Electroantennogram and Behavioral Responses of Drone Hornets to Synthetic Sex Pheromones of Vespa velutina nigrithorax under Laboratory Conditions
Abstract
Vespa velutina nigrithorax, a subspecies of the yellow-legged hornet native to southern China, has invaded several other parts of the world in recent decades. It has become a devastating predator of honey bees, particularly in Korea and Europe. Sex pheromones, namely 4-oxo-octanoic acid (4-OOA) and 4-oxo-decanoic acid (4-ODA) were reported from the queen of V. velutina in China. In this study, we investigated the responses of drone hornets to synthetic sex pheromones of V. velutina nigrithorax using electroantennogram (EAG) and Y-tube olfactometer methods under laboratory conditions. EAG experiments revealed that drone hornets showed a significantly stronger response to the pheromone, unlike worker hornets. Further analysis of drones aged 0-15 days showed a positive correlation between age and response, indicating increasing sexual maturity as the day elapsed. These results were further confirmed by Y-tube olfactometer experiments, showing that drone hornets significantly preferred the queen’s sex pheromones over the workers, and behavioral preference showed a positive correlation with age. This study clearly demonstrated that drone hornets exhibited strong responses to synthetic sex pheromones of V. velutina nigrithorax in both EAG and Y-tube olfactometer tests. In conclusion, synthetic pheromones (4-OOA and 4-ODA) could be instrumental in monitoring and controlling V. velutina nigrithorax populations in Korea. However, field trials are necessary to validate the laboratory findings.
Keywords:
Vespa velutina nigrithorax preference, Electroantennogram (EAG), Y-tube olfactometer, 4-oxo-octanoic acid (4-OOA), 4-oxo-decanoic acid (4-ODA), Sexual maturity, recognitionINTRODUCTION
Biological invasion occurs when a non-native species is unintentionally introduced to a new region, often due to human activities. The damages caused by these invasions are evident across various sectors. For instance, Aedes and Rattus pose public health threats (Morand et al., 2015; Ngoagouni et al., 2015), Gypsy moth leads to agricultural losses (Wu et al., 2020), and Coptotermes formosanus causes forest damage (Evans et al., 2019). Notably, social insects̓ invasion can significantly disrupt ecosystems. Ants and hornets, for instance, easily establish large colonies and expand their offensive habitats (Mack et al., 2000; Beggs, 2001; Ascunce et al., 2011). Vespa velutina Lepeletier, 1836 (Vespidae), originally from tropical and subtropical regions like India, southern China, and Southeast Asia, emerged as an invasive pest in Europe with the discovery of V. velutina nigrithorax insouthern France, and later Spain and Belgium in the early 2000s (Villemant et al., 2006; Rome et al., 2013; Rodríguez-Flores et al., 2019). Also, from the initial report on Mt. Bongrae in Busan in 2003, it has progressively expanded its distribution into most part of South Korea (Kim et al., 2006; Choi et al., 2012; Jung, 2012; Kim et al., 2023). Similarly, its invasion was reported from Tsushima Island and then reached the main islands of Japan (Ueno, 2014).
V. velutina cause severe damage on honeybee populations, with records indicating that around 50 out of 300 honeybee colonies have been devastated by V. velutina attacks in South Korea (Jung et al., 2008). Direct colony losses exceeding 80% due to these attacks have been reported, with 93% of severe damages occurring in southern regions of South Korea (Jeong et al., 2016). The incursion of V. velutina exacerbates existing challenges such as Colony Collapse Disorder (CCD), winter losses due to climate change, and phenological mismatches between flowers and honeybees, posing significant threats to honeybee health (VanEgelsdorp et al., 2009; Dainat et al., 2012; Kudo and Ida, 2013; Brodschneider et al., 2018). Honeybees are vital not only for honey production but also as pollinators in floral ecosystems. The economic value of their pollination services for agricultural crop production was estimated to be around 6 trillion won (Jung, 2008). Additionally, V. velutina predates various insects such as Syrphidae, Calliphoridae, Lepidoptera larvae, and Apidae, most of which are pollinators (Monceau et al., 2014; Rome et al., 2021). Thus the predation by V. velutina could indirectly affect biodiversity by lowering plant reproduction, dispersal, and pollination activities.
