Supercooling Points (SCPs) of Social Hymenopterans, Apis mellifera (Hymenoptera: Apidae) and Vespa velutina (Hymenoptera: Vespidae)
Abstract
Apis mellifera (Hymenoptera: Apidae) is an important pollinator but being threatened by Vespa velutina (Hymenoptera: Vespidae), an invasive predator in South Korea. Supercooling point (SCP) is one of indicators of cold tolerance but SCP has not studied for both A. mellifera and V. velutina. Morphological factors like body traits influence fitness of insects in terms of their thermal tolerance. Therefore, we determined SCPs of honey bee, A. mellifera workers, and wasps, V. velutina workers and V. velutina queens, and analyzed the influence of body traits; body size and body mass on SCPs of these species. This is the first report of SCPs of V. velutina queen and worker. The SCPs of A. mellifera workers, V. velutina workers, and V. velutina queens were -6.1, -6.5, and -11.3°C, respectively. Both A. mellifera and V. velutina have unimodal distribution of their SCPs. Neither body size nor body mass influenced the SCPs of A. mellifera and V. velutina. This study showed that SCPs of social insects, A. mellifera and V. velutina were much lower than those of solitary overwintering insects, and differed relative to the caste of the same species.
Keywords:
Overwinter, Cold tolerance, Caste, Body traits, InvasivenessINTRODUCTION
Temperature is one of the influential factors for development and reproduction of ectothermic invertebrates such as insects (Noor-ul-Ane and Jung, 2020), affects seasonal and geographical distribution. Ectothermic insects have colonized regions that are seasonally or perpetually cold, and must cope with temperatures that fall substantially below 0°C (Costanzo and Lee, 2013). Survival during the extreme temperature condition is crucial for sustained existence and proliferation (Teets and Denlinger, 2013). For these cold-hardy organisms, capacity for cold tolerance is tuned to the temperatures and exposure durations that a given species encounters within its habitat (Addo-Bediako et al., 2000; Noor-ul-Ane and Jung, 2021). Survival under low temperature depends on either freeze avoidance through supercooling or freeze tolerance (Sinclair et al., 2003). Supercooling is a metastable state in which body fluids remain liquid below the subzero condition, promoted by increased hemolymph cryprotectants such as glycerol or other antifreeze proteins which protect against chilling injury or remove inoculative freezing ice-nucleating agents (Duman et al., 1991). Freeze tolerance is the strategy allowing survival even with part of the body frozen, but managing tissue and cell from frost damage. Freeze tolerant insects could initiate the freezing at relatively high temperatures through inoculative freezing by ice nucleating proteins (Sinclair et al., 2003; Jakobs et al., 2015; Teets et al., 2020). In temperate regions of the northern hemisphere where the cold comes seasonally for longer periods, the main strategy is freeze avoidance while in the southern, and freeze tolerance is also more prevalent in insects (reported in 85% of species studied; Sinclair et al., 2003).
Ants, bees, termites and wasps are social insects which constitute most of terrestrial habitats (Holldobler and Wilson, 1990). Social insects not only regulate their own body temperature but also for their nest mates, whereas solitary insects only responsible to regulate their own body temperature (Kadochová and Frouz, 2013). Honey bees, plant pollinators, are well known for their contribution in the yield production of the different crops (Jung, 2008; Begna et al., 2020). Winter survival is a challenging aspect of Apis mellifera in temperate regions. A. mellifera survives in winter by forming a cluster and maintaining temperature around 20℃ (Southwick, 1985). Cold temperature may challenge A. mellifera workers during autumn for their foraging and defense purpose. Cold tolerance of sub-species of honey bees has been studied (Li et al., 2012; Qin et al., 2019) but there is no report from Korea. Vespa velutina nigrithorax, an invasive predator of honey bees, has been established in South Korea since 2003 (Park and Jung, 2016). All casts of V. velutina dies in winter except mated queens which overwinters in the soil or tree crevices (Monceau et al., 2014) but there is no report of cold tolerance of V. velutina.
