Potential Antibacterial Activity of Chestnut Honey against Paenibacillus larvae
Abstract
Honey has been proven to possess antibacterial activity and is considered a potential treatment against various bacterial diseases. It is generally known that honeys originating from certain flower sources exhibit specific properties. By using the agar-well diffusion method, we discovered that chestnut honey can inhibit the growth of Paenibacillus larvae, a causative agent of American foulbrood disease in honey bees ( Apis mellifera). We found that the purity of the chestnut honey, as indicated by the percentage of chestnut pollen in the honey, was positively correlated with the honey’s antibacterial activity. The pH of a honey sample was also correlated with its antibacterial properties.
Keywords:
Chestnut honey, Paenibacillus larvae, Antibacterial activityINTRODUCTION
The healing properties of honey and its use as a traditional medicine have been known since ancient times ( Molan, 1992a, b; Zumla and Lulat, 1989). Owing to the worldwide presence of highly resilient and drug-resistant microbes ( Levy and Marshall, 2004) and the need for more alternative approaches in disease prevention, the use of honey as a traditional remedy is once again being taken into consideration ( Basualdo et al., 2007 ). The antibacterial effect of honey results from the enzymatic production of hydrogen peroxide ( Bang et al., 2003 ), with contributions from the property of osmosis, caused by honey’s high sugar and low moisture content, along with the activity of gluconic acid ( Efem, 1988). Honey’s low pH and organic constituent compounds like methylglyoxal and bee defensin-1 ( Khan et al., 2007 ; Kwakman et al., 2010 ), as well as other non-peroxide components ( Snowdon and Cliver, 1996) also play a certain part in its activity. In addition, the water content and the color of honey have been both found to correlate positively with the content of antioxidants in honey ( Frankel et al., 1998 ). Darker, more opaque, and unprocessed honeys have been proven to be more effective in inhibiting bacterial growth than the lighter colored honeys ( Taormina et al., 2001 ). Alongside color, flavonoids and phenolic compounds, which originate from propolis, may also express certain antibiotic activity ( Marcucci, 1995). According to previous research ( Al-Mamary et al., 2002 ; Gheldof et al., 2002 ), different honeys may acquire different amounts of those compounds, which can ultimately influence their antioxidant properties.
Although there is a great deal of interest in, and a great number of accumulated studies on the use of honey against wound infection ( Cooper et al., 2002 ; Vallianou et al., 2014 ) and food spoilage bacteria ( Taormina et al., 2001 ), there is still plenty of research to be done regarding its effect on bacteria in honey bees ( Apis mellifera). Even by applying nutrigenomics to honey bees ( Alaux et al., 2011 ), little information can be found on any link between floral food sources and the effects of those food sources on against certain honey bee pathogens.
Honey bees are among the most productive livestock ( Morse and Calderone, 2000) but are threatened by various pathogens that pose a threat to their health. American foulbrood (AFB) is a fatal brood infection caused by highly infectious spores of Paenibacillus larvae, and is considered one of the most harmful honey bee diseases ( Genersch, 2010). The spores of P. larvae are resilient and long-living ( Lauro et al., 2003 ) and they are strong against heat and dryness. They present a great danger for honey bee larvae ( Neundorf et al., 2004 ). Since adult bees do not get infected, the disease can be transmitted between honey bee colonies ( Matheson and Reid, 1992a, b, c), and also via swarming of strong colonies that have not yet developed visual symptoms ( Fries and Camazine, 2001). Although approved against AFB, the use of certain antibiotics has led to the occurrence of antibiotic-resistant P. larvae in Canada, USA, and Argentina ( Alippi, 2000; Evans, 2003) which has sparked interest in alternative methods of treating and controlling AFB.
To the best of our knowledge, no reports regarding a pathogen so closely connected to a specific animal have been found within previous honey surveys. Continuing on from preliminary research previously performed in Croatia, where different honeys were tested for their antibacterial effects against various bacteria and chestnut was found to have high antibacterial activity (M. Stoi´c, University of Zagreb, unpublished data). As far as we know, there are few reports regarding antibacterial effects of chestnut honey. In addition, some Japanese beekeepers believe that keeping a chestnut orchard near the apiary is good for the health of honey bees. In the current study, we concentrated to study the inhibitory effects of samples of different raw, unprocessed chestnut honeys against P. larvae during its growth phase.
MATERIALS AND METHODS
Bacteria
P. larvae (strain P-56) in the form of isolated spores was provided by the Research Institute for Animal Science in Biochemistry and Toxicology (RIAS, Sagamihara, Kanagawa, Japan). B. subtilis (strain 3134, source of isolation not stated), E. coli (strain 3972, isolated from human feces), P. aeruginosa (strain 13257, isolated from infection of the outer ear), and S. aureus (strain 13276, isolated from a human lesion) were provided by the National Institute of Technology and Evaluation (NITE) Biological Research Center (NBRC, Kisarazu, Chiba, Japan). These bacteria are widely used as control species for antibacterial studies.