Social Hymenopteran insects rely on pheromones for communication. Queen mandibular pheromone regulates ovarian development inhibition in worker hornets, suppresses new queen emergence, and enhances foraging efficiency (Pankiw et al., 1998). Brood pheromone also inhibits ovarian development in workers and improves foraging efficiency (Le Conte et al., 2001). Additionally, alarm pheromones convey alerting information (Veith et al., 1984), while recognition pheromones facilitate nest mate identification (Ruther et al., 1998, 2002). Researches on pheromones in the Vespidae family has revealed recognition, alarm, and sex pheromones (Wyatt, 2003). Studies primarily focus on species commonly found in apiaries, such as V. mandarinia, V. crabro, and V. velutina (Ono et al., 2003; Spiewok et al., 2006; Wen et al., 2017; Thiery et al., 2018; Cheng et al., 2022; Dong et al., 2022). It is challenging to understand the role of sex pheromone of diurnally active insects such as vespa hornets (Ayasse et al., 2001; Keeling et al., 2004). While most lepidopteran moths are nocturnal, and heavily rely on antennal pheromone receptors for finding mates (Zhang and Löfstedt, 2015), diurnal insects may prioritize visual cues over olfactory cues (Winfrey and Fincke, 2017).
Wen et al. (2017) discovered and synthesized queen pheromones of V. velutina in China, identifying them as ketones (4-oxo-octanoic acid, 4-oxo-decanoic acid). This finding suggested the potential use of these sex pheromones for trap-based control of V. velutina (Wen et al., 2017). However, these chemicals have not been tested on the Korean population of V. velutina. This study aimed to assess the electroantennogram and behavioral responses of V. velutina nigrithorax to the synthetic sex pheromone components.
MATERIALS AND METHODS
1. Chemicals and reagents
All the chemicals and reagents needed for these experiments were purchased from Sigma-Aldrich, Korea.
2. GC-MS instrument
Gas chromatography-mass spectrometry (GC-MS) analysis of the synthesized pheromones was performed on an Agilent 7890B Gas Chromatography system (Agilent Technologies, USA) coupled to an Agilent 5977A Mass Spectrometer Detector system (Agilent Technologies).
3. Insects
Two V. velutina nests were collected from the middle part of South Korea, Andong (36°61ʹ41ʺN, 128°82ʹ03ʺE), and (36°36ʹ48ʺN, 128°49ʹ12ʺE). The nests were transported to the laboratory undamaged. After removing the envelope of the nest, only the internal combs were kept in an acrylic breeding chamber at 25°C in the laboratory. Emerged adults from these nests were used for the experiments. Worker hornets, queens, and drone hornets were collected from the nests.
4. Synthesis of sex pheromones
Two sex pheromones, 4-oxo-octanoic acid (4-OOA) and 4-oxo-decanoic acid (4-ODA), were synthesized using the following procedures.
γ-decanolactone (20 g, 117.5 mmol) was dissolved in 100 mL of tetrahydrofuran (THF). Then, 6N NaOH (60 mL, 352.4 mmol) was slowly added to the solution. The reaction mixture was stirred for 2 hours. Subsequently, the pH was adjusted to 1 using 6M HCl, and the resulting mixture was extracted with dichloromethane (DCM) (100 mL). The organic layer obtained from the extraction was combined with PCC (38 g, 176.2 mmol) and stirred for an additional 2 hours. The mixture was then filtered through silica gel. The organic layer obtained after filtration was concentrated, and the resulting residue was subjected to column chromatography, yielding 4-oxo-decanoic acid (4.3 g, 20%).
In 4-oxo-octanoic acid synthesize, γ-octanolactone was used as a starting material. γ-octanolactone (10 g, 70.32 mmol) was dissolved in 50 mL of THF. 6N NaOH (35 mL, 352.4 mmol) was slowly added, and the reaction mixture was stirred for 2 hours. After adjusting the pH to 1 with 6 M HCl, the resulting mixture was extracted with DCM (50 mL). The organic layer obtained was combined with PCC (30 g, 62.42 mmol) and stirred for an additional 2 hours. The mixture was then filtered through silica gel, and the resulting organic layer was concentrated. The resulting residue was subjected to column chromatography, yielding 4-oxo-octanoic acid (4 g, 40%).
5. GC-MS analysis of the synthesized sex pheromones