Body size is also another fitness trait in organisms including insects, usually larger individual benefitted more in terms of their survival, fecundity and mating success (Honek, 1993; Andersson, 1994) subsequently contributing for ecosystem functioning (Enquist et al., 2003). Stress resistance is also more evident in larger insects (Hood and Tschinkel, 1990). Larger insects show more desiccation resistance and foraging time (Kaspari, 1993) within and between species (Chown et al., 1999). Starvation is also another stress which helps larger insects for their survival as they have more energy reservoirs or lower metabolic activities (Harshman and Schmid, 1998). Body size also plays an important role in thermoregulation of organisms (Schmidt-Nielsen, 1997) including insects both in cold tolerance and heat tolerance (Baudier et al., 2008; Shepherd et al., 2008). Similarly, body mass is also important for ecosystem functioning, as it controls energy uptake and transformation in ectotherms at organismal level and it also link to stress resistance including thermal tolerance of insects (Oyen et al., 2016; Knapp and Řeřicha, 2020).
Underlying the importance of cold tolerance for these species, we determined Supercooling points of A. mellifera workers and V. velutina queen and also determined influence of body size and body mass on SCP within and across these species and then estimated SCPs compared with SCPs of social hymenopterans.
MATERIALS AND METHODS
1. Source of insect
Worker of A. mellifera were collected from winter clusters from beekeepers of Andong city, (36°33′ N 128°44′ E), Gyeongsangbuk-do, South Korea during first week of December, 2020 and maintained (@15 bees/cup) in plastic cups (bottom dia. 9 cm×top dia. 12 cm×height 8 cm, with side ventilation slots), at 25±1℃, 35±5% RH, under total dark condition in an incubator until use in experiment. Pollen patty was provided as a diet for the A. mellifera. Pollen patty diet was composed of rape seed pollen, sugar, soybean flour, yeast and nutrient supplement in 20 : 60 : 20 : 20 : 1 ratio by mass. Adults were also provided 50% sugar-water solution by volume soaked in cotton.
Workers and queens of V. velutina were collected on first week of December, 2020 from vespiculture (a semi-field rearing screen house, established at Andong National University, Andong, Gyeongsangbuk-do, South Korea), identified by mesoscutum width (MW) (Pérezde-Heredia et al., 2017) and maintained individually in above mentioned plastic cups at 4±1℃, 35±5% RH, provided with 50% sugar-water solution under total dark condition in an incubator until used in experiment.
2. Supercooling point determination
We determined SCPs of 17 workers of A. mellifera, and 11 workers and 12 queens of V. velutina. Workers and queens were attached with type-T copper thermocouple (BTM-4208SD, LT Lutron, Taipei, Taiwan) placed individually into 1.5 mL microcentrifuge tubes. Temperature was recorded at 1 second interval. The thermocouple was passed through the bottom of tube by making small hole and attached with body of insects. Four PCR tubes containing individual worker and queen were put in a styrofoam box (30×30×15 cm), which was then placed in a refrigerator at -40℃ until the thermocouples reached a temperature of -30℃. The cooling rate was measured as 0.6℃/minute. Body length was measured by digital caliper and body mass of both species were measured with an electronic balance (0.001 g) (AINSWORTH, US/Model:10) before being used for SCP determination.
3. Statistical Analysis
One-way ANOVA was applied to find out the difference between SCPs of A. mellifera workers, V. velutina workers and V. velutina queens. Linear regression was carried out to establish relationship of body length and body mass with SCP. All the analyses were conducted using SPSS v20 software.
RESULTS AND DISCUSSION
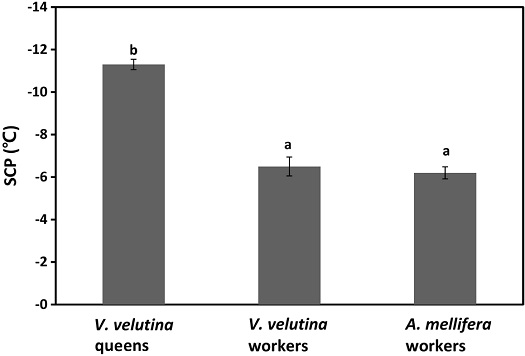
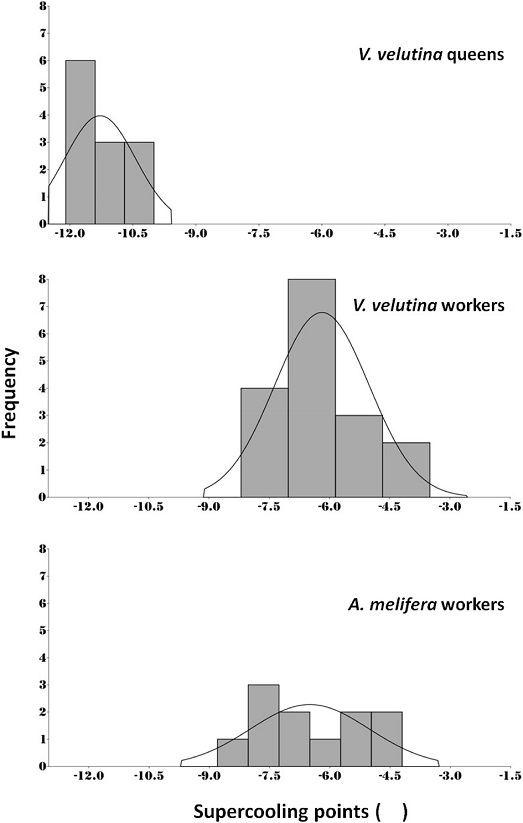
The SCPs of A. mellifera and V. velutina workers were ranged from -3.5 to -8.2℃ and -4.2 to -8.8℃, respectively, with a median value of -6.2 and -6.5℃, respectively (Fig. 1). The SCP of V. velutina queen was -11.3±0.8℃ sentand significantly higher than values of A. mellifera workers and V. velutina (ANOVA, F=74.5, df=2,37, P>0.001). Histogram shows that SCPs of both A. mellifera, V. velutina queen, and V. velutina workers have unimodal distribution (Fig. 2). SCP of A. mellifera of present study is almost similar to its subspecies of A. mellifera ligustica which has SCP of -5.3℃ calculated by Li et al. (2012) and -6.4℃ in December collected workers in another study of Qin et al. (2019). Even though winter honey bees are fully equipped with fat body and higher density of hemolymph proteins, SCPs of winter honey bees were relatively higher since the honey bees overwinter in the winter cluster within the hive in the protected space (Southwick, 1985). But still honey bees are risky and susceptible for freeze dying if winter cluster being broken or exposed to the cold even relatively shorter time associated with parasitic mites or disease (van Dooremalen et al., 2018).