Honey samples
Eight chestnut honey samples were used in this research. From the Japanese group, honey samples from Mie and Nagano prefectures were purchased, whilst samples from Tochigi and Kumamoto were provided to us as gifts. From the Croatian group, honey from Samobor was purchased, whilst honeys from Petrinja, Kupa, and Glina were gift samples. All these chestnut honeys had typical chestnut honey smell and dark brown color. Therefore, we assessed these honeys as “chestnut honeys” Alongside the chestnut honeys, one purchased acacia honey from Ibaraki and one multifloral honey, obtained from our apiary at the Institute of Livestock and Grassland Science (Tsukuba), were used in this experiment as control. To the best of our knowledge, none of the honey had been pasteurized.
Artificial honey, a solution with the same sugar content as in real honey, was used as a control to examine whether sugar is related to honey’s antimicrobial activity. It was prepared by dissolving 1.5g of sucrose, 7.5g of maltose, 40.5g of fructose, and 33.5g of glucose in 17mL of sterile deionized water ( Cooper et al., 2002 ).
Identifying chestnut pollen in honey samples
The content of pollen in honey provides reliable information of its floral components. In many studies pollen is used for identifying plant sources used by bees in their production of honey ( Louveaux et al., 1978 ; Ebenezer and Olugbenga, 2010), and is a valid indicator of honey’s origin and purity ( Yoshigaki et al., 2013 ). Taking into consideration that the purpose of the experiment was to study the antibacterial effect of raw, unprocessed chestnut honey, we identified the percentage of chestnut pollen present in each of the chosen samples. We determined which of the tested samples were the closest to pure chestnut honey, assuming that the potential antibacterial activity of chestnut honey is solely connected with the properties of chestnut honey. For counting the pollen grains, we followed the method described by Yoshigaki et al., (2013) in which honey samples were diluted and filtered through plastic membrane filters (ADVANTEC; pore size 8.0μm, Toyo Roshi Kaisha, Ltd.), dyed with gentian violet (Polysciences, Inc.) and counted under a light microscope.
pH of honey samples
For measuring the pH we used a regular pH meter (HORIBA pH Meter F-21) and prepared a 50% (v/v) solution of every honey sample with sterile deionized water.
Agar-well diffusion method
To measure the antibacterial activity of honeys we used agar-well diffusion method, which is a modified version of the disc-diffusion method ( Matuschek et al., 2014 ), in which the clear zones around the well indicated that bacteria have been affected by antimicrobial agents. For reviving and cultivating B. subtilis, S. aureus, E. coli, and P. aeruginosa, we followed specific steps prescribed by the NBRC, and for agar-well tests with these bacteria, we used a medium made up of 10g of polypepton, 2g of yeast extract, 1g of MgSO 4·7H 2O, and 15g of agar dissolved in 1L of distilled, deionized water. For P. larvae we prepared J-agar medium (5g of tryptone, 15g of yeast extract, 3g of K 2HPO 4, 100mL of 2% glucose solution, and 15g of agar with 900mL of distilled, deionized water). Bacterial suspensions were adjusted to 10 8 cells/ml and then applied to the agar media. Next, wells in size of 7mm diameter were made in the agar with reverse ends of sterile P1000 tips. The amount of raw and artificial honey used per well was 0.2g. Colonies of B. subtilis, S. aureus, E. coli and P. aeruginosa grew at 35°C in 24h, whereas P. larvae required 48 h at 35°C under a 5% CO 2 atmosphere to form colonies. Three replicates of the experiment were performed and after the incubation, inhibition zone (diameters) around the wells were measured with digital calipers (±0.002mm accuracy).
Statistical analysis
To analyze our results, we used EZR (Easy R) statistical software, which is based on R and R Commander ( Kanda, 2013). We adapted simple statistics such as t-test, regression and analyses of variance.
RESULT
Percentage of chestnut pollen in honey
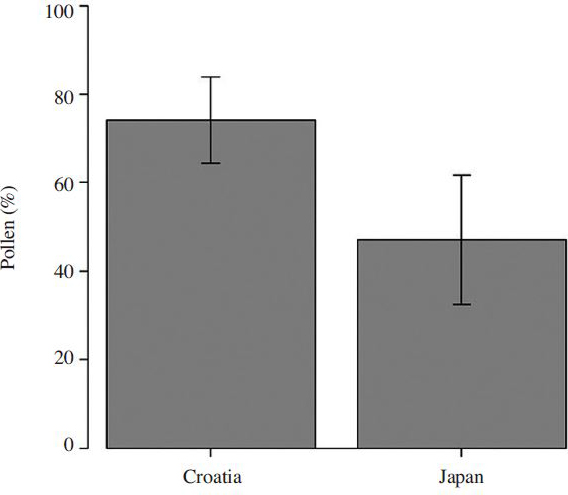
After staining and observing pollen grains through a microscope ( Fig. 1), we differentiated chestnut pollen by its shape and size ( Mert and Soylu, 2007) and determined the percentage of chestnut pollen per sample. Pollen count and percentage of chestnut pollen varied with each chestnut honey sample, and some samples contained larger amounts of chestnut pollen (Table 1). Also, group of chestnut honeys from Croatia had a significantly higher percentage of chestnut pollen ( Fig. 2; p<0.01) than the Japanese group. Samples that were defined as non-chestnut honeys did not contain any chestnut pollen. Considering that beekeepers tend to label their honeys based on their subjective decisions (smell and color of honey, observing the honey bees visiting chestnut trees, etc.) they cannot tell how much percentage of chestnut pollen, and honey, is actually present per sample. Due to those variables, we marked those “chestnut honeys” in our experiment with quotations, although they do not represent the purest samples of their kind. We decided to keep their nomenclature as chestnut honeys, since that is the name by which they are commercially present on the market.

