
Composition and Anti-Helicobacter pylori Activity of the Different Floral Honeys Form Korea
Abstract
Helicobacter pylori is a gram-negative bacterium incriminated in gastroduodenal ulcers, and mucosaassociated lymphoid tissue lymphoma imposing a major burden on health care systems worldwide. Honeys have been shown to have antibacterial activity and suitable for use in ulcers, infected wounds and burns. The aim of this study was to screen selected Korean honeys for their anti-H. pylori activity. Acacia, chestnut, linden tree, mandarin orange, and snowbell honeys from Korea were characterized by traditional physiochemical parameters, vitamins, minerals, and amino acids. The contents of moisture (17.2~19.6g/100g of honeys), ash (0.04~0.72g/100g of honey), total protein (0.13~0.46g/100g of honey), and total lipid (0.01~0.03g/100g of honey) were distinctly different by floral origin. The values of fructose (38.78~44.07g/100g of honey), glucose (22.74~32.5g/100g of honey), and fructose/glucose ratio (1.19~1.83) were a little different by floral origin. All five honey samples detected only vitamin B1, B2, and niacin. Presences of 17 kinds of the amino acids were detected in all honey samples analyzed. The highest concentration among the amino acid observed in our study was proline. Variation in trace element content in different honey types is primarily due to botanical origin rather than geographical and environmental exposition of nectar sources. Contents of Ca and K were high in chestnut and linden tree honey. All the five honey varieties exhibited anti-H. pylori activity. The strongest inhibitory activity was demonstrated by chestnut honey at a concentraion of 10% v/v. However, treatment of honey with catalase did not have H. pylori inhibitory effect as compared to honey alone. These honeys from Korea may contain compounds with therapeutic potential against H. pylori.
Keywords:
Acacia honey, Chestnut honey, Linden tree honey, Mandarin orange honey, Snowbell honey, Helicobacter pyloriINTRODUCTION
Helicobacter pylori has been considered as the relevant agent of chronic gastritis, peptic ulceration and gastric cancer (Karttunen et al., 1991; Parsonnet, 1993). About 50% of the world’s population and 59.6% population in Korea have been reported to be infected with this gramnegative bacterium (Kim et al., 2009; Ayala et al., 2014). This gram-negative curved rod bacterium colonizes the gastric epithelial surface and withstands the stomach’s hostile ambience by microaerophilic growth capacity (Goodwin and Armstrong, 1990). Treatment for the control of H. pyroli infection is continuously evolving from the standard triple therapy using a combination of therapeutic agents such as antibiotics, bismuth subsali-cylate, proton pump inhibitors and H2-blockers (Hentschel et al., 1993). However, these cure achieved is incomplete and undesirable side effects are certain to occur. Therefore, a nonantibiotic agents such as natural compounds, which are effective and free from side effects might be of utmost importance for the control of H. pyroli (Masuda et al., 2004).
Honey is being as a folk remedy for various health disorders for the past several decades, but its heterogeneous composition has limited scientific evaluation (Weston, 2000). The observation that honey produced in New Zealand, Saudi Arabia, and that can inhibit the growth of H. pyroli prompted this investigation to evaluate other honey varieties for antimicrobial effect (Ali et al., 1991; Osato et al., 1999). There are multiple mechanisms of the anti-H. pyroli effect of honey involving both hydrogen peroxide and non peroxide mediated killing. The main objectives of our work were to evaluate different honey varieties in Korea for the potential of the anti-H.pyroli, to determine whether this activity was due to presence of hydrogen peroxide in the honey, and to contribute to the knowledge of the different floral honeys.
MATERIALS AND METHODS
Materials
Five honey varieties from Korea used during 2014 were acacia (Robinia pseudoacacia L.), chestnut (Castanea crenata S.et Z.), linden tree (Tilia amurensis RUPR.), mandarin orange (Citrus unshiu S. arcov.) and snowbell (Styrax japonicas Sieb.). They were purchased from Korea Apicultural Agriculture Cooperative (Seoul, Korea).
Physicochemical analysis of honey varieties
Physicochemical parameters were analysed in duplicate according to the methods recommended by the Korean Food Standards Codex (Ministry of Food and Drug Safety, 2015). Also, vitamins, minerals, and free amino acids were determined by the Korean Food Standards Codex (Ministry of Food and Drug Safety, 2015). As brief explanation, moisture content, sugars, ash, amino acids, and vitamins were determined by HPLC (high performance liquid chromatography) method. Mineral contents measured by ICP (inductively coupled plasma) Emission spectrometer Analyzer. Total protein contents of honey calculated to nitrogen contents. Total lipid was determined by GC (Gas chromatography).
Removal of hydrogen peroxide
The different concentrations (5, 10, 20, 30, 40, and 50% v/v) of each honey constituting were made in sterile distilled water. Paired catalyse control, five honey samples with and without catalyse, was made by dissolving catalyse (0.02g, Sigma, USA) in distilled water (10ml) (Molan, 1992a, b; Snow and Manley-Harris, 2004). The honey (2.0g) was dissolved in distilled water (2.0ml) and divided into two new vials containing 1.0ml of sample each. Then either 1.00ml of distilled water (giving total activity) or catalase solution (giving non-peroxide activity) was added to the two sample vials.
Susceptibility testing of honey