The SCP of V. velutina worker from South Korean population was -6.5℃ which is almost similar to some of other hymenopteran workers of Polistes annularis (-6.8℃) and Bombus terrestris (-7.1℃) (Strassman et al., 1984; Owen et al., 2016). However, SCP of V. velutina queen (-11.3℃), was higher than SCPs of worker V. velutina (F=74.5, df=2,37, P>0.001), also close to SCPs of P. annularis, -12.9℃ and Sirex noctilio, -11.8℃ (Strassman et al., 1984; Li et al., 2019). As queens of V. velutina should overwinter, but not workers, and establish new colonies in the next spring (Monceau et al., 2014), there are physiological differences between workers and queens and that fat body appearance is also a reliable indicator of caste. For P. exclamans, 83% of the future queens survived at the lower conditioning temperature, +5℃ of 15 days exposure while 24% of the workers survived during the same exposure.
SCPs were not influenced by the body size and body mass for both A. mellifera (F=1.5, df=1,19, P=0.2354), and V. velutina worker (F=0.51, df=1,19, P=0.4919), and V. velutina queen (F=0.16, df=1,10, P=0.69554) (Fig. 3). Similarly, body length also did not influence SCPs of both A. mellifera (F=1.04, df=1,19, P=0.3217), V. velutina worker (F=0.23, df=1,11, P=0.6437), and V. velutina queen (F=2.70, df=1,10, P=0.1317) (Fig. 4). There are some observations which showed that body trait such as body size is linked with SCP (Zachariassen, 1985). Larger body size increased supercooling point in someinsects (Sinclair and Chown, 2005). While, no differences were also reported (Knapp and Uhnavá, 2014; Yunik and Chilton, 2021). Yunik and Chilton (2021) reported that the different size and weight of male and female ticks showed no difference of SCPs. We found difference in SCPs between different casts of overwintering queen and non-overwintering worker of Vespa velutina but no difference in SCPs of individuals in the same cast (worker or queen) with different body size. Also overwintering honey bee worker and non-overwintering vespa worker which differ in body size, showed similar SCPs, suggesting workers of V. velutina and A. mellifera may have similar cold tolerance. The study suggested that larger insects do not necessarily have higher SCPs.
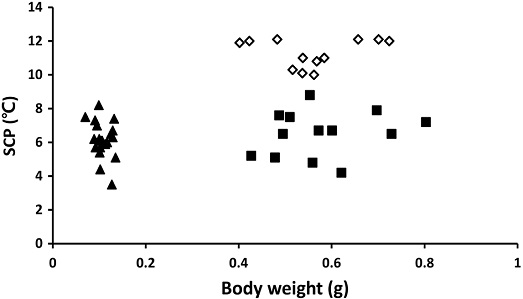
Supercooling points of A. mellifera and V. velutina of different body weights. ◊: V. velutina queens (Y=1.0685x+10.687, R2=0.0160), ▲: V. velutina workers (Y=2.5866x+5.0168, R2=0.0439) and ■: A. mellifera workers (Y= -16.601x+7.9167, R2=0.0733).
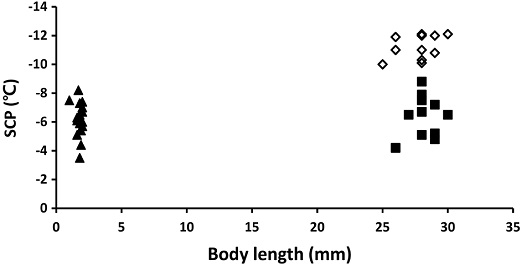
Supercooling points of A. mellifera and V. velutina of different body lengths. ◊: V. velutina queens (Y=0.2483x+4.3517, R2=0.2123), ▲: V. velutina workers (Y=0.1941x+1.0513, R2=0.0201), and ■: A. mellifera workers (Y= -1.0337x+7.9938, R2=0.0517).
There are numerous factors influencing SCPs; cryoprotectant level (Somme, 1982), developmental stage (Bouchard et al., 2006), feeding (Cannon, 1986) apart from body size. The workers of ant, Coptotermes herculeanus, have the lowest SCP (-40℃) followed by a mason bee, Osmia cornuta, (-31℃) (Table 1). The highest SCP of value -4.3 and -6.1℃, belongs to Myrmica angulinodis and A. mellifera, respectively. The queen of paper wasp, “Polistes anularis” showed SCP of value -6.8℃. While, the queens of Vespula pensylvanica showed lower SCP values (-23.3℃). In conclusion, present study shows that SCP of V. velutina queen is lower than V. velutina workers and A. mellifera workers. Body length and body mass did not have any influence on SCP of within and across species of A. mellifera, V. velutina queen, and V. velutina workers.
Acknowledgments
This study was partly supported by the BSRP through the National Research Foundation of Korea (NRF), Ministry of Education (NRF-2018R1A6A1A03024862), and RDA research grant (PJ014761022021).
References
-
Addo-Bediako, A., S. L. Chown and K. J. Gaston. 2000. Thermal tolerance, climatic variability and latitude. Proc. Biol. Sci. 267: 739-745.
[https://doi.org/10.1098/rspb.2000.1065]