Chemical analyses were conducted using a GC-MS system (HP 7890A-5975C, Agilent, US) following the procedure outlined by Wen et al. (2017). The analysis employed an HP-5ms column (30 m×250 μm×0.25 μm, Agilent, US) and the synthesized pheromones was individually analyzed in split mode with a split ratio of 1 : 10. A 1 μL injection amount was used, with the injector temperature set at 250°C. Helium was used as the carrier gas at a flow rate of 1 mL/min. The oven temperature ramp was programmed as follows: initial temperature of 50°C held for 2 minutes, followed by an increase of 10°C/min to 300°C, which was then held for 2 minutes. Mass spectra were obtained with a 70 eV electron impact and an ion source temperature of 230°C. Scanning was performed in the range of 40-500 amu.
6. Electroantennogram (EAG) analysis
Electroantennogram (EAG) (MP-15, Syntech, Netherlands) was used to examine the antennal conductivity responses of V. velutina at National institute of crop science, Suwon, South Korea. This study aimed to investigate the responses of V. velutina’s antenna to synthetic pheromone compounds (4-oxo-octanoic acid, 4-oxo-decanoic acid) in terms of caste-based and age-based differences. For the caste-based experiment, thirteen individuals from each caste, including worker hornets and drone hornets aged 72 hours, were used. In the age-based experiment, drone hornets aged 0, 1, 2, 3, 4, 5, 7, 10, 13, and 15 days after eclosion were used, with eight individuals in each age group. The insects were housed in 400 mL plastic cages and provided with a daily supply of 50% sucrose solution (50 mL).
In the EAG experiment, the scape and pedicel segments of the antennae were cut, and conductive gel (Signagel, Parker Laboratories, USA) was applied between the basal segments. The pheromone material was released for 2 seconds, with a 30-second stabilization period between repetitions to ensure amplitude stability. 4-OOA and 4-ODA were diluted to a concentration of 100 mg/mL and mixed with DCM as the solvent, at a ratio of 0.78. 10 μL of the pheromones was applied on a paper strip (4 mm by 15 mm) and allowed the solvent to evaporate. The responses were visualized using Syntech̓s EAG Pro 2.0 program.
7. Y-tube olfactometer behavioral analysis
A Y-tube olfactometer was used to assess the attraction of sex pheromones for V. velutina at Andong national university, Andong, South Korea. Before assessing responses to pheromones, we examined drone hornets̓ attraction to the queen to check selection is normal. Worker hornets, queens, and drone hornets were used for these experiments. Individuals acclimated to environmental conditions (25°C) for 30 minutes before the experiment. Ventilation was provided every 30 minutes between experiments, and red light was used to prevent visual interference. In Y-tube olfactometer experiment, air, dispensed by the SH-A3 air dispenser (Amazonl, China) at a rate of 125 mL per minute, split into two branches leading to containers (height 4.3 cm, diameter 3 cm, capacity 20 mL) for each sample. In these containers, samples were prepared using control (DCM) and sex pheromone (100 mg/mL, 0.78 ratio). A 10 μL mixture was injected onto filter paper (15 by 4 mm). Y-tubes (each 10 cm in length, with an internal diameter of 3 cm) were connected to the containers, and queens, worker hornets, and drone hornets were individually placed in containers. Each trial involved introducing 10 individuals, repeated 10 times, resulting in a total of 100 observations.
For the experiment, 3-day-old worker hornets and drone hornets aged 0, 1, 2, 5, 7, and 13 days were chosen. Twenty individuals were introduced for each experiment, repeated five times, totaling 100 observations. A choice was considered when the drone walked more than 2/3 of the length of the treated source or control arm and remained there for approximately 1 minute, or when it frequently visited the arm. A “no choice” decision was recorded if the drone had not moved after 5 minutes.
8. Date analysis
The level of antennal response measured via EAG was assessed by comparing the pre-stimulation value (2 seconds) with the peak value in the data. Due to the non-parametric distribution, the Kruskal-Wallis test analyzed differences in pheromone responses between worker hornets and drone hornets. Post hoc analysis used Dunn̓s test with Bonferroni correction to explore specific group differences further. Age-based response was examined using the Shapiro-Wilk normality test and linear regression to assess the correlation between age and response. Behavioral response choice for sex pheromones was calculated. To determine if the frequency of choice significantly varied with age, Chi-square tests were employed. All analyses were conducted using R software (Version 1.4.1106).
RESULTS
