Extraction Properties of Propolis with Ethanol Concentration
Abstract
Propolis is a sticky material made from plant growing point protected material and resin which are collected by bees, then mixed with bee saliva enzyme, it is used to keep bee colony safe by applying inside of bee hive. Propolis is consisted of 50% of resin and aromatic materials, 25% of beeswax, 10% of essential oil, and others of bee pollen and minerals, and so on. In this study, propolis is extracted with ethanol and the active component, because used as health food. We reported a summary of the main results of the extraction of propolis by ethanol. Propolis extract yield and total flavonoid content tends to increase the higher the ethanol concentration. Total phenolic content exhibited the highest value in the 50-60% EtOH, when the ethanol concentration is high, there is a tendency to lower the total phenolic content.
Keywords:
Propolis, Extraction yield, EtOH concentration, Total flavonoid contentINTRODUCTION
The propolis is a Greek word meaning the material which protects the honey bee colony safe, "pro' means protection and "polis" means city. Propolis is a sticky material made from bee collected plant growing point protected material and resin mixed with bee saliva enzyme. It is used for keep safety of bee colony by applying inside of bee hive and has various color including dark brown and yellowish brown. Propolis is sticky at warm condition but becomes hard at cool condition, so it is also called "bee glue" (Fearnley, 2011).
Bees use the propolis for colony protection to prevent fungal and viral infection by applying it on the contamination susceptible surface, to make hive water proof by filling cracks with propolis and to block hive from the outside. Also propolis is used for hive repair, entrance size adjust, antiseptic for sealing large sized insect intruder which is hard to move outside and preventing decay of carcass, and controlling disease and microorganism growth in the hive. But the most important use of propolis is larvae protection by coating thin layer of this on brood cell for keeping eggs and larvae from microbe before oviposition of queen bee, and a little amount of propolis is mixed with bee wax for brood cell sealing. The plant resin and bee salivation both contain antibiotics, so use of propolis can reduce infection of growing bee larvae and microbe growth in dead animal tissue.
Propolis is a composite material consists of various ingredients such as resin and aromatic (45~55%), bees wax (25~35%), volatile essential oil (10%), pollens, minerals (5%), tannins, and bee secretion & enzymes (Moreno et al., 2000).
The polyphenolic component and flavonoids in propolis show significant anti-oxidation effect, there are several studies referring correlation between anti-oxidation effect and polyphenolic composition in propolis (Bors et al., 1990; Heim et al., 2002; Russo et al., 2002; Kumazawa et al., 2004), and these activity get synergy effect by complex reaction between phenol compound and resin type materials (Burdock, 1998; Markham et al., 1996).
In this study, it was necessary to extract the active ingredient in crude to use of propolis, since the use of a health food and extracted with ethanol. We reported a summary of the main results of the extraction of propolis by ethanol.
MATERIALS AND METHODS
Propolis yield by ethanol concentration
Weighed 5 gram of propolis sample from Suwon, Daegu and Jeju respectively, ethanol (HPLC grade, Fisher, USA) dilutions with various concentration (0~100%) were made by diluting with triple distilled water, then propolis samples were mixed with ethanol by 1:10 volume, and digested for 48 hour at room temperature. After digestion, samples were filtered (Whatman #2 paper), concentrated and yield was measured.
Propolis yield by extraction time
Weighed 5 gram of propolis samples from Suwon, Daegu and Jeju respectively, diluted with ethanol 1:10 volume, digested with different time. After digestion, samples were filtered (Whatman #2 paper), concentrated and yield was measured.
