Nutrition for Honey Bee to Prevent Colony Collapse
Abstract
The honey bee is a globally important pollinator for wild plants and cultivated crops. On the other hand, beehives have been significantly affected by a variety of factors, including chemical exposure, pathogen infection, and a decrease in floral diversity. A healthy honeybee is able to withstand many of the stresses of modern apiculture. This review of previous studies demonstrates how healthy nutrients affect bee colonies. The development of an optimal honey bee diet may one day save the honey bees’ future. It is anticipated that the current findings will aid in the creation of a diet with appropriate nutrients for honey bees.
Keywords:
Nutrition, Macronutrient, Micronutrient, pH, Role differentiationINTRODUCTION
Honey bees as pollinators are essential to wild plants and commercial crops (Williams, 1994).
However, colony losses of up to 45% per year have been experienced by US beekeepers since 2006 (Azzouz-Olden et al., 2018). Globally, the colony collapse disorder (CCD) and the disappearance of honeybees during overwintering this year in South Korea caused great damage nationwide (Flores et al., 2021). Researchers claim that the CCD phenomenon cannot be attributed to a single source but rather to the intricate interactions of several processes (Flores et al., 2021). Increased losses from invasive mites, diseases like viruses and parasites, exposure to insecticides or mite pesticides, stress from mobile beekeeping, habitat changes, inadequate feed, and nutrition, decreased immunity, and stress from a variety of other factors are some of the causes (Fig. 1). According to Goulson et al. (2015) numerous studies on the nutrition of honey bees have suggested that malnutrition-related stress may play a significant role in CCD (Goulson et al., 2015). Many of the stresses of modern apiculture can be overcome by a well-nourished honey bee (Kim et al., 2022). Additionally, a consistent supply of pollen can help the colonies grow and encourage brood rearing (Morais et al., 2013). Thus, the hive’s food reserve and nutrition have an impact on honey bee growth (Fleming et al., 2015).
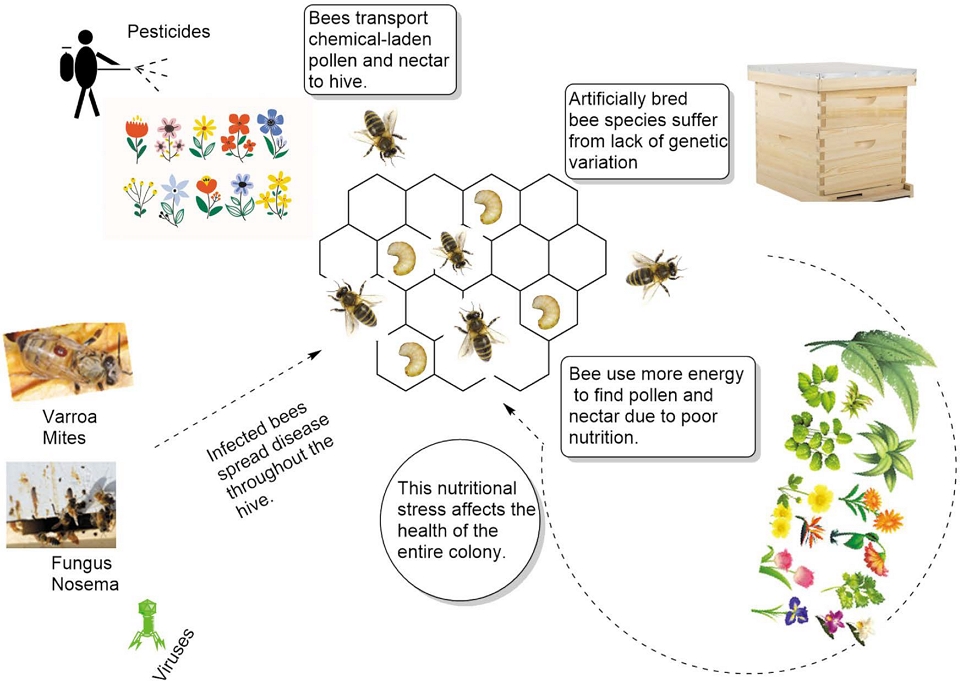
There are several reasons for the increase in honey bee losses. The reason cannot be linked to a single source but rather to the complex interactions of a number of processes. A well-nourished honey bee can overcome many of the challenges associated with modern apiculture.
Honey bees, like all other animals, require particular nutrients (Saffari et al., 2010). Vitamins, minerals, lipids (fatty acids and sterols), water, and proteins (amino acids) are necessary for honey bees. According to Standifer (1980), these nutrients must be included in the diet in the right quantities. Macronutrient-rich carbohydrates, proteins, and lipids are essential for honey bee growth and maintenance, while vitamins, minerals, phytochemicals, and other micronutrients are not only necessary but crucial for the preservation of honey bee health (Retschnig et al., 2021).
Nectar and pollen are honey bees’ primary sources of nutrition (Winston, 1991). Nectar is the main source of carbohydrates. Pollen contains lipids, amino acids, starches, sterols, vitamins, and minerals, all of which have an impact on honey bees’ lifespan and survival (Table 1). Pollen is the primary source of protein for honey bees. In this review, previous studies demonstrate how these macronutrients and micronutrients affect bee colonies and mention the relationship between nutrition and role differentiation.
MACRONUTRIENT
1. Carbohydrates
According to Winston (1991), nectar contains 82% carbohydrates and 17% water, making it a significant source of carbohydrates for honey bees (Winston, 1991). Carbohydrates are stored in a cell within the honeycomb and consist of 38% fructose, 32% glucose, and a small amount of maltose and sucrose (Vaudo et al., 2016). To make honey suitable for the colony, additional enzymes are added at this time, and moisture is reduced (Oddo et al., 1999). When they are larvae and adults, honey bees consume different amounts of carbohydrates, but adults consume fewer carbohydrates than larvae (Standifer, 1980). Throughout its development, one worker larva is fed 59.4 mg of carbohydrates (Rortais et al., 2005). An adult worker honey bee needs about 4 mg of utilizable sugars each day to survive (Barker and Lehner, 1974). Honey bees’ primary energy source during flight is nectar (Brodschneider and Crailsheim, 2010). Additionally, it is used to heat the environment in the winter and to produce wax for honeycomb construction (Hepburn et al., 2014). Honey is stored in excess. Sugar had an effect on the formation of long-term memories in honey bees (Simcock et al., 2018). Therefore, an adequate supply of carbohydrates is essential for bees.