1. Chemical analysis of the synthesized sex pheromones
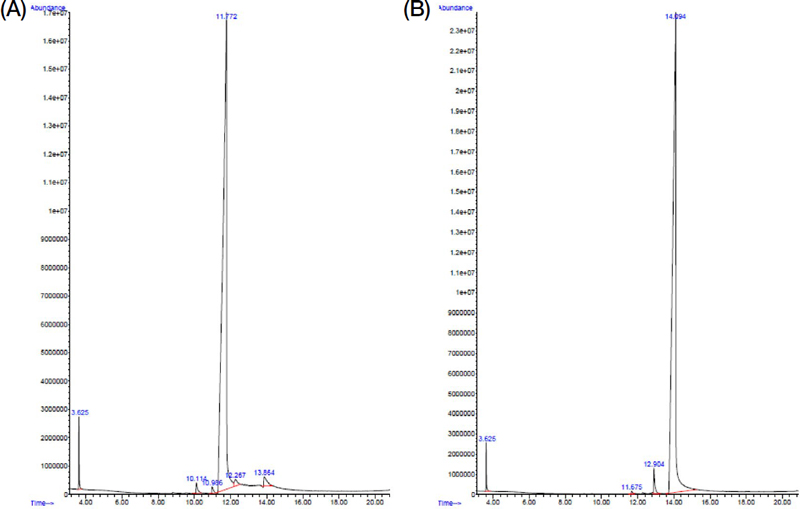
The identity of the two synthesized sex pheromones, 4-oxo-octanoic acid (4-OOA) and 4-oxo-decanoic acid (4-ODA), was confirmed by GC-MS, following the procedure outlined by Wen et al. (2017). As shown in Fig. 1, both compounds were clearly identified based of their respective molecular ion at m/z ratio of 158.0 and 186.0 mu for 4-OOA and 4-ODA, respectively.
2. EAG response by caste
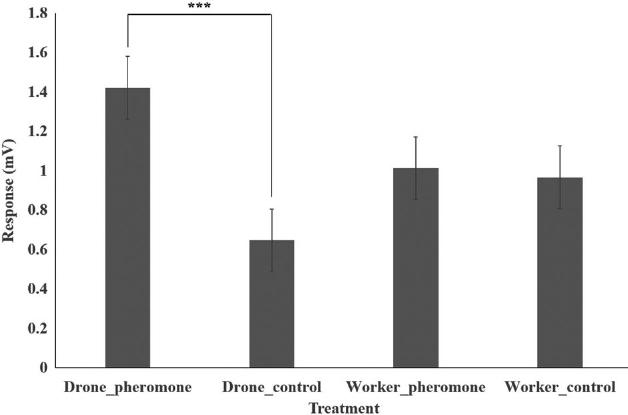
The EAG technique is highly valuable because it provides direct insights into the sensory capabilities of insects, helping researchers understand behaviors related to mating, feeding, and habitat selection. In this study, the EAG responses of V. velutina based on caste were analyzed and are presented in Fig. 2. After filtering out data with unstable amplitudes, we analyzed thirty-seven replications for each treatment. Drone hornets exhibited an average response of 1.42±0.15 (SE) mV to the sex pheromones, with a maximum recorded response of 3.72 mV. In contrast, their average response to the control was 0.65±0.08 mV. Worker hornets showed an average response of 1.01±0.13 mV to the sex pheromone, while 0.97±0.12 mV was recorded in the control group. Statistical analysis revealed that drone hornets exhibited a significantly stronger response compared to the other treatment groups (p<0.005).
3. EAG response by age
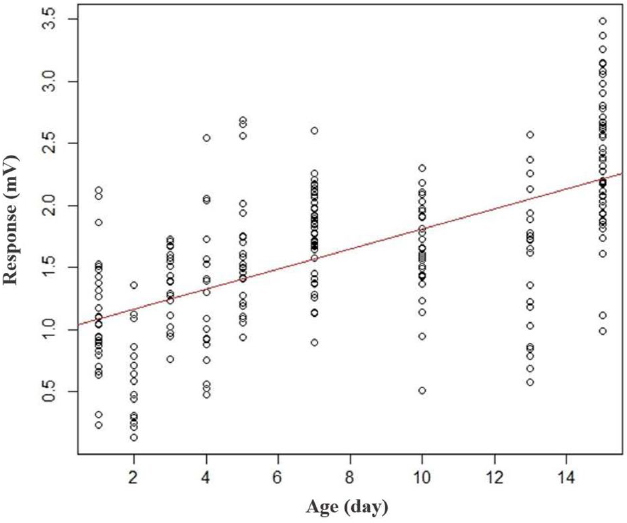
EAG analysis based on age was performed, and the results are shown in Fig. 3. In all age groups, the antennal response (mV) of drone hornets to pheromones was significantly higher than to the control (p<0.05). A positive correlation was observed between age and the antennal response to pheromones in drone hornets (y=0.08x+1.0) (p<0.001).
4. Olfactory behavioral response
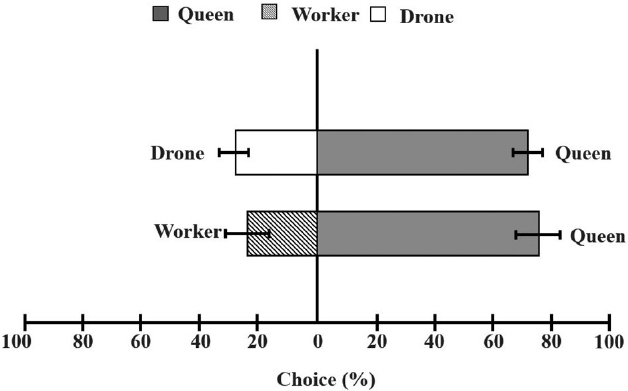
In the olfactory test, drones and workers were exposed to sex pheromone odors from queens, and their orientation responses are shown in Fig. 4. Male hornets displayed a strong attraction to the queen, with over 70% preference, while worker hornets showed no behavioral preference for the queen̓s sex pheromone. Thus, drone hornets were more attracted to the sex pheromone than to the control (X2=20.197, df=9, p=0.002).
Behavioral choice of V. velutina drone hornets between the queen and other castes of drone hornet or worker hornet in the Y-tube test. They showed higher preference to the queen over drone (X2 = 19.36, df = 1, p<0.001) or worker (X2 = 27.04, df = 1, p<0.001).
Upon close observation, drones of V. velutina exhibited the following behavioral characteristics when exposed to the sex pheromones: they were active, increased wing vibration, shook the lower part of their abdomen, and demonstrated acrobatic body bending. In contrast, the workers remained calm and showed no signs of these behavioral changes.
As drones aged, their attraction to the queen̓s sex pheromone increased, peaking at 13 days (p<0.05) (Fig. 5). This was also evident in their behavioral changes, such as increased wing vibration and shaking their lower abdomen, which increased over age. As this study only observed individuals up to 15 days, it̓s possible that beyond this period, their response could diminish. This decrease may be attributed to hormonal changes occurring towards the end of the insects̓ lifespan, which can influence sexual responsiveness, highlighting the intricate nature of mating behavior.
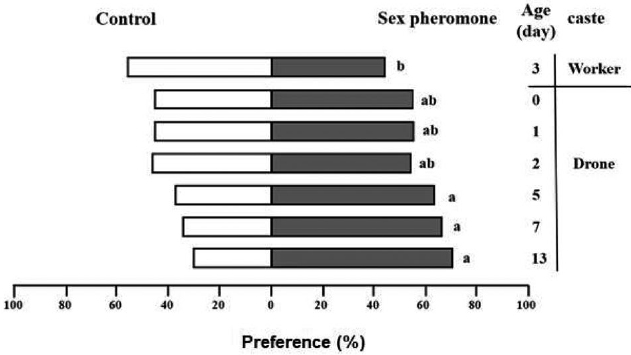
Behavioral choice to sex pheromone according to the age; workers (age in days; choice in percentage): (3; 44%) and drones (age in days; choice in percentage): (0; 55%), (1; 55%), (2; 54%), (5; 63%), (7; 66%), (13; 70%). There was a difference in the selection rate according to the treatment (X2 = 20.197, df = 6, p = 0.002).
DISCUSSION
Vespa velutina remains a widespread global predator of honey bees, posing a constant threat to the beekeeping industry in Korea and Europe (Jung, 2012; Arca et al., 2015). However, their spread in Korea has been less extensive than in Europe, possibly due to competition from other Vespa species in Korea (Jung, 2012). Drones of V. velutina are strongly attracted to the sex pheromones produced by queens, specifically 4-OOA and 4-ODA (Wen et al., 2017). Therefore, trapping based on sex pheromones could be an effective strategy to monitor and control the spread of V. velutina.
