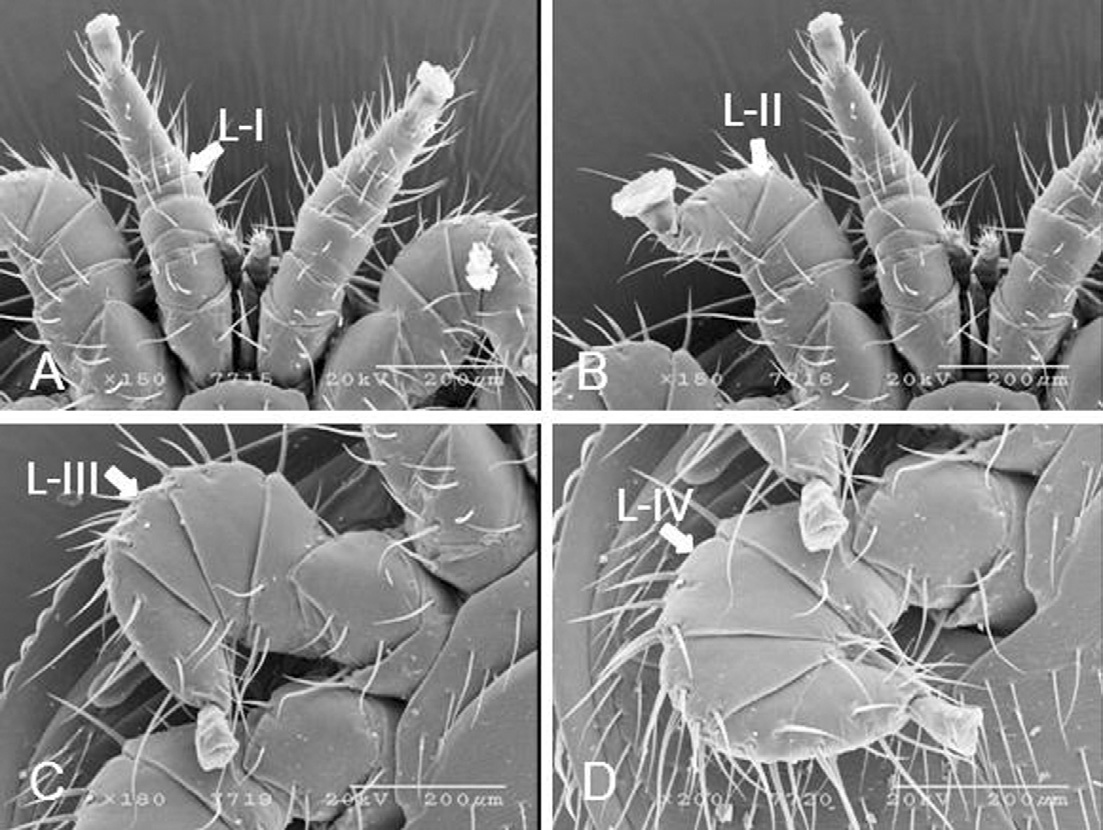
Scanning electron micrographs of legs of K-type Varroa destructor. Legs were categorized by length as Leg I, Leg II, Leg III, and Leg IV. (A) Leg-I is 624.4μm long and rounded. The condylophore is 78.94μm long. (B) Leg-II is 584.3μm long and dorso-ventrally flat with a short condylophore. (C) Leg-III is 812.8μm long, completely flattened, with short tarsi and the condylophore is 100.68μm long. (D) Leg-IV is 970.3μm long, completely flattened, and the condylophore is 86.07μm long.
COI gene sequence
The COI gene of mtDNA of K-type V. destructor is closely related to GQ379060 (100% nucleotide sequence identity), GQ379059 (99.59% nucleotide sequence identity), and GQ379063 (98.78% nucleotide sequence identity) GenBank V. destructor (Fig. 9).
DISCUSSION
Body size
Our results showed that K-type V. destructor is longer than Japan/Thai-Vietnam types including Luzon haplotype-1 and 2 in length, but smaller than Japan/Thai-Vietnam and New Zealand types in width (Table 1). This indicates that K-type V. destructor is oval rather than rounded (Japan/Thai-Vietnam types) (Table 1). The difference may be due to geographical variation. The K-type V. destructor is broader, but shorten than Japan/Thai Vietnam type of V. destructor (Anderson and Trueman, 2000) and the New Zealand type of V. destructor (Zhang, 2000) (Table 1) where the author reported that the V. destructor of New Zealand (Auckland) type is similar to the Japan/Thailand-Vietnam type. This indicates that the Japan and Korean haplotypes have different morphological features. Both haplotypes are distinguishable only by differences in mtDNA sequences (Anderson and Trueman, 2000).