2. Amino acid
Pollen is the primary source of honey bees’ natural protein (Brodschneider and Crailsheim, 2010). In 1953, De Groot stated that the essential amino acids for honey bees are arginine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine (De Groot, 1953). They are necessary for reproduction, growth, and development because they cannot be produced on their own and must be obtained through diet (De Groot, 1953). Leucine controls protein turnover through cellular mTOR signaling and gene expression, while lysine is directly involved in the synthesis of nitric oxide, a neurotransmitter that is known to improve memory in bees and moths (Gage et al., 2020). The hypopharyngeal gland’s development depends on tryptophan, which also affects honey bees’ food intake, digestion, and feeding habits (Fengkui et al., 2015). Consequently, it might be useful as a supplement (Kim et al., 2022). Methionine is essential for organism growth and development, immune enhancement, antioxidant capacity enhancement, detoxification, and methylation, and it enables worker bee larvae rather than queen bees to develop (Chen et al., 2020). Many of the non-essential compounds, it was said, aren’t necessary for growth but boost or have a “stimulatory effect” when added. In the honey bee brain, tyrosine is a precursor for dopamine, a neurotransmitter involved in memory and learning (Agarwal et al., 2011). Proline is an important amino acid for the queen bee to lay eggs. It is also essential for the wing muscles of insects. Additionally, it is thought to be a honey bee-favored ingredient (Kim et al., 2020). Bumble bee workers' foraging activity has been connected to the amount of protein in pollen, and bumble bees can taste and distinguish between diets with different amounts of protein or pollen (Vaudo et al., 2016). Therefore, a balanced supply of protein containing essential amino acids is necessary for honeybees.
3. Lipid
Lipids are typically employed as energy sources to create reserve fats, glycogen, and cell membranes (Manning, 2001). Plant pollen contains between 1-20% of lipids (oil and fats) (Manning, 2001). Additionally, lipids’ fatty acids and sterols play a significant role in honey bee development, nutrition, and production. And among them, honey bees’ diets must include fatty acids as a necessary component (Manning, 2001). Linoleic acid (LA) (C18 : 2n-6) and alpha-linolenic acid (ALA) (C18 : 3n-3) is the primary omega-3 and omega-6 fatty acids for insects, including honey bees (Arien et al., 2015). Arien et al. (2015) claim that, insects require these fatty acids (Arien et al., 2015). Learning performance may suffer when fatty acids are lacking, which may have an effect on foraging activities (Arien et al., 2015). Olfactory and tactile functions deteriorated, and the hypopharyngeal glands became smaller when the animal was fed a diet low in omega-3 for 6 weeks (Arien et al., 2015). Fatty acids such as oleic acid improve the learning and survival rate of honey bees, and the ratio of omega-6: omega-3 affects the learning performance of honey bees (Muth et al., 2018). According to Manning (2016), honey bees with a deficiency in the essential fatty acid linolenic have been found to have impaired learning and hypopharyngeal gland development (Manning, 2016). Insects use sterols, which are lipids, to make important cell membrane components and act as precursors to important ecdysis hormones. Since insects can't make cholesterol on their own, they have to eat plant sterols. According to Standifer (1980), 24-methylene cholesterol is a major sterol found in pollen and the body tissues of queen bees, brood rods, and larvae. Bumble bee larval size and growth were boosted by high sterol pollen content (Vaudo et al., 2016). Therefore, it is necessary to supply the appropriate amount of lipid to bees.
