Biological Mitigation of Sacbrood Disease on Apis cerana Colonies
Apis cerana is native honey bee species in Korea. This honey bee species has well adapted to cold winter. Apis cerana honey is preferred by domestic consumers. However, this honey bee species has been declined by sacbrood disease. Sacbrood is caused by a virus. Until now there has not been any chemical to treat this disease except biological method and the selection of sacbrood resistant bee stocks. This research was conducted from June to August, 2014 at the A. cerana apiary of NAAS, Suwon, Republic of Korea. Sacbrood disease on A. cerana colonies was observed and biological method was applied to treat the disease. The results of our study showed that the rate of infected colonies was from 26.0% to 87.0%. By applying requeen measure, the rate of recovered colonies was 57.1%, while the method of caged queen got only 28.6%.
Keywords:
Apis cerana, Sacbrood, Requeen, Caged queen, KoreaINTRODUCTION
Korean Apis cerana is a gentle, less absconding and resistant to cold winter (Thapa et al., 2012). A. cerana honey is greatly valued by the Koreans, and its price is up to four times that of A. mellifera honey (Camazine, 1989). However, this honey bee species has been rapidly declined by sacbrood disease. Sacbrood is an infectious disease of the brood of bee caused by a virus (White, 1917; Bailey et al., 1964). Sacbrood virus (SBV) is one of many insect viruses generally referred to as picornavirus-like and was the fist honeybee virus to be completely sequenced (Grabensteiner et al., 2001). At the present 4 SBV strains are identified including SBV on A. mellifera (AmSBV) (Bailey et al., 1964), SBV Thailand on A. cerana (AcTSBV), SBV China on A. cerana (AcCSBV) (Bailey, 1982; Zhang et al., 2001, Rana and Rana, 2007) and SBV Korea on A. cerana (AcSBV-Kor) (Lee et al., 2010). SBV primarily affects the brood of A. mellifera, and A. cerana resulting in perforation of sealed brood pre-pupal death due to failure in pupation (Chinh, 2008; Rana et al., 2010). SBV infection is large and can outbreak epidemic (Borchert, 1970; Bailey, 1969). SBV epidemic killed 90% A. cerana colonies in Viet Nam from 1974-1978 (Chinh, 1990). In India more than 95% of the colonies were killed by TSBV (Rana et al., 1986). In Korea sacbrood disease developed and broken out epidemic, especially serious in 2010 (Choe et al., 2012). In the field sacbrood disease can be reliably diagnosed by the characteristic symptoms produced in developing brood of both A. mellifera and A. cerana (Ball, 1999b). According to Chinh (1990) sacbrood disease on A. cerana colonies can be diagnosed base on typical sacbrood disease symptom. In the laboratory SBV diagnosis is based on traditional methods such as using electronic microcopy serology, immunology. Recently years, multiplex RT-PCR/RFLP technique has been effectively applied to diagnose early SBV infection (Trung et al., 2010). At present there is no medicine to cure sacbrood disease (Trung et al., 2010). In this paper we conducted to observe the situation of sacbrood disease on colonies at the A. cerana apiary in NAAS and control the disease by using biological method.
MATERIALS AND METHODS
Clinical diagnosis
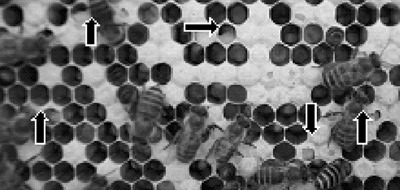
The studies were conducted from June to August, 2014. An A. cerana apiary of 33 colonies in total was established at the apiary of NAAS, Suwon, Republic of Korea. The colonies were observed and inspected weekly. SBV diseased colonies were diagnosed by expression with typical symptoms (Fig. 1) and (Fig. 2) (Chinh, 1990).
Symptoms of sacbrood disease on the surface of A. cerana brood comb. There were many pointed broods emergeing and cover of the cell was tattered.
The numbers of diseased colonies was recorded.