Anti-H. pylori was performed using the following ATCC strains(ATCC 43526) from the Korean Culture Center of Microorganisms, Seoul, Korea. H. pylori was cultured for 3 days at 37°C on trypticase soy broth (TSB, BBL, USA) containing 5% horse serum (MB cell, Korea) in an anaerobe container system (BD, USA). The agar well diffusion method was adopted according to the method of Dastouri et al. (Dastouri et al., 2008). To assess the antimicrobial activity of the crude honeys, cultured H. pylori (100μl, OD540=0.5) was added to the warm nutrient agar, and poured into plates that were allowed to set for at least 6h. Wells were made on the agar plates with sterile cork borer in a regular grid pattern. Honey samples were tested at concentration of 25% for antibacterial activity. Three replicates of phenol standards ranging from 2 to 6% and three to five replicates of the samples being tested were introduced into recorded random wells in the agar plate. After incubation for 3 days digital callipers were used to measure the diameter of inhibition halo zone around the wells.
Statistical analsysis
Data are presented as mean±standard error (SE). Experimental results were statistically analyzed using the R Statistical System.
RESULTS AND DISCUSSION
Physicochemical analysis
The analytical results of the five honey varieties are shown in Table 1. The moisture (17.2~19.6g of water/100g of honeys) of the five honey varieties was much lower than the 20% maximum established by the international standards for Apis mellifera honey (General Standard for Food Additives, 2015). The highest moisture was found in mandarin orange honey, while the lowest honey was observed in chestnut honey variety. The ash content of the five honeys varieties was in the range between 0.04 and 0.72g per 100g honey. In the case of acacia honey, the ash content was 0.04g per 100g honey, whereas chestnut honey had the ash content almost 18 times to that of acacia honey. The ash content is one of the quality criteria for honey origin, the blossom honeys having a lower content than the honeydew ones. Total protein of chestnut honey was high, comparable to the values other honeys. The five honey varieties had similar values to the total lipid content. As it is known, one of the key parameters for assessing the quality of the honey is sugar content therein. The content of fructose, glucose and oligosaccharides for each of investigated five honey varieties is presented in Table 1. According to the criteria of the international standards, the content of invert sugar (fructose+glucose) should not be less than 60% and that of saccharose not more than 5% (Codex, 2001). According to the data in Table 1, all honeys meet the first condition for exporting honey to the European Union, and the saccharose did not detect (Bagdanov et al., 1997).
Vitamins, minerals, and amino acids in honey
Honey contains a variety of vitamins and minerals. The type of vitamins and minerals and their quantity depends on the type of flowers used for apiculture. Commonly, honey contains Vitamin C, Calcium and Iron. Nutrition Facts reported that honey has vitamin C, riboflavin, niacin, vitamin B6, folate, pantothenic acid, choline, and betaine. Generally, the concentration of vitamin B3 in honey is higher than those measured for vitamin C (Hoyle, 1928; Hydak et al., 1942; Ciula et al., 2011; Self nutrition data, 2015). However, all honey samples detected only vitamin B1, B2, and niacin (Fig. 1). Also the concentration of vitamin B3 in honey is higher than other vitamins. Fig. 1 reports the amount of the vitamins in all honey samples analysed, and concentration for honeys of different botanical origin. Further, our data confirmed that honey is not a vitamin-rich food.
Five honeys of known geographic and botanic origin were analyzed in order to detect possible contamination by common air pollution or other pathways. The contamination level of the toxic elements such as Pb, Hg, and Cd were measured in our study and were not detected (Table 2). There were differences between the honeys produced in the different areas with regard to Ca, K, Mg, Mn and P contents. The trace elements determined in this study can be both from natural sources (soil and plants) and anthropogenic sources (Jonathan and White, 1957; Self nutrition data, 2015). The elements Pb, Cd, Zn, Cu, Cr, and Ni, are well known as potential air or soil contaminants of anthropogenic origin, but are of course also found as natural ingredients of soil minerals, including Fe and Mn (Self nutrition data, 2015). Variation in trace element content in different honey types is primarily due to botanical origin rather than geographical and environmental exposition of nectar sources. Contents of Ca and K were high in chestnut and linden tree honey (Table 2).
Honey has 18 kinds of amino acids such as tryptophan, threonine, and isolucine (Jonathan and White, 1957; Self nutrition data, 2015). The presence of 17 kinds of the amino acids were detected in all honey samples analyzed (Table 3). Tryptophan was not detected in all honey samples. The highest concentration among the amino acids observed in our study was proline. Nutrition facts reported that proline is one of the most important amino acids in honey. There was a greater concentration of amino acid in chestnut honey when compared to other floral different honey. Also variation in amino acids content in different honey types is primarily due to botanical origin rather than geographical and environmental exposition of nectar sources.
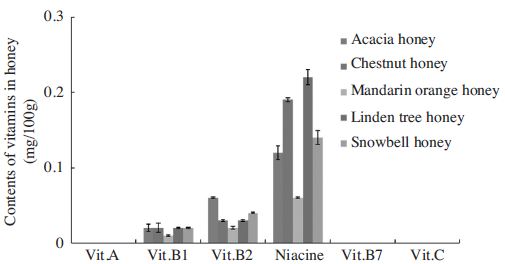
Contents of vitamins in the different floral honeys from Korea (mg/100g in the honey). Values are expressed as mean±SE.