MICRONUTRIENT
1. Organic acid
Organic acids are the major component of the taste and flavor of pollen (Park et al., 2017). Citric acid can be used to properly supplement food with minerals, making it an important part of diet and nutrition (Park et al., 2017). Lactic acid is one of the main fermentation products of the metabolism of carbohydrates (Quinto et al., 2014). Organic acids have recently been discovered to be an alternative to antibiotics for the stimulation of growth and the preservation of food (Kim and Rhee, 2015). The diet of honey bees contains a high concentration of organic acids, making it an effective antibiotic (Dranca et al., 2020). Therefore, an adequate supply of organic acids would be beneficial to honeybees.
2. Inorganic ions
Minerals are essential nutrients for physiological processes and metabolic pathways. Minerals like K, P, Mg, Ca, S, Fe, Cu, Mn, Zn, Cr, and Se can be found in pollen, making daily consumption necessary (Smith et al., 2019). Because they cannot be produced by the body, inorganic ions must be taken in through food. In order to compensate for nutritional deficiencies in the floral diet, honey bees prefer to feed on water and soil sources (Lau and Nieh, 2016; Khan et al., 2021). Honey bees prefer to feed on inorganic ions for physiological activities and functions like muscle movement (Chakrabarti et al., 2020). While Na+ ions are required for osmoregulation, Fe (iron ions) accumulate at the periphery of the abdomen and play a role in honey bee navigation (Khan et al., 2021). However, very little research has been done on the role of micronutrients like Na+, Mg2+, and Ca2+ ions in honey bee diets (Khan et al., 2021). According to Khan et al.’s research, honey bees prefer sodium chloride (NaCl), potassium chloride (KCl), and magnesium chloride (MgCl2) salt concentrations between 0.1-1.5% (Lau and Nieh, 2016; Khan et al., 2021). K+ was found to be the most common inorganic ion in bee bread. Pollen and bee bread samples contained the highest concentrations of K+, ranging from 3.6 to 6.03 mg/g (Stanciu et al., 2009). It is recommended that a final diet formulation for honeybees contains 0.1% K+, 0.05% Ca2+, 0.03% Mg2+ and less than 0.005% each of Na+, Zn2+, Mn2+, and Cu2+ (Herbert Jr and Shimanuki, 1978). Therefore, supplying the appropriate amount of inorganic ions required by bees will help bee health.
3. Polyphenolic
Polyphenolic compounds, particularly flavonoids and phenolic acids, are abundant in pollen (Kieliszek et al., 2018). According to Smetanska et al. (2021), phenolic acids are a large group of plant secondary metabolites that can be utilized as food additives, nutraceuticals, and pharmaceuticals (Smetanska et al., 2021). Due to their positive effects on health, particularly their antioxidant activity, the phenolic compound content of foods has become the focus of numerous studies. High antioxidant activity indicates a high content of phenolic antioxidants and flavonoids, which protects an organism from the harmful effects of reactive oxygen species (Kieliszek et al., 2018).
4. Vitamin
Vitamins play a variety of roles in the body and are necessary for healthy growth and development. Pollen contains almost all vitamins and is rich in B-complex (thiamine, niacin, riboflavin, pyridoxine, pantothenic acid, folic acid, biotin) and carotenoids (Table 2). Bees require a diet high in vitamins to produce royal jelly for their queen bees and larvae. Nurse bee need the vitamin B complex for brood rearing. Pantothenic acid is required for the differentiation of nurse bees and queen bees (Standifer, 1980). In addition to these vitamins, ascorbic acid (vitamin C) appears to be essential for brooding. Vitamin C is a water-soluble vitamin that is involved in the metabolism of fats, carbohydrates, and proteins (Kieliszek et al., 2018). Chestnut trees are major honey plants in many countries, and the vitamin C content of their pollen is around 0.10 mg/g (Ivanišová et al., 2015). The vitamins naturally present in pollen and bee bread are difficult to estimate due to their low concentrations, the presence of many disturbing factors, and the complexity of the matrix (Kieliszek et al., 2018). Twelve domestic pollens had an average vitamin C content of 0.56 mg/g, whereas imported pollens from China, Vietnam, and Spain had an average vitamin C content of 0.06 mg/g (Lee and Ahn, 2019). In general, the vitamins needed by a honey bee colony are met when abundantly stored in the colony’s pollen or when fresh pollen is available from the field.