-
Baudier, K. M., A. E. Mudd, S. C. Erickson and S. O’Donnell. 2015. Microhabitat and body size effects on heat tolerance: Implications for responses to climate change (army ants: Formicidae, Ecitoninae). J. Anim. Ecol. 84: 1322-1330.
[https://doi.org/10.1111/1365-2656.12388]

-
Begna, T., D. Ulziibayar, M. Noor-ul-Ane, J. H. Shin and C. Jung. 2020. Offering Pollen as Reward Enhances Foraging Activity of Honey Bee, Apis mellifera on Strawberry Greenhouse during Winter Season. J. Apic. 35: 111-118.
[https://doi.org/10.17519/apiculture.2020.06.35.2.111]

-
Berman, D. I., A. N. Leirikh and Z. A. Zhigulskaya. 2012. A Common Strategy of Cold Hardiness in Ants of the Genus Myrmica (Formicidae, Hymenoptera) in Northeast Asia,” Entomol. Rev. 92: 247-261.
[https://doi.org/10.1134/S0013873812030013]

- Berman, D. I., Z. A. Zhigulskava and A. N. Leirikh. 2017. Cold Resistance of an Ant Camponotus herculeanus (Hymenoptera, Formicidae) in Various Climates in North-east Asia. Cryo Letters 38: 17-28.
- Cannon, R. J. C. 1986. Diet and acclimation effects on the cold tolerance and survival of an Antarctic springtail. Brit. Antarct. Surv. Bull. 71: 19-30.
-
Chown, S. L., M. D. Le Lagadec and C. H. Schotz. 1999. Partitioning variance in a physiological trait: desiccation resistance in keratin beetles (Coleoptera, Trogidae). Funct. Ecol. 13: 838-844.
[https://doi.org/10.1046/j.1365-2435.1999.00373.x]