Contents of minerals in the different floral honeys. Values are expressed as mean±SE. The different letters indicate a significant difference with p<0.05
Susceptibility testing of different floral honey
All the honey samples at various concentrations demonstrated antibacterial activity against H. pylori. The zones of inhibition ranged from 1.6~3.5mm at concentrations of 20% honey. The percentage inhibition of H. pylori by different honeys at various concentrations are shown in Table 4. The greatest inhibitory effect was demonstrated by chestnut honey with zone diameter 3.5±0.7mm followed by linden tree with zone diameter 2.5±0.4mm, snowbell with zone diameter 2.4±0.4mm, acacia with zone diameter 2.3±0.4mm, and mandarin orange with zone diameter 1.6±0.6mm at concentration of 10%. However, treatment of honey with catalase did not have H. pylori inhibitory effect as compared to honey alone at concentration of 10% (Table 4). These results suggested that the inhibitory effect of different five floral honeys from Korea was the result of hydrogen peroxide content.
Honey is a folk remedy for a number of ailments. Studies have shown natural honeys to have significant inhibitory activity against a number of bacterial genera and various enteropathogens. Honey from New Zealand and Saudi Arabia has been shown to inhibit H. pylori in concentrations of 20-30% v/v (Dustman, 1979; McGoven et al., 1999; Nzeako and Namaani, 2006). On the other hand, in comparison with this in our study, we report the five different floral honeys were screened for their antimicrobial activity against H. pylori at a lower concentration of honey. Our data showed the all honey varieties demonstrated antimicrobial action from concentrations as low as 20% v/v. In comparison, the manuka honey inhibited both with and without catalase (Molan, 1992a, 1992b). However all honey samples didn’t inhibit with catalyse.
In conclusion, Acacia, chestnut, linden tree, mandarin orange, and snowbell honey from Korea were effective in inhibiting the growth of H. pylori at concentration of >10% v/v. Osmotic effects, not hydrogen peroxide content, were shown to be responsible for the anti-H. pylori effect of the Korean honeys.
Acknowledgments
This work was carried out with the support of "Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ01083701)" Rural Development Administration, Republic of Korea.
LITERATURE CITED
-
Ayala, G., Escobedo-Hinojosa, W. I., de la Cruz-Herrera, C. F., Romero, I., (2014), Exploring alternative treatments for helicobacter pylori infection, World journal of gastroenterology, 20, p1450-1469.
[https://doi.org/10.3748/wjg.v20.i6.1450]

