
Effects of Species and Larvae Weights on Royal Jelly Secreted by Honey Bees (Apis mellifera ligustica and Apis cerana koreana) Bred in Korea
Abstract
Honey bees control the cast determination and colony development through the larvae’s nutritional state by the Royal Jelly (RJ). However, the study of RJ dominates for RJ production of Apis mellifera honey bees. In any other case, RJ secretion didn’t apply as a general selection characteristic to estimate the potential and to breed strong colonies. The purpose of this research was to relate the secretion of RJ against the different species and weights of grafted larvae of honey bees collected after 22 hrs. Larvae of different instars (0-2 days old) were grafted into queen cell cups and inserted into rearing colonies for the RJ collection within 22 hrs. The frames were later removed and taken to the bee breeding laboratory for measurements. It was found that the secreted RJ for feeding 1 mg A. m. ligustica larvae significantly differed from A. c. koreana: (57.84±23.32 mg) and (31.25±19.72 mg) respectively. Also, the group with the highest weight of larvae A. m. ligustica (16.04±5.66 mg) got significantly more RJ (147.94±30.04 mg) which was 2.59 times higher than that of larvae from the youngest group. These findings led us to understand that the weight of grafted larvae and species of honey bees influences the secretion of RJ into queen cell cups. Practically, this data can be used to estimate the RJ secretion by workers of honey bees in a short time to breed healthy strong colonies for different purposes.
Keywords:
Larval weight, Species, Royal Jelly, Honey bee, Apis mellifera ligustica, Apis cerana koreanaINTRODUCTION
Royal Jelly (RJ) is secreted by the hypopharyngeal and mandibular glands of worker honey bees to feed the larvae. Scientists have researched the outstanding physiological activity of RJ and its components which contain protein, acids, vitamins, lipids, and sugar (Fratini et al., 2016; Maleszka, 2018; Ahmad et al., 2020). Due to its numerous nutritional and medicinal content (antibiotic, anti-inflammatory, antioxidant, anti-tumor, and immune-modulatory activities), coupled with the fact that RJ is fed upon by young larvae and queen bees, some researchers have attributed the long lifespan of queen bees to RJ consumption (Maleszka, 2018). Therefore, honey bees control colony development and the cast determination of worker and queen bees through the larvae nutritional state by the nurses during the ontogenetic development (Weaver, 1957; Page et al., 1995; De Sousa et al., 2018). It was found that the start of the genetic health program of larvae takes place from the first to the third day of larvae development, after which larvae of queen bees feed on RJ, larvae of worker bees feed on mix of RJ, honey, and pollen. However, Wang et al. (2015) reported the weight of larvae in queen cells above first instars was more than larvae in worker cells of Apis mellifera ligustica despite the same feeding condition. Regarding the fact that queen bees emerge on the 16th day while worker bees emerge on the 21st day, the mean weight of queen bees is 57.3% more than that of the worker bees (Wang et al., 2015). This reveals the outstanding values of RJ to the metabolic pathways in its natural state.
To collect RJ for humans’ purposes, subspecies of A. mellifera honey bees were selected and bred in different countries for high-level of RJ secretion (Robinson, 2011; Cao et al., 2016; Gemeda et al., 2020). It was found that RJ yields were significantly more with A. m. ligustica than A. m. carnica (238.46±1.96 mg/cap), and (192.33±1.06 mg/cap) respectively (Khan et al., 2021). Moreover, the feeding of larvae depends on the subspecies, colony type (queen-less or queen-right) (Van Toor and Littlejohn, 1994), age of grafted larvae (Sahinler and Kaftanoglu, 1997), and harvesting interval (El-Din, 2010). Irrespective of the species of honey bees, the amount of the RJ secreted by worker bees and their number influences the honey bee colony development (Szabo and Lefkovitch, 1988) because the number of simultaneously fed larvae are restricted. Hence, RJ secretion should be studied in other honey bee species including A. cerana.
The purpose of our study was to relate the secretion of RJ collected after 22 hrs against the different species of honey bees (A. m. ligustica, and A. c. koreana) and different weights of grafted larvae of honey bees. Practically, this data can be used to decrease the time of the RJ collection to estimate the RJ yield in a short time. In the future, the selection of high RJ secretion colonies is the way to breed healthy strong colonies for different purposes.
MATERIALS AND METHODS
1. Study site
This study was carried out in the Bee breeding Laboratory, Department of Agricultural Biology, NationalInstitute of Agricultural Science (NIAS), the Republicof Korea.