Propolis yield by extraction temperature
Weighed 5 gram of propolis samples from Suwon, Daegu and Jeju respectively, diluted with ethanol 1:10 volume, digested for 48hour at freezing (-20°C), cold (4°C) and room temperature (25°C). After digestion, samples were filtered (Whatman #2 paper), concentrated and yield was measured.
Propolis extraction by ultrasonic treatment
Weighed 5 gram of propolis samples from Suwon, Daegu and Jeju respectively, diluted with ethanol 1:10 volume, treated with ultrasonic cleaner (Branson 8510, USA) for 100 min at room temperature. After treatment, samples were filtered (Whatman #2 paper), concentrated and yield was measured.
Total flavonoid content analysis in propolis
Weighed 0.1g of extracted and concentrated propolis, dissolved with 80% ethanol 20ml, then centrifuged (3,000 rpm, 10 min). Then supernatant was collected, residue was extracted 3 times with 80% ethanol, then all extract was united one sample and 80% ethanol was added to make 50ml sample.
Put 0.5ml of sample into test tube, added ethanol 1.5ml, 10% aluminum nitrate (Sigma, USA) solution 0.1ml, water 2.8 ml, stirred sufficiently and stationed for 40 min, and another process which aluminum nitrate solution was substituted with 0.1ml water was done absorbance of both sample fluid bed was measured using 10mm cell with 415nm wave length using water as control. Using the value by subtracting latter process absorbance value from former one, then total flavonoid content (mg/ml) was calculated using calibration curve acquired by quercetin (Sigma, USA).
Total flavonoid contents(%)=
Total phenol content analysis
Total phenol content was measured using modified Folin-Ciocalteau method (Kuyala et al., 2000). Sample solution 0.5ml (three times extracted with 80% ethanol) was mixed with 0.5ml of 1N Folin-Ciocalteau (Sigma, USA) solution 0.5ml and 0.5ml of 10% Na2CO3 solution then stationed for 50 min. Sample was centrifuged at 150g for 10 min, absorbance of supernatant was measured by UV/VIS Spectrophotometer (Perkin-Elmer Lambda 10, USA) at 760nm wave length. Total phenolic content (mg/ml) was calculated using calibration curve acquired by gallic acid (Sigma, USA).
RESULTS AND DISCUSSION
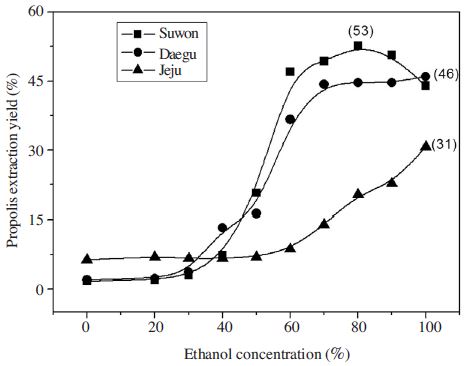
Extraction yield of propolis by ethanol concentration
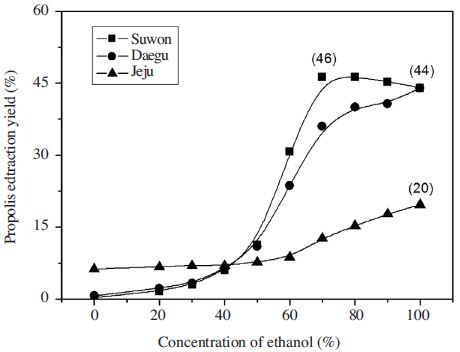
The extraction yield of propolis by ethanol concentration were identified (Fig. 1), the extraction yield of propolis from Suwon showed 10% until 40% EtOH concentration, increased to over 30% with 60% EtOH conc. and 44~46% over 70% EtOH conc. without significant difference. The extraction Yield of Daegu originated showed over 35% at 60% EtOH, more than 40% over 70% EtOH. But Jeju originated propolis yield showed different tendency, which was less than 10% yield until 60% EtOH conc. The yield curve showed gradual increase at 10~20% yield section over 70% EtOH conc. The propolis yield value from Daegu and Suwon were more than 40% over 70% EtOH but that of Jeju was more than 15% over 80% EtOH concentration. So the propolis yield sampled from Jeju was lower than other samples from Daegu and Suwon.
Extraction yield of propolis by extraction time
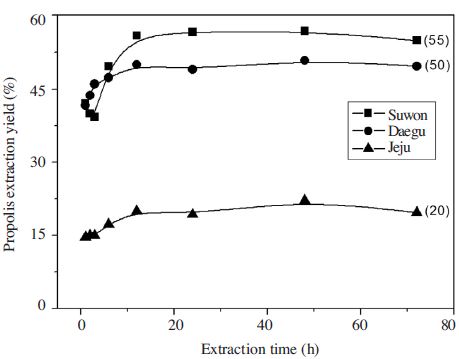
The yield of propolis by extraction time was examined (Fig. 2), yield of sample from Suwon was about 40% 1 hour after extraction, reached 60% after 6 hours, and showed 56% after 12 hours. The 56% yield was maintained without significant fluctuation after 24, 48 and 72 hours extraction. Sample from Daegu showed 42% yield 1 hour after extraction, reached 50% after 12 hours, and showed little difference after extended extraction. The yield of 1 hour extraction of Jeju sample was 15% and maintained about 20% after 12 hours. The yield of Jeju sample was lower than other samples but did not showed significant difference in extraction time.
Extraction yield of propolis by extraction temperature
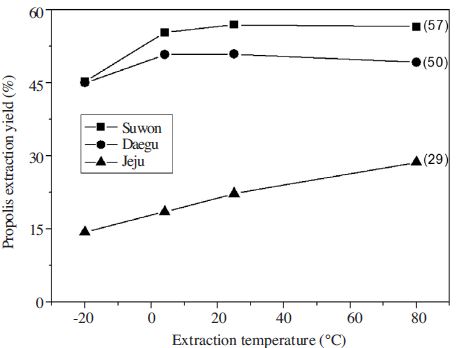