SBV treatment
Biological method was applied to treat sacbrood disease on A. cerana colonies. The principle of this method is to make the colony broodless for 7-8 days particularly 2-day-old larvae, because at this age larvae are most susceptible to the virus (Bailey, 1981). A total of 14 diseased colonies including 4 heavy infected colonies (Fig. 3) and 10 light infected colonies (Fig. 4) were equilibrated by exchanging the combs each other. Each colony had 3 combs kept in a modal hive.
Then diseased colonies were divided in to two groups, 7 colonies of each. Queen in the colony belonging to groups 1 was replaced by a queen cell reared from healthy colony collected in Cheon An province (Fig. 5).
In groups 2, queen was caged for 8 days by using a plastic cage containing sugar powder and 5 young worker bees (Fig. 6). This queen was reared from the colony collecting in Cheon An province in May.
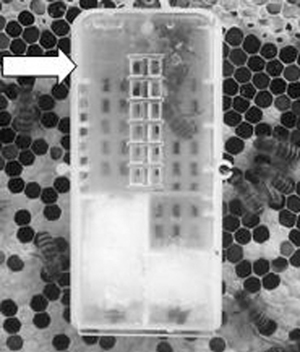
Queen in the diseased colony was caged by a plastic cage containing sugar powder and 5 young worker bees.
Every week colonies in both groups were continuously fed for three times with 400ml sugar syrup/colony/time, ratio of sugar/water is 2.5/3 (w/v).
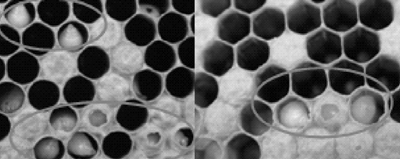
Re-infected colonies and collapsed colonies were recorded at the time of 3 weeks, 5 weeks and 7 weeks post treatment. Re-infected colonies were characterized by typical sacbrood symptom. Recovered colonies were indicated by the presence of sealed brood combs (Fig. 7) and the number of worker combs remained in the colony.
Collapsed colonies were characterized by the combs containing a lot of empty cells and the cells with dead larvae. The population of the colony dwindled down (Fig. 8).
RESULTS AND DISCUSSION
The development and spread of sacbrood disease on A. cerana colonies
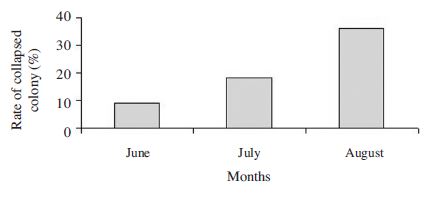
Fig. 9 demonstrates the development and spread of sacbrood disease on colonies at the A. cerana apiary of NAAS. The observations on 33 A. cerana colonies showed that there were SBV infected colonies in all the observed months.

The infection rate with sacbrood disease on the colonies was observed over the months from June to August, 2014 at the A. cerana apiary. Each bar represented the average rate of infected colonies for 4 times observations in a month.
The rate of infected colonies in June was 26.0%. This rate increased rapidly on July with 78.1% of infected colonies and remained at high infection rate in August (87.0%). Our results demonstrate that sacbrood disease spread very quickly and remained with high infection rate on A. cerana colonies during months of summer season. Sacbrood occurs most frequently in spring when the colony is growing most rapidly and large numbers of susceptible larvae and young adults are available (Bailey, 1969). Under natural circumstances sacbrood abates and usually disappears spontaneously during summer even though larvae are easily infected by feeding them the virus at any time of the year (Ball, 1999). However, the infection of sacbrood disease on honey bee colonies was different between the years (Borchet, 1970; Chinh, 2008). The present experiment colonies were established at the end of May. Therefore, colonies produced rapidly a large quantity of larvae in June. This may be responsible for causing outbreak of sacbrood disease on experiment colonies in the next months. It suggests that the number of larvae, especially young larvae in the colony plays an important role in the development, spread and outbreak of this disease.