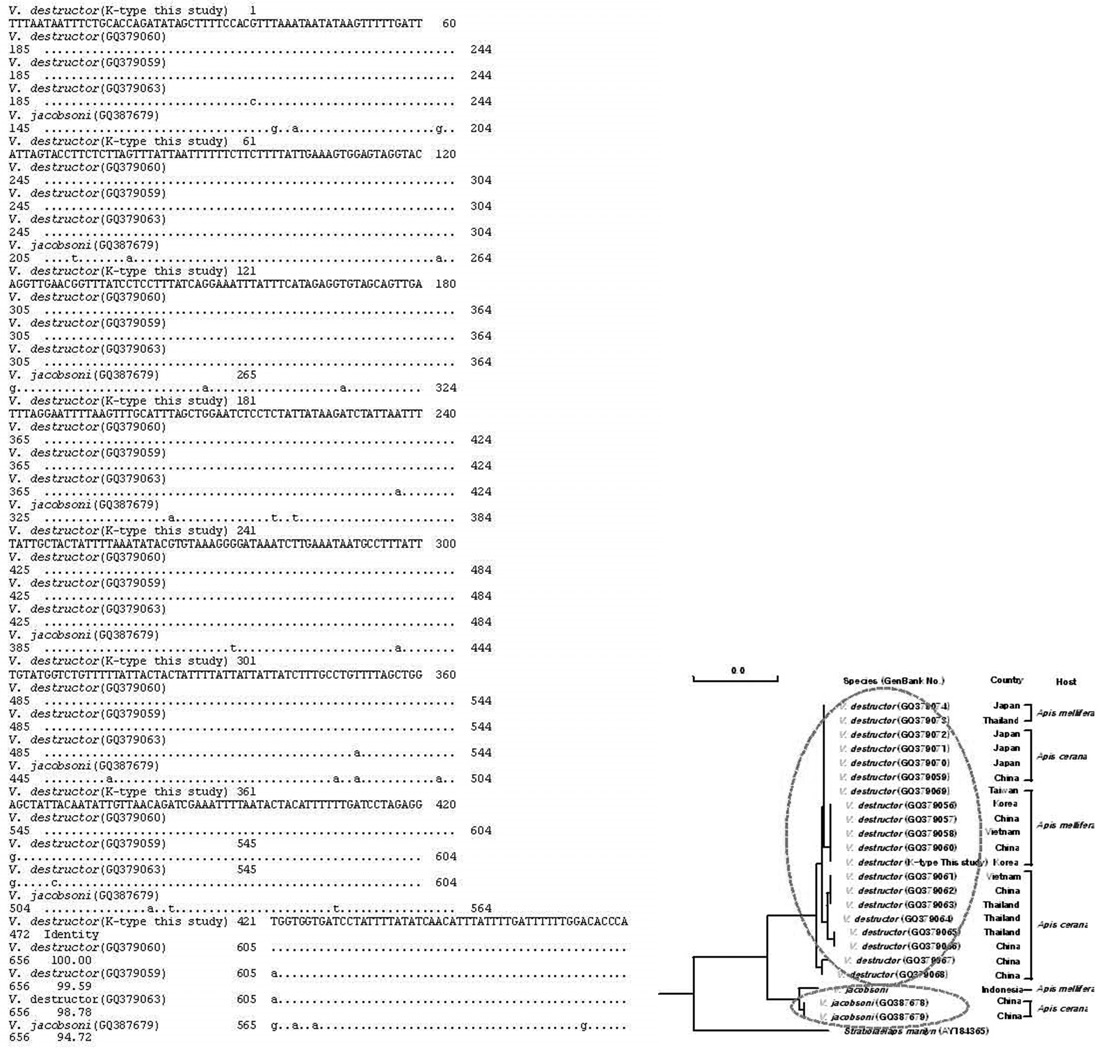
Multiple alignment of mitochondrial COI gene (472bp) of the K-type V. destructor with known GenBank Varroa mite mitochondrial COI genes. Identical nucleotide sequences are dotted. K-type V. destructor was closely related to GQ379060 (100%), GQ379059 (99.59%), and GQ379063 (98.78%) of GenBank accession number of V. destructor cox1 genes.
Body setae
SEM micrographs showed dorsally serrated setae dorsally and entire simple setae ventrally. The most possible explanation is that the dorsal setae are probably used to interlock with the body hairs of bees during the phoretic period. The ventrally entire spinescent setae may help V. destructor to walk on brood cell and on bee’s bodies. All these setae are reflexed posteriorly.
Marginal setae
SEM micrographs of the marginal setae showed that the tips are smooth and flexible. The morphological plasticity suggest that K-type V. destructor also uses the marginal setae to grab the integument of the larvae during the feeding periods inside the sealed cells, and also use to grap the adult bees during hibernation times inside the tergits of winter bees, and the phoretic period. Davis and Camin (1976) have demonstrated that the primary role of marginal setae in Dermanyssus prognephilias mites, ectoparasites of birds, is for attachment.