In this study, we evaluated the antennal response and attraction of drone hornets to synthetic sex pheromones under laboratory conditions. EAG experiments revealed that drone hornets showed a significantly stronger reaction to the pheromone, whereas worker hornets showed no significant difference in response between the pheromone and solvent. The sexual maturity of drone hornets at different ages was assessed, and the findings showed a positive correlation between age (from 0 to 15 days) and their response in EAG experiments.
The EAG analysis findings were corroborated by Y-tube olfactometer experiments, revealing drones̓ significant attraction to the sex pheromones, whereas no notable differences were observed among workers exposed to the same stimuli. Throughout the experiments, drones exhibited various behavioral changes, such as restlessness, shaking of their lower abdomen, and increased wing vibration. In contrast, none of these behaviors were observed in workers exposed to the sex pheromones. Age-specific behavior studies also demonstrated a positive correlation between age and pheromone sensitivity in drone hornets, emphasizing the importance of timing in reproductive activities.
Drone hornets, like honey bee drones, are eusocial insects primarily dedicated to mating (Metz and Tarpy, 2019). Therefore, reaching full sexual maturity is crucial for successful mating. Previous studies on V. similima and V. affinis have shown that they initiate reproductive activities after spending approximately 8-11 days within the nest (Martin, 1991, 1993). Given the similarities in life characteristics and genetics between V. similima and V. velutina (Choi et al., 2012; Namin and Jung, 2020), it is likely that their reproductive release characteristics are similar. Additionally, research by Poidatz et al. (2018) indicated that the testes of V. velutina gradually reduce in size and compartmentalization over time, with seminal vesicles eventually filling with sperm. As a result, mating is deemed impossible before 10 days post-eclosion. Similar findings have been reported in other Hymenopteran species (Boomsma et al., 2005; Fiorillo et al., 2008; Araújo et al., 2017).
Previous studies have shown that sexual maturity in insects typically increases with age, a trend also observed in this study. However, as insects near the end of their lifespan, hormonal changes can lead to a decrease in sexual responsiveness. For example, in Agrotis ipsilon, behavioral response and neural sensitivity to sex pheromones initially increase with age but decline after reaching sexual maturity. This decline is attributed to hormonal changes involving dopamine and ecdysteroid receptors, which regulate sexual sensitivity (Gadenne et al., 1993). Similarly, in Megoura viciae, pheromone release increases by the second day of adulthood but decreases after the sixth day (Hardie et al., 1990). In indoor rearing conditions, the average lifespan of drone hornets is approximately 17.9±7.1 days (SD), with a maximum of 30 days (Unpublished data), implying an optimal age and timing for mating. Although our study only observed individuals up to 15 days post-emergence, it is expected that beyond this optimal period, sexual responsiveness may decline.
After laboratory verification, the next step involves conducting a field test. Currently, pest control methods based on pheromones aim to reduce mating opportunities through mass trapping and mating disruption (Rizvi et al., 2021). However, commercially used pheromones are mostly volatile (Witzgall et al., 2010), making them susceptible to degradation under outdoor conditions such as rain, wind, and ultraviolet rays (Hamilton and Carlson, 2003; Byers, 2008; Jaoui et al., 2016). Therefore, careful selection of dispensers is crucial, and objectives must be chosen with caution.
Acknowledgments
This research was supported by the Korea Research Foundation (NRF-2018R1A6A1A03024862) and the Rural Development Administration research project (RS-2024-00397542).
References
- Araújo, V. A., U. Zama, H. Dolder and J. Lino-Neto. 2017. Morphology and ultrastructure of the spermatozoa of Scaptotrigona xanthotricha Moure (Hymenoptera, Ap- idae, Meliponini). J. Morphol. Sci. 22(3): 137-141.
-
Arca, M., F. Mougel, T. Guillemaud, S. Dupas, Q. Rome, A. Perrard, F. Muller, A. Fossoud, C. Capdevielle-Dulac, M. Torres-Leguizamon and X. X. Chen. 2015. Recon- structing the invasion and the demographic history of the yellow-legged hornet, Vespa velutina, in Europe. Biol. Invasions 17: 2357-2371.
[https://doi.org/10.1007/s10530-015-0880-9]