2. Colony selection, larvae choice
Fourteen strong queenright of A. m. ligustica and A. c. koreana honey bee colonies were studied from March to June 2021. These colonies were used for breeding and neither were adapted for special RJ production. In the apiary, three and four colonies were used for the selection and rearing of larvae respectively. Two queen bees were excluded for two consecutive days on the built combs without eggs for the queen to deposit eggs within 24 hours. Four days later, the larvae at the age of 0-2 days old were checked in the frames.
3. Grafting of larvae, collection of data
The study was done according to the standard methods for A. mellifera L. RJ research (Hu et al., 2017) and the adapted method for A. c. koreana (Vung et al., 2017). The larvae of different instars (0-2 days old) had been grafted into queen cell cups for the RJ collection and were put into the rearing colonies. Frames with grafted larvae were removed from rearing colonies after 22 hours and taken to the bee breeding laboratory for RJ collection and measurements. An electric scale balance was set to collect measurements in milligrams. The plastic queen cell cup, wax, larvae, and RJ were weighed consistently. The wax covering of the cells was studied because it keeps the environment for larval development. The data on larvae weight were grouped into six groups according to their weight which has been documented to be positively correlated with age till 28 days of age (Sahinler and Kaftanoglu, 1997; Wang et al., 2015; Rinkevich et al., 2016). The first number of groups 1 (1.1, 1.2), 2 (2.1, 2.2), 3 (3.1, 3.2) signifies the age of 1, 2, and 3 instars, respectively. The second number of groups 1 (1.1, 2.1, 3.1), and 2 (1.2, 2.2, 3.2) means the increased larvae weight inside the instar’s development.
4. Data analysis
All data were analyzed by the Principal Component Analysis (PCA) to visualize the grouped data, by Descriptive Statistics to characterize the groups, by the Regression analysis with Pearson correlation to estimate the relations between the data, by One-way ANOVA followed by Tukey’s posthoc test for comparing three and more groups, and paired samples t-test to compare two groups, where the significant levels were set at α=0.05. The SAS Enterprise Guide 7.1 statistical software (ver.25), MS Excel with the XLSTAT, AtteStat application, program R were used for statistical calculations and visualization of data.
RESULTS AND DISCUSSION
1. Effect of species (Apis mellifera ligustica and Apis cerana koreana) on the royal jelly collection for 1 mg larvae
The study of the RJ secretion was carried out on two species of honey bees A. m. ligustica and A. c. koreana which were kept in the same location and used for breeding but neither were adapted for special RJ production. A paired samples t-test was performed to compare the weights of Royal Jelly (RJ) and wax covering queen cells for 1 mg larvae for both honey bees. As expected, there was a significant difference in RJ secretion between A. m. ligustica (m=57.84 mg, SD=23.32, n=69) and A. c. koreana (m=31.25 mg, SD=19.72, n=8); t=3.467, df=75, p=0.001. Also, a significant difference was found in wax covering between A. m. ligustica (m=38.30 mg, SD=12.77, n=69) and A. c. koreana (m=22.50 mg, SD=3.34, n=8); t=3.094, df=75, p=0.003. The amount of RJ which A. m. ligustica worker bees secrete to feed 1 mg larvae during 22 h, was 1.85 times more than the amount of RJ for the same 1 mg larvae of A. c. koreana worker bees. The species features were fixed like the more intensive feeding of A. m. ligustica larvae in queen cells, which queen development takes 16 days (Wang et al., 2015), than A. c. koreana larvae in queen cells provided by less feeding whose development is 15 days (Vung et al., 2020). These findings confirm that the feeding and secretion of RJ by worker honey bees are influenced by the species of the honey bee. Moreover, the RJ collection trait can be recommended for the selection of honey bees for breeding strong colonies for various purposes.
2. Effect of larva weight on royal jelly, and wax covering queen cells collection of Apis mellifera ligustica honey bees
Data were collected after grafting larvae of different weights and rearing into the colonies of A. m. ligustica honey bees after 22 hours. The activity of honey bees to feed larvae depending on their weight was studied. Raw data were grouped by weight according to the body weight of European honey bee A. mellifera queens and workers as 1, 2, and 3 instars (Wang et al., 2015), each of them was grouped in relation to instar development as 1.1 and 1.2, 2.1 and 2.2, 3.1 and 3.2 by the increased larvae weight. The means and standard deviations of the groups are shown on Table 1.