Body lengths and widths of female K-type V. destructor compared with those of V. destructor reported by Anderson and Trueman (2000) and Zhang (2000)
Ventral shields
The sternal shield has a fixed number of sternal setae. The St3 and St4 are adjacent, whereas other sternal setae separated and asymmetric. SEM micrographs indicate that the endopodal shield has a fixed number of endopodal setae and no marginal setae. These are diagnostic characters of K-type V. destructor. The number of endopodal setae of K-type V. destructor is similar to V. jacobsoni, but less than V. rindereri (Table 2). A comparison of endopodal setae needs to be made with V. destructor reported from other parts of the world. The metapodal shield has two types of setae, long and short. The long setae protrude ventrally outside of the body margin and are used to protect the longitudinal muscles running along the outer margin of the body from rubbing. These setae may also assist in locomotion. The number of metapodal setae in K-type V. destrcutor is more than V. jacobsoni and V. rendereri. In contrast, the number of marginal setae of K-type V. destructor is very close to V. jacobsoni, but less than V. rindereri (Table 2).
Stigma and peritreme
Our observations showed that one end of the peritreme opens into the stigma and the other end is a bulb-like structure without an opening. The peritreme groove is internally lined with setules and micropapillae (Bruce et al., 1997). The peritreme of K-type V. destructor is smaller than V. jacobsoni and V. rindereri (Table 2). The stigma has an opening indicated by a ring-like structure (Fig. 4D). We are doubtful that air reaches into the atrium directly through the peritreme. Our observations did not support the theory that the air comes from directly through the peritreme because the tip of the peritreme is closed, covered by peritreme sheath, and flexible. It seems likely that the peritremes may be used to move air into the atrium through the stigma opening. More observations are needed to clarify the functions of peritremes.
Gnathosoma and palptarsi
SEM micrographs showed that V. destructor has two apotele claws, long and short, originating from a single base at the palptarsus. The tip of the long apotele claw is pointed and slightly incurved, whereas the tip of the small apotele claw is blunt and straight. The structure of the long apotele claw tip indicates that they are used to pierce the integument of adult bees and larvae. The short apotele claw with a rounded tip has no piercing function, but probably helps to grip the integument during feeding and phoretic periods. SEM micrographs showed that the palptarsi have 16 sensory setae at different layers. These may function as the sensilla on the palptarsus of female V. jacobsoni which considered to be chemoreceptors to perceive chemicals in drone cells (Liu, 1988; Liu and Peng, 1990).
Legs
SEM micrographs showed that leg I is more rounded compared to legs II, III and IV. The most plausible explanation is that V. destructor, due to the presence of mechano-chemoreceptor sensory setae on leg I, rarely uses for walking. Legs II-IV are dorso-ventrally flattened with pairs of short and long spiny setae are used for walking. The short spiny setae on the lateral side of legs III and IV indicate that these setae may protect the mite from bee grooming. V. destructor has two types of setae, long and short, compared with V. jacobsoni, which has only one type of leg with long setae (Akratanakul, 1975). The leg chaetotaxis of K-type V. destructor has some differences in the number of setae on particular segments (Table 3).
Conclusion
The K-type of V. destructor has more oval shape with three distinct types of body setae, viz serrated type dorsally, barbs type ventrally and smooth type setae on the body margins. The anal opening is protruded and semibounded by tiny hinged plates from the top of opening. Cheliceral bear two teeth of similar size teeth. The apotele claws are short and long types which may use to pinch the integument of developing larvae and adult honey bees during the feeding time. The body shape, seate, and cheliceral morphology is central to the evolutionary success of survival of V. destructor mites.
Acknowledgments
This work was supported by Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ010487) from the Rural Development Administration (RDA) of the Republic of Korea.
LITERATURE CITED
- Akratanakul, P., (1975), Biology and Systematic of Bee Mites of the family Varroidae (Acari: Mesostigmata), Thesis, Oregon State University, p64.
- Allen, MF., Ball, BV., White, RF., Antoniw, JF., (1986), The detection of acute paralysis virus in Varroa jacobsoni by the use of a simple indirect ELISA, J. Apic. Res, 25, p100-105.
- Anderson, DL., Fuchs, S., (1998), Two genetically distinct populations of Varroa jacobsoni with contrasting reproductive abilities on Apis mellifera, J. Apic. Res, 37, p69-78.
-
Anderson, DL., (2000), Variation in the parasitic bee mite Varroa jacobsoni Oud, Apidologie, 31(2), p281-292.
[https://doi.org/10.1051/apido:2000122]