-
Ascunce, M. S., C. C. Yang, J. Oakey, L. Calcaterra, W. J. Wu, C. J. Shih and D. Shoemaker. 2011. Global inva- sion history of the fire ant Solenopsis invicta. Science 331(6020): 1066-1068.
[https://doi.org/10.1126/science.1198734]

-
Ayasse, M., R. J. Paxton and J. Tengö. 2001. Mating behavior and chemical communication in the order Hymenop- tera. Annu. Rev. Entomol. 46(1): 31-78.
[https://doi.org/10.1146/annurev.ento.46.1.31]

-
Beggs, J. 2001. The ecological consequences of social wasps (Vespula spp.) invading an ecosystem that has an abun- dant carbohydrate resource. Biol. Conserv. 99(1): 17-28.
[https://doi.org/10.1016/S0006-3207(00)00185-3]

-
Boomsma, J. J., B. Baer and J. Heinze. 2005. The evolution of male traits in social insects. Annu. Rev. Entomol. 50: 395-420.
[https://doi.org/10.1146/annurev.ento.50.071803.130416]

-
Brodschneider, R., A. Gray, N. Adjlane, A. Ballis, V. Brusbardis, J. D. Charrière and J. Danihlík. 2018. Multi-country loss rates of honey bee colonies during winter 2016/2017 from the COLOSS survey. J. Apic. Res. 57(3): 452-457.
[https://doi.org/10.1080/00218839.2018.1460911]

-
Byers, J. A. 2008. Active space of pheromone plume and its relationship to effective attraction radius in applied models. J. Chem. Ecol. 34(9): 1134-1145.
[https://doi.org/10.1007/s10886-008-9509-0]

-
Cheng, Y. N., P. Wen, K. Tan and E. Darrouzet. 2022. Designing a sex pheromone blend for attracting the yellow-legged hornet (Vespa velutina), a pest in its native and invasive ranges worldwide. Entomol. Gen. 42(3): 427-438.
[https://doi.org/10.1127/entomologia/2022/1395]

-
Choi, M. B., S. J. Martin and J. W. Lee. 2012. Distribution, spread and impact of the invasive hornet Vespa veluti- na in South Korea. J. Asia-Pac. Entomol. 15(3): 473-477.
[https://doi.org/10.1016/j.aspen.2011.11.004]

-
Dainat, B., D. Vanengelsdorp and P. Neumann. 2012. Colony collapse disorder in Europe. Environ. Microbiol. Rep. 4(1): 123-125.
[https://doi.org/10.1111/j.1758-2229.2011.00312.x]

-
Dong, S., A. Sun, K. Tan and J. C. Nieh. 2022. Identification of giant hornet Vespa mandarinia queen sex pheromone components. Curr. Biol. 32(5): R211-R212.
[https://doi.org/10.1016/j.cub.2022.01.065]

-
Evans, T. A., B. T. Forschler and C. C. Trettin. 2019. Not just urban: The Formosan subterranean termite, Coptoter- mes formosanus, is invading forests in the Southeast- ern USA. Biol. Invasions. 21: 1283-1294.
[https://doi.org/10.1007/s10530-018-1899-5]

-
Fiorillo, B. S., J. Lino-Neto and S. N. Báo. 2008. Structural and ultrastructural characterization of male reproduc- tive tracts and spermatozoa in fig wasps of the genus Pegoscapus (Hymenoptera, Chalcidoidea). Micron. 39(8): 1271-1280.
[https://doi.org/10.1016/j.micron.2008.03.005]

-
Gadenne, C., M. Renou and L. Sreng. 1993. Hormonal control of pheromone responsiveness in the male black cut- worm Agrotis ipsilon. Experientia 49: 721-724.
[https://doi.org/10.1007/BF01923960]