Mean and standard deviation of the larvae weights Apis mellifera ligustica, Royal Jelly (RJ), and wax covering per queen cell
The visualization of grouped data was done by Data Mining Plots which most separated the larvae 3.2 groups from other data by the principal component 1 (PC1) (66%) than by the PC2 (26%) (Fig. 1A).
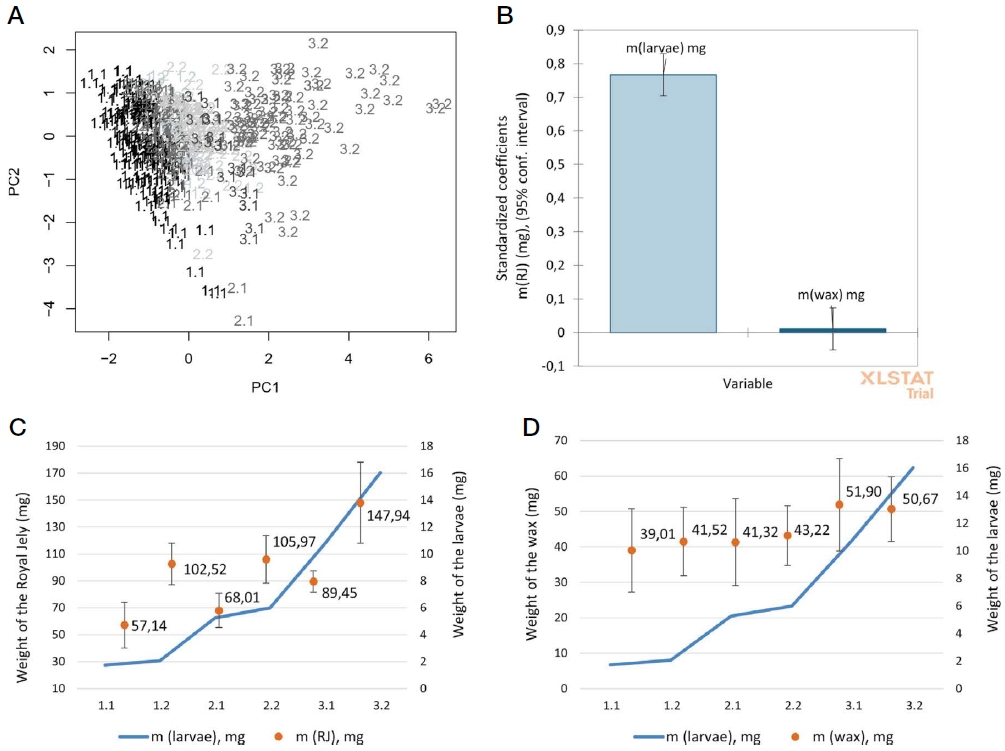
Analysis of grouped data by the larvae weight of Apis mellifera ligustica honey bees (1.1-3.2); A - PCA analysis; B - Regression analysis of the relation of the weights of larvae, wax, and RJ. The estimation of the predictive power of two predictors showed the different scales of measurement (m (larvae) and m (wax)) in the RJ research; C, D - Effect of larva size on RJ secretion and wax covering within 22 h, respectively.
The grouping of the larvae by weight from the same instar showed that the weight of RJ in cells with larvae didn’t maintain by worker bees at the same amount. The weights of RJ (1.1 and 3.2) had significant differences from all other data (Table 2). The low amount of RJ food in group 1.1 could be attributed to larvae weight because older larvae in the same colony got more RJ 2.59 times. Group number 3.2 with the highest weight of larvae (16.04±5.66 mg) had significantly higher RJ food of (147.94±30.04 mg) than larvae from other groups within 22 hours by one-way ANOVA (F=389.44, df=5, P<0.0001), Tukey post hoc test (α=0.05) (Table 1, Fig. 1C).
In a similar scenario as for RJ, wax from groups 1.1, 1.2, 2.1, and 2.2 were significantly different from groups 3.1 and 3.2 by one-way ANOVA (F=16.09, df=5, P<0.0001), Tukey post hoc test (α=0.05) (Fig. 1D). More wax around queen cell cups (51.90±13.06 mg), and (50.61±9.15 mg) were collected with older larvae of group 3.1 (10.8±0.83 mg), group 3.2 (16.04±5.66 mg) respectively after 22 hours. Obviously, the nurse honey bees distinguished the weight of larvae, which were not only fed a suitable diet but also covered the older larvae (3.1 and 3.2) with significantly more wax cover. However, a strong positive correlation between the weight of larvae and RJ was observed (r=0.771), but low with the weight of wax (Table 2).
The relation of the weights of larvae, and RJ were confirmed by regression analysis. The weights of larvae and RJ had higher standardized coefficients near 1, which showed the dependence of the weight of RJ food on the weight of larvae more than the weight of the wax cover (Fig. 1B). These results discern our research from other studies on RJ, which have identified factors affecting the production and queen rearing in Apis mellifera honey bees including the weight of transferred larvae (Sahinler and Kaftanoglu, 1997; Jianke, 2000; Gemeda et al., 2020).