-
Anderson, DL., Trueman, JWH., (2000), Varroa jacobsoni (Acari: Varroidae) is more than one species, Expert. Appl. Acarolo, 24(3), p165-189.
[https://doi.org/10.1023/A:1006456720416]

- Ball, BV., (1985), Acute paralysis virus isolates from honeybee colonies infested with Varroa jacobsoni, J. Apic. Res, 24, p115-119.
-
Bradbear, N., (1988), World distribution of major honeybee diseases and pests, Bee World, 69, p15-39.
[https://doi.org/10.1080/0005772X.1988.11098943]

-
Bruce, WA., Delfinado-Baker, M., Vincent, DL., (1997), Comparative morphology of the peritremes Varroa and Euvarroa (Varroidae), parasite of honeybees (Apidae), Inter, J. of Acarology, 23(1), p13-20.
[https://doi.org/10.1080/01647959708684114]

-
Chen, YP., Siede, R., (2007), Honeybee viruses, Adv. Virus Res, 70, p33-80.
[https://doi.org/10.1016/S0065-3527(07)70002-7]

-
Cox-Foster, DL., Conlan, S., Holmes, EC., Palacios, G., Evans, JD., Moran, NA., Quan, PL., Biese, T., Hornig, M., Geiser, DM., Martinson, V., vanEngelsdorp, D., Kalkstein, AL., Drysdale, A., Hui, J., Zhai, J., Hutchison, SK., Simons, JF., Egholm, M., Pettis, JS., Lipkin, WI., (2007), A metagenomic survey of microbes in honeybee colony collapse disorder, Science, 318, 5848, p283-287.
[https://doi.org/10.1126/science.1146498]

- Davis, JC., Camin, JH., (1976), Setae of the anterior tarsi of the Martin mite; Dermanyssus prognephilus (Acari: Dermanyssidae), J. Kansas Entomol. Sco, 49(3), p441-449.
-
de Guzman, LI., Rinderer, TE., Stelzer, JA., (1997), DNA evidence of the origin of Varroa jacobsoni Oudemans in the Americas, Biochem. Genet, 35, p325-335.
[https://doi.org/10.1023/A:1021821821728]

-
de Guzman, LI., Rinderer, TE., (1999), Identification and comparison of Varroa species infesting honeybees, Apidologie, 30, p85-95.
[https://doi.org/10.1051/apido:19990201]

- De-Jong, D., (1997), Mites: Varroa and other parasites of brood, In: Honey bee pests, predators, and diseases, Morse, RA., and Flottum, K. (eds), AI Root Co, Medina, OH., USA, p279-327.
-
De Jong, D., Morse, RA., Eickwort, GC., (1982), Mite pests of honeybees, Annul. Review of Entomology, 27, p229-252.
[https://doi.org/10.1146/annurev.en.27.010182.001305]

-
De-Jong, D., De Jong, PH., (1983), Longevity of Africanized honeybees (Hymenoptera: Apidae) infested by Varroa jacobsoni (Parasitiformes: Varroidae), J. Eco. Entomol, 76, p766-768.
[https://doi.org/10.1093/jee/76.4.766]

- Delfinado-Baker, M., (1988), The tracheal mite of honeybees: a crisis in beekeeping, in: Needham, G., Page, R., Delfinado-Baker, M., Bowman, C. (eds), Africanized honeybees and bee mites, Halsted Press, Chichester, England, p483-497.
-
Garrido, C., Rosenkranz, P., Paxton, RJ., Gonclaves, LS., (2003), Temporary changes in Varroa destructor fertility and haplotype in Brazil, Apidologie, 34, p535-541.
[https://doi.org/10.1051/apido:2003041]

- Harbo, JR., Harris, JW., (2005), Suppressed mite reproduction explained by the behavior of adult bees, J. Apic. Res, 44(1), p21-23.
-
Johnson, RM., Evans, JD., Robinson, GE., Berenbaum, MR., (2009), Changes in transcript abundance relating to colony collapse disorder in honeybees (Apis mellifera), Proc. Nat. Acad. Sci. USA, 106, p14790-14795.
[https://doi.org/10.1073/pnas.0906970106]