The propolis yield of Suwon sample by extraction temperature was 45% at freezing condition (-20°C), 54% at cold condition (4°C), 57% at room temperature (25°C), and 57% at high temperature, respectively. The yield of Daegu sample did not show significant difference by temperature, it was 45% at freezing condition and 50% over the cold condition (>4°C). The result of Jeju samples were 15% at freezing condition, 18% at cold condition (4°C), 21% at room temperature, and 27% at boiling temperature, the results showed that extraction yield increased to temperature rise (Fig. 3).
Propolis extraction by sonication
The extraction of propolis by sonication showed similar tendency of extraction by ethanol concentration (Fig. 4). The Suwon sample showed 7% extraction yield at 40% EtOH, about 20% at 50% EtOH, and more than 46% over 60% EtOH. The result of propolis extraction yield from Daegu was about 15% at 40~50% EtOH, 35% at 60% EtOH, more than 40% over 70% EtOH. And Jeju sample's extraction yield showed higher value by increase of EtOH concentration. The extraction yield of propolis by sonication was lower than that of ethanol extraction.
Total contents of flavonoid and phenolics by ethanol concentration
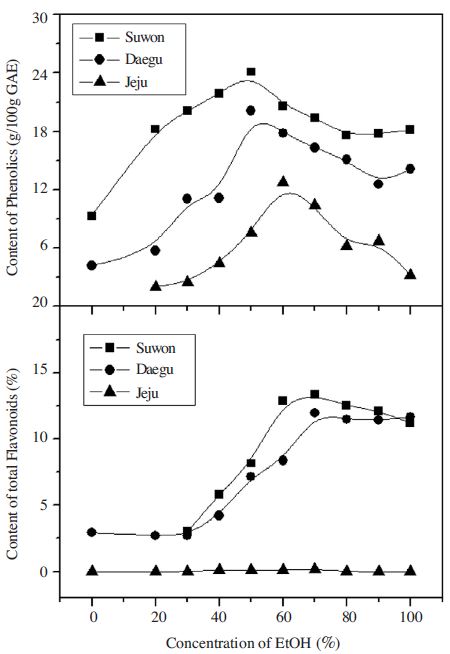
The total flavonoid content of Suwon & Daegu propolis samples by EtOH concentration was about 3% under 20% EtOH, then increased from 40% EtOH and showed the highest value at 70% EtOH. Little total flavonoid was detected from Jeju samples.
The extraction yield and total flavonoid content were excellent at 70% ethanol (When used as food is edible alcohol use). And this was consistent with the result (Kujumgiev et al., 1999; Santos et al., 2002; Lu et al., 2005; Mani et al., 2006) which used 70~100% ethanol for propolis extraction. On the contrary, total phenolic content of Suwon and Daegu samples showed the highest content (24, 20g/100g gallic acid) at 50% EtOH and decreased by EtOH concentration rise. Jeju sample showed highest content (12g/100g gallic acid) at 60% EtOH, and decreased by EtOH concentration rise (Fig. 5).
Propolis extraction yield and total flavonoid content had tendency to increase by EtOH concentration rise until 70%, but total phenolic content was the highest at 50~60% EtOH, and decreased by EtOH concentration rise.
The total phenolic content of propolis extract was highest when 50 to 60% ethanol, 50% ethanol, 24% in Suwon, Daegu in 50% ethanol, 20%, Jeju total phenolic content of 12% in 60% ethanol are shown.
The extraction yield of EtOH concentration by sonication for propolis extraction (at 25°C, 100 min).
Total flavonoid content by ethanol concentration and extraction method
Total flavonoid content of propolis extracted by EtOH concentration was determined (Table 1). The total flavonoid content of Suwon sample was 3.54% by sonication at 40% EtOH, and 7~9% with little fluctuation over 60% EtOH. Propolis extract at room temperature showed the content of 4.62% in the 40% ethanol, appeared to around 9% in more than 60%EtOH.
The content of sonicated sample Daegu was higher (3.37~5.34%) at 40~60% EtOH than extracted sample at room temperature (2.50~4.12%), but it was lower (6.28~ 6.46) than extracted sample at room temperature (6.97~ 7.71) at 70~90% EtOH.
Most of Jeju sample showed very low value (less than 1%), sonicated sample showed higher value than extracted sample at room temperature.
The lower efficiency of sonication for propolis extraction comparing room temperature extraction was confirmed because of similar tendency of low total flavonoid content with lower extraction yield by sonicated extraction compare to room temperature treatment.
This suggests that propolis extraction is more efficient than just using alcohol spirit when proper amount of water is mixed with ethanol. Twelve hour was enough for full extraction of propolis, and extraction temperature was no matter except freezing condition.
Acknowledgments
This research was funded by RDA agenda research project PJ010912.
LITERATURE CITED
-
Bors, W., W. Heller, C. Michel, and M. Saran, (1990), Flavonoids as antioxidants: determination of radicalscavenging efficiencies, Meth. Enzymol, 186, p343-355.
[https://doi.org/10.1016/0076-6879(90)86128-I]