Weights of grafted larvae had a significantly positive impact on the amount of RJ secreted by nurse bees which is most important when estimating the ability of honey bee colonies to healthy and quickly development. However, two instar larvae cannot be used for queen rearing, because newly emerged queens raised from younger larvae had higher characteristics than queens raised from older larvae (De Souza et al., 2018).
CONCLUSION
The relation of the secretion of RJ against the different species of honey bees and different weights of grafted larvae of honey bees collected after 22 hrs was the purpose of our study. We refined that the weight of honey bee larvae (16.04±5.66 mg) got significantly more RJ (147.94±30.04 mg). However, RJ secretion by nurse bees differs among species (A. cerana and A. mellifera). The amount of RJ secreted into queen cells containing 1 mg A. m. ligustica larvae was significantly different from those of A. c. koreana: (57.84±23.32 mg) and (31.25±19.72 mg) respectively. Practically, our study would help breeders to select the larvae of honey bees for estimation of RJ secretion by honey bee colonies within 22 hrs and assess the potential development rate of honey bee colonies in the foraging season. In the future, the selection of high RJ secretion honey bee colonies is the way to breed healthy strong colonies for different purposes.
Acknowledgments
We gratefully thank the bee breeding laboratory, especially Mr. Kim Ho Jin, National Institute of Agricultural Science (NIAS), Rural Development Administration (RDA), Korea Africa Food and Agricultural Cooperation Initiative (KAFACI) Secretariat, and the Institute of Agricultural Research for Development (IRAD), for their collaboration toward the success of this project. This research was funded by a grant from the Research Program for Agricultural Science & Technology Development (Project No. PJ01575503), National Institute of Agricultural Sciences, Rural Development Administration, Republic of Korea.
CONTRIBUTION OF AUTHORS
Conceptualization, O.F. and P.N.A.; methodology, O.F. and P.N.A.; software, O.F.; validation, P.N.A., Y.-S.C.; formal analysis, D.K., E.-J.K.; investigation, resources, B.-S.P., D.K.; data curation, B.-S.P.; writing-original draft preparation, O.F., P.N.A.; writing-review and editing, P.N.A.; visualization, B.-S.P.; supervision, E.-J.K., D.K., Y.-S.C.; project administration, funding acquisition, Y.-S.C.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the correspondent author. The data are not publicly available due to privacy or ethical restrictions.
References
-
Ahmad, S., M. Campos, F. Fratini, S. Altaye and J. Li. 2020. New Insights into the Biological and Pharmaceutical Properties of Royal Jelly. Int. J. Mol. Sci. 21: 1-28.
[https://doi.org/10.3390/ijms21020382]