View of pollen grains after filtration and staining with 0.01% gentian violet. (a) Elongated oval shape of chestnut pollen grains. (b) Various shapes of other pollen grains present in the sample along with unidentified particles.
Antibacterial potency of chestnut honey
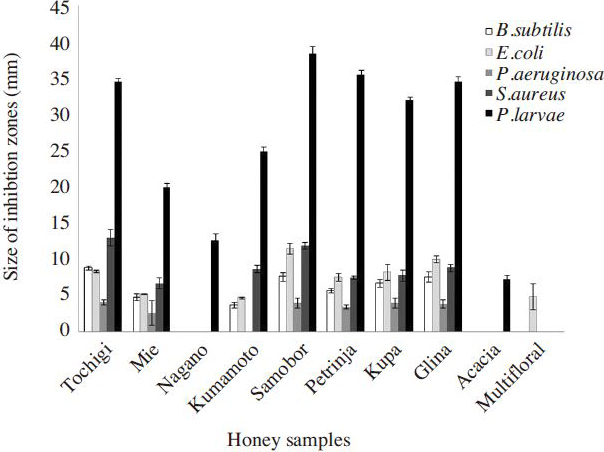
All chestnut honey showed high antibacterial potency against P. larvae ( Fig. 3). Chestnut honey also inhibited the growth of other tested bacteria ( Fig. 4), but the antibacterial potency against other bacteria’s was significantly weaker than one against P. larvae. Acacia honey managed to inhibit the growth of P. larvae, but the potency was not as high as chestnuts honey. Artificial honey did not show any antibacterial potency against any tested bacteria species.
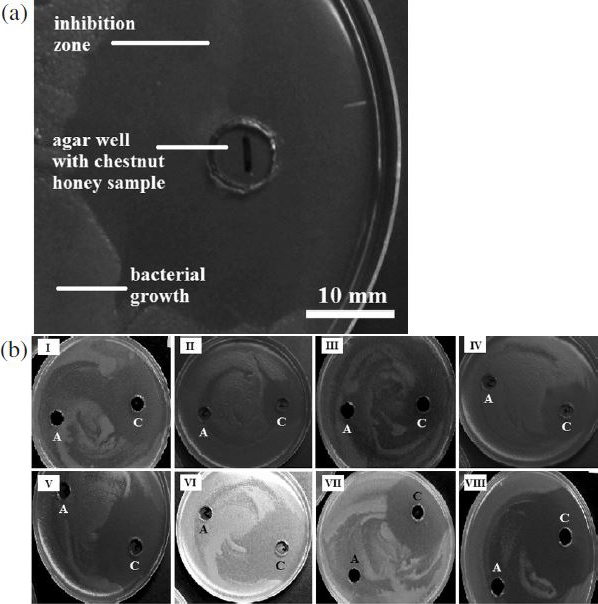
(a) Inhibition zone of P. larvae after a 2-day incubation with chestnut honey. (b) Antibacterial activity of chestnut honey samples from Japan (I-IV) and Croatia (V-VIII) against P. larvae; A-artificial honey, C-chestnut honey.
Differences in honey sample activity
The effectiveness of chestnut honey was shown in Fig. 5a. We discovered that the antibacterial activity of chestnut honey was significantly stronger than the antibacterial activity of non-chestnut honeys ( t-test; p<0.01).
Within the chestnut group, certain chestnut honey samples showed noticeably better activity than the others. After comparing the Japanese and Croatian groups, we concluded that the Croatian group was more effective than the Japanese group in inhibiting the growth of P. larvae ( Fig. 5b; t-test; p<0.05).
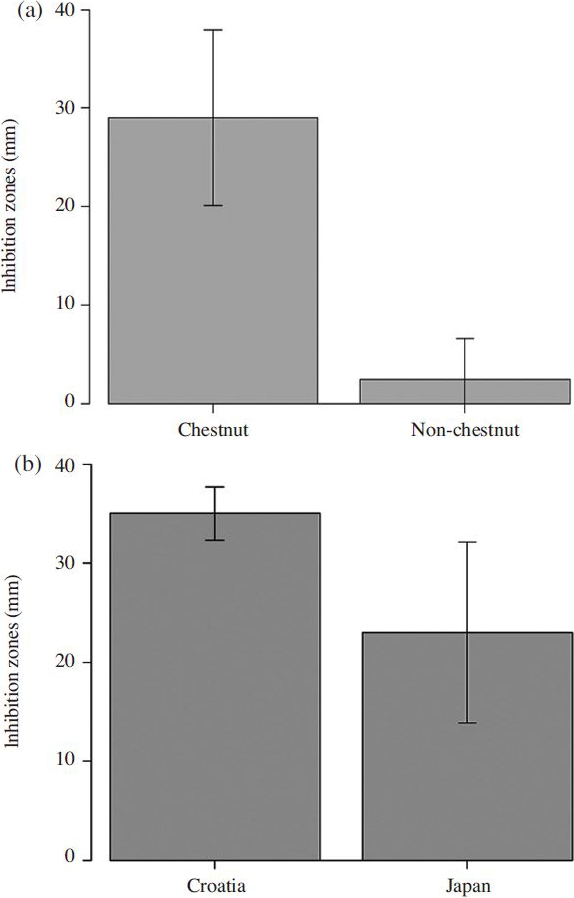
(a) Antibacterial activity of chestnut and non-chestnut honeys against P. larvae ( p<0.01). Bars represent the standard error of means of twelve measurements. (b) Antibacterial activity of two chestnut honey groups against P. larvae ( p<0.05). Bars represent the standard error of means of all measurements.
Antibacterial activity and pollen percentage
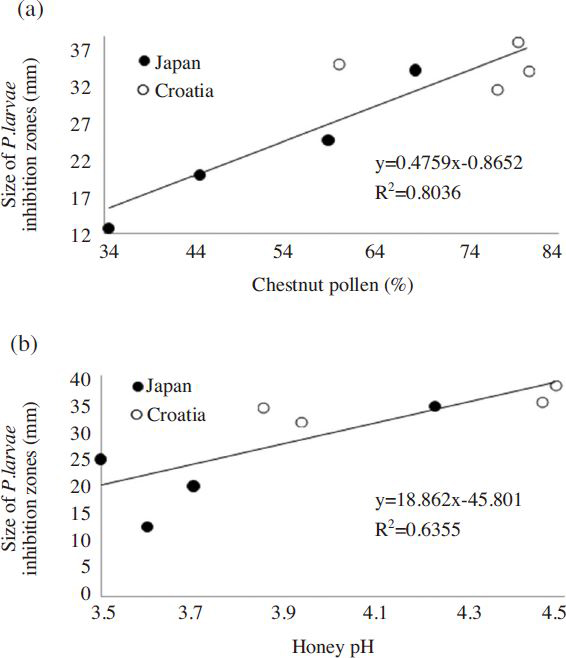
Higher amounts of chestnut pollen per sample caused wider inhibition zones ( Fig. 6a). In our experiment, the purity of chestnut honey was explained by the percentage of pollen counts, which defined the proportion of chestnut honey present in the total amount of each tested sample. By using linear regression, we investigated the connection between antibacterial activity and purity ( R 2 =80.36%, p<0.05). Purity of samples significantly affected the potency, and was in positively correlated with antibacterial activity.