- Bagdanov, S., Martin, P., Lullmann, C., (1997), Harmonised methods of the European Honey Commission, Apidologie, 55, p1-59.
-
Ciulu, M., Solinas, S., Floris, I., Panzanelli, A., Pilo, M. I., Piu, P. C., Spano, N., Sanna, G., (2011), RP-HPLC determination of water-soluble vitamins in honey, Talanta, 83, p924-929.
[https://doi.org/10.1016/j.talanta.2010.10.059]

- Codex Standard for honey, (2001), Codex Alimentarious, FAO.
- Dastouri, M. R., Fakhimzadeh, K., Shayeg, J., Dolgari-sharaf, J., Valilou, M. R., Maheri-sis, N., (2008), Evaluating antibacterial activity of the Iranian honey through MIC method on some dermal and intestinal pathogenic bacteria, Journal of animal and veterinary advances, 7, p409-412.
- Dustman, J. H., (1979), Antibacterial effect of honey, Apiacta, 14, p7-11.
-
Goodwin, C. S., Armstrong, J. A., (1990), Microbiological aspects of Helicobacter pylori (campylobacter pylori), The European Journal of Clinical Microbiology and Infectious Diseases, 9, p1-13.
[https://doi.org/10.1007/BF01969526]

- Haydak, M. H., Palmer, L. S., Tanquary, M. C., Vivino, A. E., (1942), Vitamin content of honeys, Journal of nutrition, 23, p581-588.
-
Hentschel, E., Brandstatter, G., Fragosics, B., Hirschl, A. M., Nemec, H., Schutze, K., Taufer, M., Wurzer, H., (1993), Effect of ranitidine and amoxicillin plus metronidazole on the eradication of Helicobacter pylori and the recurrence of duodenal ulcer, The N ew England journal of medicine, 328, p308-312.
[https://doi.org/10.1056/NEJM199302043280503]

-
Hoyle, E., (1929), The vitamin content of honey, Biochemical journal, 23, p54-60.
[https://doi.org/10.1042/bj0230054]

- Honey standard, (1990), International honey commission.
- Jee, H.S., Chang, K.H., Moon, S.H., Park, S.H., Paik, H.D., (2008), Anti-Helicobacter pylori, cytotoxic, and antiinflammatory activities of white ginseng extract, Food science and biotechnology, 17, p1106-1109.
-
Jonathan, W., White, J. R., (1957), The composition of honey, Bee World, 38, p57-66.
[https://doi.org/10.1080/0005772X.1957.11094976]

-
Kim, N., Kim, J.J., Choe, Y.H., Kim, H.S., Kim, J.I., Chung, I.S., (2009), Diagnosis and treatment guidelines for helicobacter pylori infection in Korea, The Korean journal of gastroenterology, 54, p269-278.
[https://doi.org/10.4166/kjg.2009.54.5.269]

- Korea Food Act, (2015), Ministry of Food and Drug Safety, Osong, Korea.
-
Kusters, J. G., van Vliet, A. H., Kuipers, E. J., (2006), Pathogenesis of Helicobacter pylori infection, Clinical microbiology reviews, 19, p449-490.
[https://doi.org/10.1128/CMR.00054-05]

-
Manyi-Loh, C. E., Clarke, A. M., Munzhelele, T., Green, E., Mkwetshana, N. F., Ndip, R. N., (2010), Selected South African honeys and their extracts possess in vitro anti-Helicobacter pylori activity, Archives of medical research, 41, p324-331.
[https://doi.org/10.1016/j.arcmed.2010.08.002]

-
Matongo, F., Nwodo, U.U., (2014), In vitro assessment of Helicobacter pylori ureases inhibition by honey fractions, Archives of medical research, 45, p540-546.
[https://doi.org/10.1016/j.arcmed.2014.09.001]

- McGoven, D. P., Abbas, S. Z., Vivian, G., Dalton, H. R., (1999), Manuka honey against Helicobacter pylori, Journal of The Royal Society of Medicine, 92, p439.
-
Molan, P. C., (1992), The antibacterial activity of honey: 2. Variation in the potency of the antibacterial activity, Bee world, 73, p59-76.
[https://doi.org/10.1080/0005772X.1992.11099118]

- Molan, P. C., (1992a), The antibacterial activity of honey 1. The nature of the antibacterial activity 1. The nature of the antibacterial activity, Journal of Apicultural Research, 73, p5-28.
-
Molan, P. C., (1992b), The antibacterial activity of honey 2. Variation in the potency of the antibacterial activity, Bee World, 73, p59-76.
[https://doi.org/10.1080/0005772X.1992.11099118]

- Nzeako, B. C., Namaani, F. A., (2006), The antibacterial activity of honey on Helicobacter pylori, Sultan Qaboos University Medical Journal, 6, p71-76.
-
Osato, M. S., Reddy, S. G., Graham, D. Y., (1999), Osmotic effect of honey on growth and viability of helicobacter pylori, Digestive diseases and sciences, 44, p462-464.
[https://doi.org/10.1023/A:1026676517213]

- Self Nutrition Data, Nutrition Facts, Honey, http://nutritiondata.self.com.
-
Snow, M. J., Manley-Harris, M., (2004), On the nature of non-peroxide antibacterial activity in New Zealand manuka honey, Food chemistry, 84, p145-147.
[https://doi.org/10.1016/S0308-8146(03)00258-9]

-
Torres, J., Perez-Perez, G., Goodman, K. J., Atherton, J. C., Gold, B. D., Harris, P. R., la Garza, A. M., Guarner, J., Muñoz, O., (2000), A comprehensive review of the natural history of Helicobacter pylori infection in children, Archives of medical research, 31, p431-469.
[https://doi.org/10.1016/S0188-4409(00)00099-0]

-
Weston, R. J., (2000), The contribution of catalase and other natural products to the antibacterial activity of honey: a review, Food chemistry, 71, p235-239.
[https://doi.org/10.1016/S0308-8146(00)00162-X]