The effect of disease
Fig. 10 demonstrates the rate of collapsed colonies by sacbrood disease. In June, the number of collapsed colonies was 3 colonies (9.1%) in total of 33 observed colonies. In July and August, those were 6 colonies (18.1%) and 12 colonies (36.4%) respectively. In the infected colonies sacbrood virus killed old larvae and pre-pupae stage and leads to a general weakening of the colony and colony collapse (Ball, 1999b; Chinh, 1990). According to Bailey (1981) the larval liquid containing 1mg virus can infect whole larvae of 1000 healthy colonies. A comparatively small number of sacbrood germs ingested by a larva of two day old is sufficient to produce the disease (White, 1917). Epidemic sacbrood disease caused seriously colonies loss for beekeeping with honey bee A. cerana. In Viet Nam, 70,000 A. cerana colonies (90%) in total 74,000 colonies were killed by sacbrood disease from 1974-1978 (Anh, 1983; Chinh, 1990). In Mahabaleshwar, India this virus infected the brood of A. cerana and caused destruction of 21 colonies out of a total of 44 colonies in summer of 2008 (Deshpande and Chaphalkar, 2013).
Control sacbrood disease by biological technique
Table 1 shows the effect of caging queen and requeen measure on controlling sacbrood disease on A. cerana colonies. There was difference on the rate of re-infected colonies by using requeen and caged queen measure at the time of 3 weeks and 5 weeks post treatment. For requeen and caged queen measure at the time of 3 weeks post treatment this rate was 14.3% and 71.4% respectively. After 5 weeks treatment the rate of re-infected colonies by using requeen measure was 42.9% whereas, of this by using caged queen measure was 100%. However, there were 100% of re-infected colonies after 7 weeks treatment with both requeen and caged queen measure.
Currently, there is no chemical to treat sacbrood disease so requeen and caged queen measure are being applied to treat this disease. According to Chinh (1990) there was 90% of diseased colonies have not been re-infected by replacing queen with a virgin queen but most of those by caging queen were re-infected after a new worker generation. The effect of cage queen measure to treat sacbrood disease on A. cerana in this study is rather similar to those reported by Chinh (1990). However, the effect of requeen measure on treating sacbrood disease is different. This may be due to virgin queen for replacing queen in diseased colony on studies conducted by Chinh (1990) has been selected. While virgin queen for replacing the queen in diseased colony on our studies has not been selected yet. The results of studies on sacbrood disease conducted by Verma et al., (1990) suggested that some mechanism of resistance to Thai sacbrood virus exists in A. cerana.
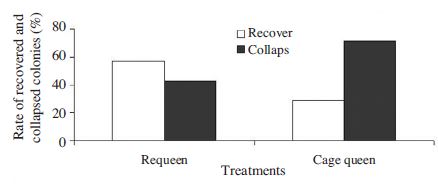
Fig. 11 demonstrates the rate of recovered and collapsed colonies after 7 weeks treatment. The rate of recovered colony by using requeen measure was 57.1%. Meanwhile of this by using cage queen measure was only 28.6%. Our results suggest that by using requeen measure to treat sacbrood disease although there was 100% of re-infected colonies, 57% of diseased colonies were able to recover. This is probably because there were more adult worker bees in the re-queened colonies in compared to caged queen ones. These worker bees quickly detected and removed diseased larvae from the colony.
Acknowledgments
This research was supported from Rural Development Administration, Republic of Korea (No. PJ0086222). We would like to thank technicians in the Honey bee Laboratory of Department of Agricultural Biology, NAAS, RDA for their kind helps to our research.
References
- Anh, M., (1983), Prevention methods of honey bee larvae diseases in Viet Nam, the beekeeping Congress 2 Viet Nam 1983.
- Bailey, L., (1981), Honeybee pathology, p124, Academic Press, Inc., USA.
-
Bailey, L., (1969), The multiplication and spread of sacbrood virus of bees, Ann. Appl. Biol, 63, p483-491.