Antibacterial activity and pH
We found that the antibacterial activity was significantly positively correlated with the honey’s pH ( Fig. 6b; R 2 =63.55%, p<0.01). On the other hand, pH did not show any correlation with the number of chestnut pollen ( R 2 =26.65%)
DISCUSSION
This experiment showed that raw chestnut honey possessed antibacterial activity which distinguished it from other honeys, even though the sample size was limited. We also established that its purity was connected with its antibacterial activity. Artificial honey did not express any antibacterial activity; therefore, we concluded that the sugar content of honey does not influence the sizes of the inhibition zones. We observed positive correlation between the pH of the honey and the size of the inhibition zones, showing that pH also played a certain role; however, pollen percentage was not correlated with pH.
Previous research has shown that dark-colored honeys possess high contents of pigments that could also be linked to their strong antibacterial effect ( Taormina et al., 2001 ). Amber is the second darkest honey in the category of seven colors ( http://eccentricbeekeeper.com), and since chestnut honey is also a dark-colored honey, some of its properties may be due to its levels of plant pigments. We could not check the antibacterial activity of other dark-colored honeys, however it is important to perform such survey in the future. Also, it would be interesting to investigate the antibacterial activity of various pigments in honey.
Our results showed that antibacterial activity of chestnut honey positively correlated to the pH. Since honey’s antibacterial potency is generally connected to its overall content of hydrogen peroxide, a compound responsible for influencing the pH of the solutions, antibacterial activity of chestnut honey may just be related to the concentration of that particular compound. Furthermore, antibacterial component of mãnuka honey for example, appears to be methylglyoxal. Therefore, chestnut honey may contain some specific, yet un-surveyed antibacterial components.
Japanese and Croatian chestnut honeys did not have the same antibacterial effect. Numbers of chestnut pollen grains between Japanese and Croatian honeys were significantly different ( Fig. 2, Fig. 6a). Therefore, we could assume that purity of chestnut honey influenced the difference. Moreover, it is possible that this difference may be related to distinctiveness in floral species. In