- Hamilton, D. W. and J. D. Carlson. 2003. Movement of Odors Off-Farm. Div. Agric. Sci. Nat. Resour., Oklahoma State Univ.
-
Hardie, J., M. Holyoak, J. Nicholas, S. F. Nottingham, J. A. Pickett, L. J. Wadhams and C. M. Woodcock. 1990. Aphid sex pheromone components: age-dependent release by females and species-specific male response. Chemoecology 1: 63-68.
[https://doi.org/10.1007/BF01325230]

-
Jaoui, M., M. Lewandowski, K. S. Docherty, E. W. Corse, W. A. Lonneman, J. H. Offenberg and T. E. Kleindienst. 2016. Photooxidation of farnesene mixtures in the presence of NOx: analysis of reaction products and their implication to ambient PM2.5. Atmos. Environ. 130: 190-201.
[https://doi.org/10.1016/j.atmosenv.2015.10.091]

-
Jeong, S. M., C. Y. Lee, D. W. Kim and C. Jung. 2016. Ques- tionnaire study on the overwintering success and pest management of honeybee damage assessment of Vespa hornets in Korea. Korean J. Apic. 31(3): 201-210.
[https://doi.org/10.17519/apiculture.2016.09.31.3.201]

- Jung, C. 2008. Economic value of honeybee pollination on ma- jor fruit and vegetable crops in Korea. J. Apic. 23(2): 147-152.
- Jung, C. 2012. Spatial expansion of an invasive hornet Vespa velutina nigrithorax Buysson (Hymenoptera: Vespidae) in Korea. J. Apic. 27(2): 87-93.
- Jung, C., M. S. Kang and D. W. Kim. 2008. Vespid wasps (Hymenoptera) occurring around apiaries in Andong, Korea. Korean J. Apic. 22(1): 63-70.
-
Keeling, C. I., E. Plettner and K. N. Slessor. 2004. Hymenop- teran semiochemicals. Chem. Pheromones. Other Se- miochemicals I. 133-177.
[https://doi.org/10.1007/b95452]

-
Kim, J. K., M. B. Choi and T. Y. Moon. 2006. Occurrence of Vespa velutina Lepeletier from Korea and a revised key for Korean Vespa species (Hymenoptera: Vespi- dae). Int. J. Entomol. Res. 36(2): 112-115.
[https://doi.org/10.1111/j.1748-5967.2006.00018.x]

-
Kim, M. J., S. Bak and C. Jung. 2023. Modeling abundance and risk impact of Vespa velutina nigrithorax (Hyme- noptera: Vespidae) in Korea: application of a species abundance model. Sci. Rep. 13(1): 13616.
[https://doi.org/10.1038/s41598-023-40016-9]

-
Kudo, G. and T. Y. Ida. 2013. Early onset of spring increases the phenological mismatch between plants and pollina- tors. Ecology 94(10): 2311-2320.
[https://doi.org/10.1890/12-2003.1]

-
Le Conte, Y., A. Mohammedi and G. E. Robinson. 2001. Primer effects of a brood pheromone on honeybee behavioural development. Proc. R. Soc. Lond. B Biol. Sci. 268(1463): 163-168.
[https://doi.org/10.1098/rspb.2000.1345]

-
Mack, R. N., D. Simberloff, W. M. Lonsdale, H. Evans, M. Clout and F. A. Bazzaz. 2000. Biotic invasions: causes, epidemiology, global consequences, and control. Ecol. Appl. 10(3): 689-710.
[https://doi.org/10.1890/1051-0761(2000)010[0689:BICEGC]2.0.CO;2]

- Martin, S. J. 1991. A simulation model for colony develop- ment of the hornet Vespa simillima (Hymenoptera, Vespidae). Jpn. J. Entomol. 59(1): 105-124.
-
Martin, S. J. 1993. Weight changes in adult hornets, Vespa af- finis (Hymenoptera: Vespidae). Insectes Soc. 40: 363-368.
[https://doi.org/10.1007/BF01253899]

-
Metz, B. N. and D. R. Tarpy. 2019. Reproductive senescence in drones of the honey bee (Apis mellifera). Insects 10(1): 11.
[https://doi.org/10.3390/insects10010011]

-
Monceau, K., O. Bonnard and D. Thiéry. 2014. Vespa velutina: a new invasive predator of honeybees in Europe. J. Pest Sci. 87(1): 1-16.
[https://doi.org/10.1007/s10340-013-0537-3]

-
Morand, S., F. Bordes, H. W. Chen, J. Claude, J. F. Cosson, M. Galan and A. Ribas. 2015. Global parasite and Rattus rodent invasions: The consequences for rodent borne diseases. Integ. Zool. 10(5): 409-423.
[https://doi.org/10.1111/1749-4877.12143]

-
Namin, S. M. and C. Jung. 2020. Genetic diversity of genus Vespa including an invaded species of V. velutina (Hy- menoptera: Vespidae) in Korea inferred from DNA barcoding data. J. Asia-Pac. Entomol. 23(2): 540-545.
[https://doi.org/10.1016/j.aspen.2020.04.004]