[https://doi.org/10.1111/j.1744-7348.1969.tb02844.x]

- Bailey, L., A.J. Gibbs, R.D. Woods, (1964), Sacbrood virus of the larvae of honeybee (Apis mellifera L.), Virology, 23, p425-429.
- Ball, B.V., (1999b), Sacbrood, p91-97, in Bee disease diagnosis, eds. by M.E. Colin, B.V. Ball, M. Kilani. Options Mésditerranéennes: Series B: Etudes et Researchers. N. 25 CIHEAM publication, Zaragoza, Espana.
- Borchert, A., (1970), Disease and parasites on bees, p425, Agricultural Publishing House, Hanoi.
- Camazine, S., (1989), Beekeeping in South Korea, Bee World, 70, p66-74.
- Chinh, P.H., (2008), Sacbrood disease on exotic honey bee A. mellifera in Viet Nam, Journal of Agriculture and Rural Development, 1, p2-5.
- Chinh, P.H., (1990), A disease of bee larvae A, cerana, p24, Agricultural Publishing House, Hanoi.
-
Choe, S.E., L.K.T. Nguyen, J.H. Noh, C.H. Kweon, K.E. Reddy, H.B. Koh, K.Y. Chang, S.W. Kang, (2012), Analysis of the complete genome sequence of two Korean sacbrood viruses in the honey bee, Apis mellifera, Virology, 432, p155-161.
[https://doi.org/10.1016/j.virol.2012.06.008]

- Deshpande, M.T., and R.C. Chaphalkar, (2013), Antiviral activity of plant extracts against sacbrood virus in vitro - a preliminary report, International Journal of Institutional Pharmacy and Life Sciences, 3, p1-15.
- Grabensteiner, E., W. Ritter, M.J. Carter, S. Davison, H. Pechhacker, J. Kolodziejek, O. Boecking, I. Derakhshifar, R. Moosbeckhofer, E. Licek, N. Nowotny, (2001), Sacbrood virus of the honeybee (Apis mellifera): rapid identification and phylogenetic analysis using reverse bee virus and sacbrood virus in the honey bee (Apis mellifera), Ann. Appl. Biol, 114, p1-7.
- Lee, C.H., S.E. Choe, B.H. Hyun, S.W. Kang, S.C. Jung, J.Y. Song, (2010), GenBank accession number HQ322114.
- Rana, R., and B.S. Rana, (2007), Molecular characterization of Thai sacbrood virus of honey bee Apis cerana, Sequences directly submitted to Gene Bank.
- Rana, B.S., I.D. Garg, K.S.M. Paul, L.R. Verma, H.O. Agrawal, (1986), Thai sacbrood virus of honeybees (Apis cerana indica F) in North- west Himalayas, Indian J. Virol, 2, p127-131.
- Thapa, R., P. Kongpitak, M.L. Lee, and Y.S. Choi, (2012), Recognition of sacbrood viral disease of Apis cerana F, Kor. J. Apic, 27, p201-207.
- Trung, Q.L., D.Q. Tam, T.V. Toan, T.A. Tuan, T.T. Phuong, D.T. The, P.H. Chinh, (2010), Application Multiplex RT-PCR/RFLP for early detection and genetics relationship research of sacbrood viruses on honey bees in Viet Nam, Journal of Agriculture and Rural Development, 1, p34-40.
- Verma, L.R., B.S. Rana, S. Verma, (1990), Observations on Apis cerana colonies surviving from Thai sacbrood virus infestation Apidologie, 21, p169-174.
- White, G.F., (1917), Sacbrood, United States Department of Agriculture, Bulletin No. 341.
-
Zhang, J., J. Feng, Y. Liang, D. Chen, Z.H. Zhou, Q. Zhang, X. Lu, (2001), Three-dimensional structure of the Chinese sacbrood bee virus, Science in China, 44, p443-449.
[https://doi.org/10.1007/BF02879612]