pH
Although pH is not nutritional, it is considered an important factor to consider when feeding bees. The literature showed that the pH values of bee pollen varied from 3.8 to 6.3 and bee bread from 3.8 to 4.4 (Kieliszek et al., 2018). Royal jelly is crucial for the development of a honey bee larva into a queen (Buttstedt et al., 2018). In the natural system, hypopharyngeal gland secretion has a pH of 5.1, and just after the addition of the mandibular gland secretion (pH 3.9), the pH of the final product is lowered to around pH 4.0 (Jantakee and Tragoolpua, 2015). Thus, royal jelly is the mixture of the hypopharyngeal and mandibular gland secretions that reduce the pH levels to around pH 4.0 (Jantakee and Tragoolpua, 2015). It has been reported that the pH value of royal jelly (3.6 to 4.1) and honey (3.4 to 4.1) varies (Adaškevičiūtė et al., 2019; Mureşan and Buttstedt, 2019). Because the pH of bee bread, royal jelly, and honey, the main food for honey bees, is around 4.0, one should consider adjusting the pH to around 4.0 when developing honey bee artificial diets (Adaškevičiūtė et al., 2019; Mureşan and Buttstedt, 2019).
NUTRITION AND ROLE DIFFERENTIATION
When the foraging bee is removed, the 5-day-old nurse bee becomes a foraging bee. Even if the bee doesn’t have enough pollen or honey stored in it, it becomes an early foraging bee. Schulz et al. (1998) conducted research on role differentiation and nutrition, and the results showed that food-deprived bees foraged earlier than well-fed bees (Schulz et al., 1998). Baby bees store fewer lipids than adults, but after a few days, the amount of lipids increases and stays high during brooding. In honey bees, lipid storage in the abdomen is cut in half during the transition from the nurse bee to the foraging bee (Ament et al., 2010). Interestingly, this did not decrease due to flight behavior. These well-fed bees showed earlier foraging activity than the control group when the lipid content was reduced experimentally (Toth and Robinson, 2005). It was found that changes in lipid content induce behavioral changes in honeybees. Similar to the storage of lipids, vitellogenin, a stored protein, was abundant in the nurse bee and decreased prior to foraging activity (Ament et al., 2010). Experimentally, inhibition of vitellogenin synthesis induced early foraging activity (Marco Antonio et al., 2008) (Fig. 2). Thus, the timing of honey bee behavioral maturation is influenced by the body's stored protein and lipid content.
CONCLUSION
Honey bees need an adequate supply of carbohydrates and lipids and balanced amino acids. And the supply of organic acid, inorganic ions, polyphenolics, and vitamins could be beneficial to the honey bee. Insufficient nutrition resulted in an abnormal division of roles in bees, resulting in weak colonies. A healthy diet is essential for a healthy colony. Stressors like pathogens, pesticides, and a decrease in floral diversity can be overcome by well-nourished honeybees. Providing bees with a diet rich in the nutrients they require at just the right time is critical. It is regarded as a pressing necessity to develop a bee-friendly diet rich in well formulated nutrients. It is anticipated that by providing bees with a diet rich in the necessary nutrients, it will be possible to aid in the prevention of bee colony decay.
Acknowledgments
This work was carried out with the support of the “Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ015755022022)” and the Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2020R1A6A1A03041954). Rural Development Administration, Republic of Korea and the Center for Women In Science, Engineering and Technology (WISET) Grant funded by the Ministry of Science and ICT (MSIT) under the Program for Re- turners into R&D (Project No. WISET-2022-726).
References
-
Adaškevičiūtė, V., V. Kaškonienė, P. Kaškonas, K. Barčauskaitė, and A. Maruška. 2019. Comparison of Physicochemical Properties of Bee Pollen with Other Bee Products. Biomolecules 9(12): 819.
[https://doi.org/10.3390/biom9120819]