Europe, chestnut honey is mostly produced from Castanea sativa tree, and those were the honey samples we used from the Croatian group. In Japan, Castanea crenata is the source of chestnut honey. Thus, the botanical background of those honeys might have contributed to the variety of their activity. Lastly, different countries have also different ways of processing the honey after it is harvested, and that may also be the cause of occurred divergence.
In this research, we examined the sensitivity of P. larvae to different types of chestnut honey during its growth phase of P. larvae. However, since it is known that the honey bees only contract AFB when bee larvae swallow P. larvae spores ( Neundorf et al., 2004 ), it would be interesting to test the susceptibility of P. larvae spores to antibacterial substances with a similar experiment. If results obtained from that type of experiment were able to confirm the connection between chestnut honey and its inhibitory activity against P. larvae, a diet based on a chestnut floral source could be developed as a possible means of defense against AFB. For example, in migratory beekeeping, beekeepers may transfer their hives into chestnut orchard in the flowering season, for prevention purpose.
AFB is a highly contagious disease of bees that can be transmitted within and between colonies ( Fries and Camazine, 2001). Although some countries still resort to the use of antibiotics in the fight against AFB, the use of antibiotics for this purpose is prohibited in European countries. Therefore, finding and developing alternative strategies and methods of treatment has become crucial in beekeeping. Since the present chestnut honey experiment was, to the best of our knowledge, the first of its kind, the findings from this survey will be of benefit as a basis for more detailed research in the future, and will contribute to the general battle against AFB. In addition, to elucidate the reasons of antibacterial activity of chestnut honey should be proceeded.
ACKNOWLEDGEMENTS
We thank the beekeepers of Japan and Croatia who generously provided us with their chestnut honey samples. Also, we thank Mrs. Ayumi Nakamura for extracting and offering her assistance with multifloral honey from Tsukuba.
LITERATURE CITED
-
Alaux, C., C. Dantec, H. Parrinello, and Y. Le Conte, (2011), Nutrigenomics in honey bees: digital gene expression analysis of pollen’s
nutritive effects on healthy and varroa-parasitized bees
, BMC Genomics, 12, p496.
[https://doi.org/10.1186/1471-2164-12-496]