- Corrigan, S. T. and J. Irwin. 2009. Supercool social wasps: lower lethal limits to cold tolerance [Conference presentation abstract]. Annual Meeting of Society of Integr. Comp. Biol. Central Washington University. Retrieved from https://sicb.burkclients.com/meetings/2009/schedule/abstractdetails.php3?id=1320, .
-
Costanzo, J. P. and R. E. Jr Lee. 2013. Avoidance and tolerance of freezing in ectothermic vertebrates. J. Exp. Biol. 216: 1961-1967.
[https://doi.org/10.1242/jeb.070268]

-
Duman, J. G., D. W. Wu, L. Xu, D. Tursman and T. M. Olsen. 1991. Adaptations of insects to subzero temperatures. Q. Rev. Biol. 66: 387-410.
[https://doi.org/10.1086/417337]

-
Enquist, B. J., E. P. Economo, T. E. Huxman, A. P. Allen, D. D. Ignace and J. F. Gillooly. 2003. Scaling metabolism from organisms to ecosystems. Nature 423: 639-642.
[https://doi.org/10.1038/nature01671]

-
Harshman, L. G. and J. L. Schmid. 1998. Evolution of starvation resistance in Drosophila melanogaster: aspects of metabolism and counter-impact selection. Evol. 52: 1679-1685.
[https://doi.org/10.1111/j.1558-5646.1998.tb02247.x]

- Holldobler, D. B. and E. O. Wilson. 1990. The Ants, p. 746. Harvard University Press, Boston.
-
Hood, W. G. and W. R. Tschinkel. 1990. Desiccation resistance in arboreal and terrestrial ants. Physiol. Entomol. 15: 23-35.
[https://doi.org/10.1111/j.1365-3032.1990.tb00489.x]

-
Jakobs, R., T. D. Gariepy and B. J. Sinclair. 2015. Adult plasticity of cold tolerance in a continental-temperate population of Drosophila suzukii. J. Insect Physiol. 79: 1-9.
[https://doi.org/10.1016/j.jinsphys.2015.05.003]

- Jung, C. 2008. Economic Value of Honeybee Pollination on Major Fruit and Vegetable Crop in Korea. Kor. J. Apic. 23: 147-152.
-
Kadochová, S. and J. Frouz. 2013. Thermoregulation strategies in ants in comparison to other social insects, with a focus on red wood ants (Formica rufa). F1000Research 2: 280.
[https://doi.org/10.12688/f1000research.2-280.v1]

-
Kaspari, M. 1993. Body size and microclimate use in neotropical granivorous ants. Oecologia 96: 500-507.
[https://doi.org/10.1007/BF00320507]

-
Knapp, M. and K. Uhnava. 2014. Body size and nutrition intake effects on fecundity and overwintering success in Anchomenus dorsalis (Coleoptera: Carabidae). J. Insect Sci. 14: 240.
[https://doi.org/10.1093/jisesa/ieu102]

-
Krunić, M. and L. Stanisavljevic. 2007. Supercooling points and diapause termination in overwintering adults of orchard bees Osmia cornuta and O. rufa (Hymenoptera: Megachilidae). Bull. Entomol. Res. 96: 323-326.
[https://doi.org/10.1079/BER2006423]

-
Li, C., L. Wang, J. Li, C. Gao, Y. Luo and L. Ren. 2019. Thermal survival limits of larvae and adults of Sirex noctilio (Hymenoptera: Siricidae) in China. PLoS ONE 14(6): e0218888.
[https://doi.org/10.1371/journal.pone.0218888]

-
Li, Y., S. Wang and Y. Qin. 2012. Supercooling Points of Apis mellifera ligustica when Performing Different Age-Related Tasks. J. Apic. Sci. 56: 107-113.
[https://doi.org/10.2478/v10289-012-0012-z]

-
Monceau, K., O. Bonnard and D. Thiéry. 2014. Vespa velutina: a new invasive predator of honeybees in Europe. J. Pest Sci. 87: 1-16.
[https://doi.org/10.1007/s10340-013-0537-3]

-
Noor-ul-Ane, M. and C. Jung. 2020. Temperature-dependent development and survival of small hive beetle, Aethina tumida (Coleoptera: Nitidulidae). J. Apic. Res. 59: 807-816.
[https://doi.org/10.1080/00218839.2020.1740406]

-
Noor-ul-Ane, M. and C. Jung. 2021. Characterization of Cold Tolerance of Immature Stages of Small Hive Beetle (SHB) Aethina tumida Murray (Coleoptera: Nitidulidae). Insects 12:459.
[https://doi.org/10.3390/insects12050459]