-
Agarwal, M., M. Giannoni Guzmán, C. Morales-Matos, R. A. Del Valle Díaz, C. I. Abramson and T. Giray. 2011. Dopamine and Octopamine Influence Avoidance Learning of Honey Bees in a Place Preference Assay. PLoS One 6(9): e25371.
[https://doi.org/10.1371/journal.pone.0025371]

-
Ament, S. A., Y. Wang and G. E. Robinson. 2010. Nutritional regulation of division of labor in honey bees: toward a systems biology perspective. WIREs Syst. Biol. Med. 2(5): 566-576.
[https://doi.org/10.1002/wsbm.73]

-
Arien, Y., A. Dag, S. Zarchin, T. Masci and S. Shafir. 2015. Omega-3 deficiency impairs honey bee learning. Proc. Natl. Acad. Sci. U. S. A. 112(51): 15761-15766.
[https://doi.org/10.1073/pnas.1517375112]

-
Azzouz-Olden, F., A. Hunt and G. DeGrandi-Hoffman. 2018. Transcriptional response of honey bee (Apis mellifera) to differential nutritional status and Nosema infection. BMC Genom. 19(1): 628.
[https://doi.org/10.1186/s12864-018-5007-0]

-
Barker, R. J. and Y. Lehner. 1974. Acceptance and sustenance value of naturally occurring sugars fed to newly emerged adult workers of honey bees (Apis mellifera L.). J. Exp. Zool. 187(2): 277-285.
[https://doi.org/10.1002/jez.1401870211]

-
Brodschneider, R. and K. Crailsheim. 2010. Nutrition and health in honey bees. Apidologie 41(3): 278-294.
[https://doi.org/10.1051/apido/2010012]

-
Buttstedt, A., C. I. Mureşan, H. Lilie, G. Hause, C. H. Ihling, S.-H. Schulze, M. Pietzsch and R. F. A. Moritz. 2018. How Honeybees Defy Gravity with Royal Jelly to Raise Queens. Curr. Biol. 28(7): 1095-1100.
[https://doi.org/10.1016/j.cub.2018.02.022]