- Koch, W., Ritter, W., (1989), Examination of artificially infested brood with Varroa mites for secondary infections, In Cavaloro, R. (eds), Present status of Varroatosis in Europe and progress in the Varroa mite control, Proceedings of a meeting of the Experts’ group/Udine, Italy, November 1988. Commission of the European Communities, Luxembourg, p245-251.
-
Kocher, TD., Thomas, WK., Meyer, A., Edwards, SV., Paabo, S., Villablanca, FX., Wilson, AC., (1989), Dynamics of mitochondrial DNA evolution in animals: Amplification and sequencing with conserved primers, Proc. Nat. Acad. Sci, vol. 86, p6196-6200, PMID: 2762322.
[https://doi.org/10.1073/pnas.86.16.6196]

- Liu, TP., (1988), The morphology of the tarsal sensilla in the female mite Varroa jacobsoni, Pan-Pacific Entomol, 64(3), p261-265.
- Liu, TP., Peng, YS., (1990), Palpal tarsal sensilla of the female mite, Varroa jacobsoni Oudemans (Acari: Varroidae), Can. Entomol, 122, p295-300.
-
Matheson, A., (1995), World bee health update, Bee World, 76, p31-39.
[https://doi.org/10.1080/0005772X.1995.11099235]

- Munoz, I., Garrido-Bailon, E., Martin-Hernandez, R., Meana, A., Higes, M., De La Rua, P., (2008), Genetic profile of Varroa destructor infesting Apis mellifera ibriensis colonies, J. Apic. Res, 47(4), p310-313.
-
Oldroyd, BP., (1999), Coevolution while you wait: Varroa jacobsoni, a new parasite of western honeybees, Trends Ecol. Evol, 8, p312-315.
[https://doi.org/10.1016/S0169-5347(99)01613-4]

- Piccirillo, GA., De Jong, D., (2003), The influence of brood comb cell size on the reproductive behavior of the ectoparasitic mite, Varroa destructor in Africanized honeybee colonies Genet, Mol. Res, 2(1), p36-42, PMID: 1291 7800.
-
Rosenkranz, P., Aumeier, P., Ziegelmann, B., (2010), Biology and control of Varroa destructor, Jr Inverte.Pahto, 103, pS96-S119.
[https://doi.org/10.1016/j.jip.2009.07.016]

-
Rinderer, TE., de Guzman, LI., Delatte, GT., Stelzer, JA., Lancaster, VA., Kuznetsov, V., Beaman, L., Watts, R., Harris, JW., (2001), Resistance to the parasitic mite Varroa destructor in honeybees from far-eastern Russia, Apidologie, 32, p381-394.
[https://doi.org/10.1051/apido:2001138]

- Sakai, T., Okada, I., (1973), The present beekeeping in Japan, Glean Bee Cult, 101, p356-357.
- Shimanuki, H., Knox, DA., Furgala, B., Caron, DM., Williams, JL., (1992), Diseases and pests of honeybees, In Graham, JM. (eds), The hive and the honey bee, Dadant and Sons, Hamilton, Illinois, USA, p1083-1152.
-
Simon, C., Frati, F., Beckenbach, A., Crespi, B., Liu, H., Flook, P., (1994), Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers, Annals. Entomol. Soc. Amer, 87(6), p651-700.
[https://doi.org/10.1093/aesa/87.6.651]

-
Solignac, M., Cornuet, JM., Vautrin, D., Le Conte, Y., Anderson, D., Evans, J., Cros-Arteil, S., Navajas, M., (2005), The invasive Korean and Japan types of Varroa destructor, ectoparasitic mites of the western honeybees (Apis mellifera), are two partly isolated clones, Proc. Bio. Sci, 272(1561), p411-419, PMID: 15734696.
[https://doi.org/10.1098/rspb.2004.2853]

-
Spivak, M., Reuter, GS., (2001), Varro destructor infestation in untreated honeybee (Hymenoptera : Apidae) colonies selected for hygienic behavior, J. Econ. Entomol, 94(2), p320-331, PMID: 11332821.
[https://doi.org/10.1603/0022-0493-94.2.326]

- USDA, (1987), Varroa jacobsoni - Detection techniques, Amer. Bee Jr., 127, p755-757.
-
Zhang, ZQ., (2000), Notes on Varroa destructor (Acari: Varroidae) parasitic honeybees in New Zealand, Sys. Appl. Acarol. Spec. Pub, 5, p9-14.
[https://doi.org/10.11158/saasp.5.1.2]