-
Ohyama, Y. and E. Asahina. 1972. Frost resistance in adult insects. J. Insect Physiol. 18: 267-282.
[https://doi.org/10.1016/0022-1910(72)90127-8]

-
Owen, E. L., J. S. Bale and S. A. L. Hayward. 2013. Can Winter-Active Bumblebees Survive the Cold? Assessing the Cold Tolerance of Bombus terrestris audax and the Effects of Pollen Feeding. PLoS ONE 8(11): e80061.
[https://doi.org/10.1371/journal.pone.0080061]

-
Oyen, K. J., S. Giri and M. E. Dillon. 2016. Altitudinal variation in bumblebee (Bombus) critical thermal limits. J. Therm. Biol. 59: 52-57.
[https://doi.org/10.1016/j.jtherbio.2016.04.015]

-
Park, J. and C. Jung. 2016. Risk Prediction of the Distribution of Invasive Hornet, Vespa velutina nigrothorax in Korea using CLIMEX Model. J. Apic. 31: 293-303.
[https://doi.org/10.17519/apiculture.2016.11.31.4.293]

-
Pérez-de-Heredia, I., E. Darrouzet, A. Goldarazena, P. Romón and J. C. Iturrondobeitia. 2017. Differentiating between gynes and workers in the invasive hornet Vespa velutina (Hymenoptera, Vespidae) in Europe. J. Hymenopt. Res. 60: 119-133.
[https://doi.org/10.3897/jhr.60.13505]

-
Qin, M., H. Wang, Z. Liu, Y. Wang, W. Zhang and B. Xu. 2019. Changes in cold tolerance during the overwintering period in Apis mellifera ligustica. J. Apic. Res. 58: 702-709.
[https://doi.org/10.1080/00218839.2019.1634461]

-
Schmidt-Nielsen, K. 1997. Animal Physiology: Adaptation and Environment Cambridge University Press, Cambridge.
[https://doi.org/10.1017/9780511801822]

-
Shepherd, B. L., H. D. Prange and A. P. Moczek. 2008. Some like it hot: body and weapon size affects thermoregulation in horned beetles. J. Insect Physiol. 54: 604-611.
[https://doi.org/10.1016/j.jinsphys.2007.12.007]

-
Sinclair, B. J., A. Addo-Bediako and S. L. Chown. 2003. Climatic variability and the evolution of insect freeze tolerance. Biol. Rev. 78: 181-195.
[https://doi.org/10.1017/S1464793102006024]

-
Sinclair, B. J. and S. L. Chown. 2005. Deleterious effects of repeated cold exposure in a freeze-tolerant sub-Antarctic caterpillar. J. Exp. Biol. 208: 869-879.
[https://doi.org/10.1242/jeb.01455]

-
Sommer, L. 1982. Supercooling and winter survival in terrestrial arthropods. Comp. Biochem. Physiol. 73A: 519-543.
[https://doi.org/10.1016/0300-9629(82)90260-2]

-
Southwick, E. E. 1985. Allometric relations, metabolism and heat conductance in clusters of honey bees at cool temperatures. J. Comp. Physiol. B. 156: 143-149.
[https://doi.org/10.1007/BF00692937]

-
Teets, N. M., J. D. Gantz and Y. Kawarasaki. 2020. Rapid cold hardening: ecological relevance, physiological mechanisms and new perspectives. J. Exp. Biol. 223: jeb203448.
[https://doi.org/10.1242/jeb.203448]

-
Teets, N. M. and D. L. Denlinger. 2013. Physiological mechanisms of seasonal and rapid cold-hardening in insects. Physiol. Entomol. 38: 105-116.
[https://doi.org/10.1111/phen.12019]

-
Van Dooremalen, C., B. Cornelissen, C. Poleij-Hok-Ahin and T. Blacquière. 2018. Single and interactive effects of Varroa destructor, Nosema spp., and imidacloprid on honey bee colonies (Apis mellifera). Ecosphere 9: 02378.
[https://doi.org/10.1002/ecs2.2378]

-
Yunik, M. E. M. and N. B. Chilton. 2021. Supercooling points of adult Dermacentor variabilis (Acari: Ixodidae) from a population near the northern distribution limit. J. Med. Entomol. 58: 961-964.
[https://doi.org/10.1093/jme/tjaa223]

-
Zachariassen, K. E. 1985. Physiology of cold tolerance in insects. Physiol. Rev. 65: 799-832.
[https://doi.org/10.1152/physrev.1985.65.4.799]