-
Campos, M. G. R., S. Bogdanov, L. B. de Almeida-Muradian, T. Szczesna, Y. Mancebo, C. Frigerio and F. Ferreira. 2008. Pollen composition and standardisation of analytical methods. J. Apic. Res. 47(2): 154-161.
[https://doi.org/10.1080/00218839.2008.11101443]

-
Chakrabarti, P., H. M. Lucas and R. R. Sagili. 2020. Evaluating Effects of a Critical Micronutrient (24-Methylenecholesterol) on Honey Bee Physiology. Ann. Entomol. Soc. Am. 113(3): 176-182.
[https://doi.org/10.1093/aesa/saz067]

-
Chen, W.-F., Y. Wang, W.-X. Zhang, Z.-G. Liu, B.-H. Xu and H.-F. Wang. 2020. Methionine as a methyl donor regulates caste differentiation in the European honey bee (Apis mellifera). Insect Sci. 28.
[https://doi.org/10.1111/1744-7917.12788]

- De Groot, A. P. 1953. Protein and amino acid requirements of the honeybee (Apis mellifica L.).
-
Dranca, F., F. Ursachi and M. Oroian. 2020. Bee Bread: Physicochemical Characterization and Phenolic Content Extraction Optimization. Foods 9(10): 1358.
[https://doi.org/10.3390/foods9101358]

-
Fengkui, Z., X. Baohua, Z. Ge and W. Hongfang. 2015. The Appropriate Supplementary Level of Tryptophan in the Diet of Apis mellifera (Hymenoptera: Apidae) Worker Bees. J. Insect Sci. 15(1): 161.
[https://doi.org/10.1093/jisesa/iev142]

-
Fleming, J., D. Schmehl and J. Ellis. 2015. Characterizing the Impact of Commercial Pollen Substitute Diets on the Level of Nosema spp. in Honey Bees (Apis mellifera L.). PLoS One 10: e0132014.
[https://doi.org/10.1371/journal.pone.0132014]

-
Flores, J. M., V. Gámiz, Á. Jiménez-Marín, A. Flores-Cortés, S. Gil-Lebrero, J. J. Garrido and M. D. Hernando. 2021. Impact of Varroa destructor and associated pathologies on the colony collapse disorder affecting honey bees. Res. Vet. Sci. 135: 85-95.
[https://doi.org/10.1016/j.rvsc.2021.01.001]

-
Gage, S. L., S. Calle, N. Jacobson, M. Carroll and G. DeGrandi-Hoffman. 2020. Pollen Alters Amino Acid Levels in the Honey Bee Brain and This Relationship Changes With Age and Parasitic Stress. Front. Neurosci. 14: 231.
[https://doi.org/10.3389/fnins.2020.00231]

-
Goulson, D., E. Nicholls, C. Botías and E. L. Rotheray. 2015. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347(6229): 1255957.
[https://doi.org/10.1126/science.1255957]

-
Hepburn, H., C. Pirk and O. Duangphakdee. 2014. Nectar flows and comb-building. pp. 175-206. in Honeybee nests. Springer.
[https://doi.org/10.1007/978-3-642-54328-9_9]

-
Herbert Jr, E. W. and H. Shimanuki. 1978. Chemical composition and nutritive value of bee-collected and bee-stored pollen. Apidologie 9(1): 33-40.
[https://doi.org/10.1051/apido:19780103]

-
Ivanišová, E., M. Kačániová, H. Frančáková, J. Petrová, J. Hutková, V. Brovarskyi, S. Velychko, L. Adamchuk, Z. Schubertová and J. Musilová. 2015. Bee bread - perspective source of bioactive compounds for future. Potr. S. J. F. Sci. 9(1): 592-598.
[https://doi.org/10.5219/558]

-
Jantakee, K. and Y. Tragoolpua. 2015. Activities of different types of Thai honey on pathogenic bacteria causing skin diseases, tyrosinase enzyme and generating free radicals. Biol. Res. 48(1): 4.
[https://doi.org/10.1186/0717-6287-48-4]