- Alippi, A. M., (2000), Is Terramycin R losing its effectiveness against AFB? The Argentinian experience , Bee Biz, 11, p27-29.
-
Al-Mamary, M., A. Al-Meeri, and M. Al-Habori, (2002), Antioxidant activities and total phenolics of different types of honey
, Nutr. Res, 22, p1041-1047.
[https://doi.org/10.1016/S0271-5317(02)00406-2]

-
Bang, L.M., C. Buntting, and P. Molan P., (2003), The effect of dilution on the rate of hydrogen peroxide production in honey and its
implications for wound healing
, J. Altern. Complement Med, 9, p267-273.
[https://doi.org/10.1089/10755530360623383]

-
Basualdo, C., V. Sgroy, M. S. Finola, and J. M. Marioli, (2007), Comparison of the antibacterial activity of honey from different provenance against
bacteria usually isolated from skin wounds
, Vet. Microbiol, 124, p375-381.
[https://doi.org/10.1016/j.vetmic.2007.04.039]

-
Cooper, R.A., P. C. Molan, and K. G. Harding, (2002), The sensitivity to honey of Gram-positive cocci of clinical significance isolated
from wounds
, J. Appl. Microbiol, 93, p857-863.
[https://doi.org/10.1046/j.1365-2672.2002.01761.x]

- Ebenezer, I. O., M. T. Olugbenga, (2010), Pollen 17. Characterization of honey samples from North Central Nigeria , J. Biol. Sci, 10, p43-47.
-
Efem, S. E., (1988), Clinical observations on the wound healing properties of honey, Br. J. Surg, 75, p679-681.
[https://doi.org/10.1002/bjs.1800750718]

- Evans, J. D., (2003), Diverse origins of tetracycline resistance in the honeybee bacterial pathogen Paenibacillus larvae, J. Invertebr. Pathol, 83, p46-50.
-
Frankel, S., G. E. Robinson, and M. R. Berenbaum, (1998), Antioxidant capacity and correlated characteristics of 14 unifloral honeys
, J. Apic. Res, 37, p27-31.
[https://doi.org/10.1080/00218839.1998.11100951]

-
Fries, I., and S. Camazine, (2001), Implications of horizontal and vertical pathogen transmission for honey bee
epidemiology
, Apidologie, 32, p199-214.
[https://doi.org/10.1051/apido:2001122]

-
Genersch, E., (2010), American Foulbrood in honeybees and its causative agent,
Paenibacillus larvae, J. Invertebr. Pathol, 103, pS10-S19.
[https://doi.org/10.1016/j.jip.2009.06.015]

-
Gheldof, N., X. H. Wang, and N. J. Engeseth, (2002), Identification and quantification of antioxidant components of honeys from various
floral sources
, J. Agric. Food Chem, 50, p5870-5877.
[https://doi.org/10.1021/jf0256135]

- Kanda, Y., (2013), Investigation of the freely available easy-touse software ‘EZR’ for medical statistics , Bone Marrow Transplant, 48, p452-458.
-
Khan, F. R., Z. Ul Abadin, and N. Rauf, (2007), Honey: nutritional and medicinal value, Int. J. Clin. Pract, 61, p1705-1707.
[https://doi.org/10.1111/j.1742-1241.2007.01417.x]