-
Burdock, G.A., (1998), Review of the biological properties and toxicity of bee propolis, Food Chem. Toxicol, 36, p347-363.
[https://doi.org/10.1016/S0278-6915(97)00145-2]

-
Heim, K.E., A.R. Tagliaferro, and D.J. Bobilya, (2002), Flavonoids antioxidants: chemistry, metabolism and structure-activity relationship, J. Nutr. Biochem, 13, p572-584.
[https://doi.org/10.1016/s0955-2863(02)00208-5]

- James, Fearnley, (2001), Bee propolis, Souvenir press Ltd.
-
Kujala, T.S., J.M. Loponen, K.D. Klika, and K. Pihlaja, (2000), Phenolics and Betacyanins in Red Beetroot (Beta vulgaris) Root: Distribution and Effect of Cold Storage on the Content of Total Phenolics and Three Individual Compounds, J. of Agriculture Food Chemistry, 48, p5338-5342.
[https://doi.org/10.1021/jf000523q]

-
Kujumgiev, A., I. Tsvetkova, Y. Serkedjieva, V. Bankova, R. Christov, and S. Popov, (1999), Antibacterial, antifungal, and antiviral activity of propolis of different geographic origin, Journal of Ethnopharmacology, 64, p235.
[https://doi.org/10.1016/S0378-8741(98)00131-7]

-
Kumazawa, S., H. Tomoko, and N. Tsutomu, (2004), Antioxidant activity of propolis of various geographic origins, Food Chemistry, 84, p329-339.
[https://doi.org/10.1016/S0308-8146(03)00216-4]

-
Lu, L.C., Y.W. Chen, and C.C. Chou, (2005), Antibacterial activity of propolis against Staphylococcus aureus, International Journal of Food Microbiology, 102, p213-220.
[https://doi.org/10.1016/j.ijfoodmicro.2004.12.017]

-
Mani, F., H.C.R. Damasceno, E.L.B. Novelli, E.A.M. Martins, and J.M. Sforcin, (2006), Propolis: Effect of different concentrations, extracts and intake period on seric biochemical variables, Journal of Ethnopharmacology, 105, p95-98.
[https://doi.org/10.1016/j.jep.2005.10.011]

-
Markham, K. R., K. A. Mitchell, A. L. Wilkins, J. A. Daldy, and Y. Lu, (1996), HPLC and GC-MS identification of the major organic constituents in New Zealand propolis, Phytochemistry, 42, p205-211.
[https://doi.org/10.1016/0031-9422(96)83286-9]

-
Moreno, M. I. N., M. I. Isla, A.R. Sampietro, and M. A. Vattuone, (2000), Comparison of the free radicalscavenging activity of propolis from several regions of Argentina, Journal of Ethnopharmacology, 71, p109-114.
[https://doi.org/10.1016/S0378-8741(99)00189-0]

-
Russo, A., R. Longo, and A. Vanella, (2002), Antioxidant activity of propolis: role of caffeic acid phenethyl ester and galangin, Fitoterapia, 73, pS21-S29.
[https://doi.org/10.1016/S0367-326X(02)00187-9]

-
Santos, F.A., E.M. Bastos, P.H. Rodrigues, M. de Uzeda, M.A. de Carvalho, M. Farias Lde, E.S. Moreira, (2002), Susceptibility of Prevotella intermedia/Prevotella nigrescens (and Porphyromonas gingivalis) to propolis (bee glue) and other antimicrobial agents, Anaerobe, 8.
[https://doi.org/10.1006/anae.2002.0411]