-
Khan, K. A., H. A. Ghramh, Z. Ahmad, M. A. A. El-Niweiri and M. E. A. Mohammed. 2021. Honey bee (Apis mellifera) preference towards micronutrients and their impact on bee colonies. Saudi J. Biol. Sci. 28(6): 3362-3366.
[https://doi.org/10.1016/j.sjbs.2021.02.084]

-
Kieliszek, M., K. Piwowarek, A. M. Kot, S. Błażejak, A. Chlebowska-Śmigiel and I. Wolska. 2018. Pollen and bee bread as new health-oriented products: A review. Trends Food Sci. Technol. 71: 170-180.
[https://doi.org/10.1016/j.tifs.2017.10.021]

-
Kim, H., J. Hwang, Z. Ullah, B. Mustafa and H. W. Kwon. 2022. Comparison of physicochemical properties of pollen substitute diet for honey bee (Apis mellifera). J. Asia-Pac. Entomol. 25(4): 101967.
[https://doi.org/10.1016/j.aspen.2022.101967]

-
Kim, S. A. and M. S. Rhee. 2015. Synergistic Antimicrobial Activity of Caprylic Acid in Combination with Citric Acid against both Escherichia coli O157 : H7 and Indigenous Microflora in Carrot Juice. Food Microbiol. 49: 166-172.
[https://doi.org/10.1016/j.fm.2015.02.009]

-
Kim, Y. K., S. Lee, J. H. Song, M. J. Kim, U. Yunusbaev, M.-L. Lee, M. S. Kim and H. W. Kwon. 2020. Comparison of Biochemical Constituents and Contents in Floral Nectar of Castanea spp. Molecules 25(18): 4225.
[https://doi.org/10.3390/molecules25184225]

-
Lau, P. W. and J. C. Nieh. 2016. Salt preferences of honey bee water foragers. J. Exp. Biol. 219(6): 790-796.
[https://doi.org/10.1242/jeb.132019]

-
Lee, G. and M. Ahn. 2019. The characteristics and analysis of nutritional compositions of bee pollen from Korea. J Apic. 34: 73-86.
[https://doi.org/10.17519/apiculture.2019.04.34.1.73]

-
Manning, R. 2001. Fatty acids in pollen: a review of their importance for honey bees. Bee World 82(2): 60-75.
[https://doi.org/10.1080/0005772X.2001.11099504]

-
Manning, R. 2016. Artificial feeding of honeybees based on an understanding of nutritional principles. Anim. Prod. Sci. 58(4): 689-703.
[https://doi.org/10.1071/AN15814]

-
Marco Antonio, D. S., K. R. Guidugli-Lazzarini, A. M. do Nascimento, Z. L. P. Simões and K. Hartfelder. 2008. RNAi-mediated silencing of vitellogenin gene function turns honeybee (Apis mellifera) workers into extremely precocious foragers. Naturwissenschaften 95(10): 953-961.
[https://doi.org/10.1007/s00114-008-0413-9]

-
Morais, M. M., A. P. Turcatto, R. A. Pereira, T. M. Francoy, K. R. Guidugli-Lazzarini, L. S. Goncalves, J. De Almeida, J. Ellis and D. De Jong. 2013. Protein levels and colony development of Africanized and European honey bees fed natural and artificial diets. Genet. Mol. Res. 12(4): 6915-6922.
[https://doi.org/10.4238/2013.December.19.10]

-
Mureşan, C. I. and A. Buttstedt. 2019. pH-dependent stability of honey bee (Apis mellifera) major royal jelly proteins. Sci. Rep. 9(1): 9014.
[https://doi.org/10.1038/s41598-019-45460-0]

-
Muth, F., P. R. Breslow, P. Masek and A. S. Leonard. 2018. A pollen fatty acid enhances learning and survival in bumblebees. Behav. Ecol. 29(6): 1371-1379.
[https://doi.org/10.1093/beheco/ary111]

-
Oddo, L. P., M. G. Piazza and P. Pulcini. 1999. Invertase activity in honey. Apidologie 30(1): 57-65.
[https://doi.org/10.1051/apido:19990107]