-
Kwakman, P. H., A. A. te Velde, L. de Boer, D. Speijer, C. M. Vandenbroucke-Grauls, and S. A. Zaat, (2010), How honey kills bacteria, FASEB. J, 24, p2576-2582.
[https://doi.org/10.1096/fj.09-150789]

-
Lauro, F. M., M. Favaretto, L. Covolo, M. Rassu, and G. Bertoloni, (2003), Rapid detection of Paenibacillus larvae from honey and hive samples with a novel
nested PCR protocol
, Int. J. Food Microbiol, 81, p195-201.
[https://doi.org/10.1016/S0168-1605(02)00257-X]

-
Levy, S. B., and B. Marshall, (2004), Antibacterial resistance worldwide: causes, challenges and responses, Nat. Med, 10, pS122-S129.
[https://doi.org/10.1038/nm1145]

-
Louveaux, J., A. Maurizio, and G. Vorwohl, (1978), Methods of melissopalynology, Bee World, 59, p139-153.
[https://doi.org/10.1080/0005772X.1978.11097714]

-
Marcucci, M. C., (1995), Propolis: chemical composition, biological properties and therapeutic activity
, Apidologie, 26, p83-99.
[https://doi.org/10.1051/apido:19950202]

- Matheson, A., and M. Reid, (1992a), Strategies for the prevention and control of American foulbrood. Part I , Am. Bee J, 132, p399-402.
- Matheson, A., and M. Reid, (1992b), Strategies for the prevention and control of American foulbrood. Part II , Am. Bee J, 133, p471-475.
- Matheson, A., and M. Reid, (1992c), Strategies for the prevention and control of American foulbrood. Part III , Am. Bee J, 143, p534-547.
-
Matuschek, E., D. F. J. Brown, and G. Kahlmeter, (2014), Development of the EUCAST disk diffusion susceptibility testing method and its
implementation in routine microbiology laboratories
, Clin Microbiol. Infect, 20, p0255-0266.
[https://doi.org/10.1111/1469-0691.12373]

- Mert, C., and A. Soylu, (2007), Morphology and anatomy of pollen grains from male-fertile and male-sterile cultivars of chestnut ( Castanea sativa Mill) , J. Hortic. Sci. Biotechnol, 82, p474-480.
- Molan, P. C., (1992a), The antibacterial activity of honey. 1. The nature of antibacterial activity , Bee World, 73, p5-28.
- Molan, P. C., (1992b), The antibacterial activity of honey. 2. Variation in the potency of the antibacterial activity , Bee World, 73, p59-76.
- Morse, R. A., and N. W. Calderone, (2000), The value of honey bees pollination in the United States, Bee Cult, 128, p1-15.
-
Neundorf, S., K. Hedtke, G. Tangen, and E. Genersch, (2004), Biochemical characterization of different genotypes of
Paenibacillus larvae
subsp.
larvae, a honey bee bacterial pathogen
, Microbiology, 150, p2381-2390.
[https://doi.org/10.1099/mic.0.27125-0]

-
Snowdon, J. A., and D. O. Cliver, (1996), Microorganisms in honey, Int. J. Food Microbiol, 31, p1-26.
[https://doi.org/10.1016/0168-1605(96)00970-1]

-
Taormina, P. J., B. A. Niemira, and L. R. Beuchat, (2001), Inhibitory activity of honey against foodborne pathogens as influenced by the
presence of hydrogen peroxide and level of antioxidant power
, Int. J. Food Microbiol, 69, p217-225.
[https://doi.org/10.1016/S0168-1605(01)00505-0]

- Vallianou, N. G., P. Gounari, A. Skourtis, J. Panagos, and C. Kazazis, (2014), Honey and its anti-inflammatory, antibacterial and anti-oxidant properties , Gen. Med. (Los Angel), 2, p132.
- Yoshigaki, S., Y. Komagata, J. Nakamura, O. Uchida, T. Nagashima, and T. Ando, (2013), A comparison of pollen analysis and trace element analysis of honey, J. Jpn. Health Med. Assoc, 21, p277-282, (in Japanese with English summary).
- Zumla, A., and A. Lulat, (1989), Honey-a remedy rediscovered, J. R. Soc. Med, 82, p384-385.