-
Cao, L. F., H. Q. Zheng, C. W. W. Pirk, F. L. Hu and Z. W. Xu. 2016. High Royal Jelly producing honeybees (Apis mellifera ligustica) (Hymenoptera: Apidae) in China. J. Econ. Entomol. 109: 1-5.
[https://doi.org/10.1093/jee/tow013]

-
De Souza, D. A., M. H. Huang and D. R. Tarpy. 2018. Experimental improvement of honey bee (Apis mellifera) queen quality through nutritional and hormonal supplementation. Apidologie 50: 14-27.
[https://doi.org/10.1007/s13592-018-0614-y]

- El-Din, H. 2010. Studies on Royal Jelly production in honeybee colonies: Cairo University.
-
Fratini, F., G. Cilia, S. Mancini and A. Felicioli. 2016. Royal Jelly: An ancient remedy with remarkable antibacterial properties. Microbiol. Res. 192: 130-141.
[https://doi.org/10.1016/j.micres.2016.06.007]

-
Gemeda, M., G. Legesse, T. Damto and D. Kebaba. 2020. Harvesting Royal Jelly Using Splitting and Grafting Queen Rearing Methods in Ethiopia. Bee World 97: 114-116.
[https://doi.org/10.1080/0005772X.2020.1817657]

-
Hu, F.-L., K. Bılikova, H. Casabianca, D. Gaelle, F. S. Espindola, M. Feng, C. Guan, B. Han, T. K. Krakova, J. K. Li, L. Li, X. A. Li, J. Simuth, L. M. Wu, Y. Q. Wu, X. F. Xue, Y. B. Xue, K. Yamaguchi, Z. J. Zeng, H. Q. Zheng and J. H. Zhou. 2017. Standard methods for Apis mellifera Royal Jelly research. J. Apic. Res. 56: 1-68.
[https://doi.org/10.1080/00218839.2017.1286003]

- Jianke, L. 2000. Technology for Royal Jelly production. Am. Bee J. 1: 469-472.
-
Khan, K. A., A. Hamed, Z. A. Ghramh, A. A. Mogbel, El-Niweiri and E. A. M. Mohamed. 2021. Queen cells acceptance rate and royal jelly production in worker honey bees of two Apis mellifera races. PLoS ONE 16: e02448593.
[https://doi.org/10.1371/journal.pone.0248593]

-
Maleszka, R. 2018. Beyond Royalactin and a master inducer explanation of phenotypic plasticity in honey bees. Commun. Biol. 1: 8.
[https://doi.org/10.1038/s42003-017-0004-4]

-
Page, R. E., K. D. Waddington, G. J. Hunt and M. K. Fondrk. 1995. Genetic determinants of honey bee foraging behavior. Anim. Behav. 50: 1617-1625.
[https://doi.org/10.1016/0003-3472(95)80015-8]

-
Rinkevich, F. D., W. M. Joseph, M. P. Jean, A. O. James and B. H. Kristen. 2016. Pteridine levels and head weights are correlated with age and colony task in the honey bee, Apis mellifera. PeerJ 4: 1-15.
[https://doi.org/10.7717/peerj.2155]

-
Robinson, G. 2011. Royal aspirations. Nature 473: 454-455.
[https://doi.org/10.1038/473454a]

-
Sahinler, N. and O. Kaftanoglu. 1997. Effects of feeding, age of the larvae, and queenlessness on the production of Royal Jelly. pp. 173-178. in Bee Products., eds. by A. Mizrahi, Y. Lensky. 269 p. Springer, Boston.
[https://doi.org/10.1007/978-1-4757-9371-0_21]

-
Szabo, T. I. and L. P. Lefkovitch. 1988. Fourth generation of closed population honey bee breeding. Apidologie 19: 259-274.
[https://doi.org/10.1051/apido:19880306]

-
Van Toor, R. and R. Littlejohn. 1994. Evaluation of hive management techniques in the production of Royal Jelly by honey bees (Apis mellifera) in New Zealand. J. Apic. Res. 33: 160-166.
[https://doi.org/10.1080/00218839.1994.11100864]

-
Vung, N. N., M. L. Lee, M. Y. Lee, H. K. Kim, E. J. Kang, J. E. Kim and Y. S. Choi. 2017. Breeding and Selection for Resistance to Sacbrood Virus for Apis cerana. J. Apic. 32: 345-352.
[https://doi.org/10.17519/apiculture.2017.11.32.4.345]

-
Vung, N. N., Y. S Choi and I. Kim. 2020. High Resistance to Sacbrood Virus Disease in Apis cerana (Hymenoptera: Apidae) Colonies Selected for Superior Brood Viability and Hygienic Behavior. Apidologie 51: 61-74.
[https://doi.org/10.1007/s13592-019-00708-6]

-
Wang, Y., L. T. Ma and B. H. Xu. 2015. Diversity in live history of queen and worker honey bees, Apis mellifera L. J. Asia Pac. Entomol. 18: 145-149.
[https://doi.org/10.1016/j.aspen.2014.11.005]

-
Weaver, N. 1957. Effects of Larval Age on Dimorphic Differentiation of the Female Honey Bee. Ann. Entomol. Soc. Am. 50: 283-294.
[https://doi.org/10.1093/aesa/50.3.283]