-
Park, Y., J.-H. Kim and S.-H. Kim. 2017. Free Sugar and Organic Acid Contents of Pollens from Quercus spp. in Korea. J. Apic. 32(4): 375-379.
[https://doi.org/10.17519/apiculture.2017.11.32.4.375]

-
Quinto, E. J., P. Jiménez, I. Caro, J. Tejero, J. Mateo and T. Girbés. 2014. Probiotic lactic acid bacteria: a review. Food Nutr. Sci. 5(18): 1765.
[https://doi.org/10.4236/fns.2014.518190]

-
Retschnig, G., J. Rich, K. Crailsheim, J. Pfister, V. Perreten and P. Neumann. 2021. You are what you eat: relative importance of diet, gut microbiota and nestmates for honey bee, Apis mellifera, worker health. Apidologie 52(3): 632-646.
[https://doi.org/10.1007/s13592-021-00851-z]

-
Rortais, A., G. Arnold, M.-P. Halm and F. Touffet-Briens. 2005. Modes of honeybees exposure to systemic insecticides: estimated amounts of contaminated pollen and nectar consumed by different categories of bees. Apidologie 36(1): 71-83.
[https://doi.org/10.1051/apido:2004071]

- Saffari, A., P. G. Kevan and J. L. Atkinson. 2010. Palatability and consumption of patty-formulated pollen and pollen substitutes and their effects on honeybee colony performance. J. Apic. Sci. 54(2): 63-71.
-
Schulz, D. J., Z.-Y. Huang and G. E. Robinson. 1998. Effects of colony food shortage on behavioral development in honey bees. Behav. Ecol. Sociobiol. 42(5): 295-303.
[https://doi.org/10.1007/s002650050442]

-
Simcock, N., H. Gray, S. Bouchebti and G. A. Wright. 2018. Appetitive olfactory learning and memory in the honeybee depend on sugar reward identity. J. Insect Physiol. 106(1): 71-77.
[https://doi.org/10.1016/j.jinsphys.2017.08.009]

-
Smetanska, I., O. Tonkha, T. Patyka, D. Hunaefi, D. Mamdouh, M. Patyka, A. Bukin, M. Mushtruk, N. Slobodyanyuk and A. Omelian. 2021. The influence of yeast extract and jasmonic acid on phenolic acids content of in vitro hairy root cultures of Orthosiphon aristatus. Potr. S. J. F. Sci. 15: 1-8.
[https://doi.org/10.5219/1508]

-
Smith, D. B., F. Solano, L. G. Woodruff, W. F. Cannon and K. J. Ellefsen. 2019. Geochemical and mineralogical maps, with interpretation, for soils of the conterminous United States. USGS Scientific Investigations Report 2017-5118.
[https://doi.org/10.3133/sir20175118]

- Stanciu, O. G., L. A. Marghitas and D. Dezmirean. 2009. Macro- and oligo-mineral elements from honeybee-collected pollen and beebread harvested from Transylvania (Romania). Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Anim. Sci. Biotechnol. 66: 1-2.
- Standifer, L. 1980. Honey bee nutrition and supplemental feeding. Beekeeping in the United States Agriculture Handbook, 335: 39-45.
-
Toth, A. L. and G. E. Robinson. 2005. Worker nutrition and division of labour in honeybees. Anim. Behav. 69(2): 427-435.
[https://doi.org/10.1016/j.anbehav.2004.03.017]

-
Vaudo, A. D., H. M. Patch, D. A. Mortensen, J. F. Tooker and C. M. Grozinger. 2016. Macronutrient ratios in pollen shape bumble bee (Bombus impatiens) foraging strategies and floral preferences. Proc. Natl. Acad. Sci. 113(28): E4035-E4042.
[https://doi.org/10.1073/pnas.1606101113]

- Williams, I. H. 1994. The dependence of crop production within the European Union on pollination by honey bees. Agric. Zool. Rev. 6: 229-257.
- Winston, M. L. 1991. The biology of the honey bee. Harvard University press.