-
Ngoagouni, C., B. Kamgang, E. Nakouné, C. Paupy and M. Kazanji. 2015. Invasion of Aedes albopictus (Diptera: Culicidae) into central Africa: what consequences for emerging diseases?. Parasites Vectors 8: 1-7.
[https://doi.org/10.1186/s13071-015-0808-3]

-
Ono, M., H. Terabe, H. Hori and M. Sasaki. 2003. Components of giant hornet alarm pheromone. Nature 424(6949): 637-638.
[https://doi.org/10.1038/424637a]

-
Pankiw, T., M. L. Winston and G. E. Robinson. 1998. Queen mandibular gland pheromone influences worker honey bee (Apis mellifera L.) foraging ontogeny and juvenile hormone titers. J. Insect Physiol. 44(7-8): 685-692.
[https://doi.org/10.1016/S0022-1910(98)00040-7]

-
Poidatz, J., K. Monceau, O. Bonnard and D. Thiéry. 2018. Activity rhythm and action range of workers of the invasive hornet predator of honeybees Vespa velutina, measured by radio frequency identification tags. Ecol. Evol. 8(15): 7588-7598.
[https://doi.org/10.1002/ece3.4182]

-
Rizvi, S. A. H., J. George, G. V. Reddy, X. Zeng and A. Guer- rero. 2021. Latest developments in insect sex phero- mone research and its application in agricultural pest management. Insects 12(6): 484.
[https://doi.org/10.3390/insects12060484]

-
Rodríguez-Flores, M. S., A. Seijo-Rodríguez, O. Escuredo and M. D. C. Seijo-Coello. 2019. Spreading of Vespa velutina in northwestern Spain: influence of elevation and meteorological factors and effect of bait trapping on target and non-target living organisms. J. Pest Sci. 92: 557-565.
[https://doi.org/10.1007/s10340-018-1042-5]

-
Rome, Q., A. Perrard, F. Muller, C. Fontaine, A. Quilès, D. Zuccon and C. Villemant. 2021 January. Not just hon- eybees: predatory habits of Vespa velutina (Hymenop- tera: Vespidae) in France. Ann. Soc. Entomol. Fr. (NS) 57(1): 1-11.
[https://doi.org/10.1080/00379271.2020.1867005]

-
Rome, Q., L. Dambrine, C. Onate, F. Muller, C. Villemant, A. García-Pérez and E. Bruneau. 2013. Spread of the inva- sive hornet Vespa velutina Lepeletier 1836 in Europe in 2012 (Hym. Vespidae). Bull. Soc. Entomol. Fr. 118(1): 21-22.
[https://doi.org/10.3406/bsef.2013.2580]

-
Ruther, J., S. Sieben and B. Schricker. 1998. Role of cuticular lipids in nestmate recognition of the European hornet Vespa crabro L. (Hymenoptera Vespidae). Insectes Soc. 45: 169-179.
[https://doi.org/10.1007/s000400050077]

-
Ruther, J., S. Sieben and B. Schricker. 2002. Nestmate recogni- tion in social wasps: manipulation of cuticular hydrocarbon profiles induces aggression in the European hornet. Naturwissenschaften 89: 111-114.
[https://doi.org/10.1007/s00114-001-0292-9]

-
Spiewok, S., E. Schmolz and J. Ruther. 2006. Mating system of the European hornet Vespa crabro: male seeking strategies and evidence for the involvement of a sex pheromone. J. Chem. Ecol. 32: 2777-2788.
[https://doi.org/10.1007/s10886-006-9162-4]

-
Thiéry, D., O. Bonnard, L. Riquier, G. De Revel and K. Mon- ceau. 2018. An alarm pheromone in the venom gland of Vespa velutina: evidence revisited from the Europe- an invasive population. Entomol. Gen. 38(2).
[https://doi.org/10.1127/entomologia/2018/0719]

- Ueno, T. 2014. Establishment of the invasive hornet Vespa ve- lutina (Hymenoptera: Vespidae) in Japan. Int. J. Chem. Environ. Biol. Sci. 2(4): 220-222.
-
VanEngelsdorp, D., J. D. Evans, C. Saegerman, C. Mullin, E. Haubruge, B. K. Nguyen and J. S. Pettis. 2009. Colony collapse disorder: a descriptive study. PLoS One 4(8): e6481.
[https://doi.org/10.1371/journal.pone.0006481]

-
Veith, H. J., N. Koeniger and U. Maschwitz. 1984. 2-Methyl-3-butene-2-ol, a major component of the alarm phero- mone of the hornet Vespa crabro. Naturwissenschaften 71(6): 328-329.
[https://doi.org/10.1007/BF00396622]

-
Villemant, C., J. C. Streito and J. Haxaire. 2006. Premier bilan de l'invasion de Vespa velutina Lepeletier en France (Hyme- noptera, Vespidae). Bull. Soc. Entomol. Fr. 111(4): 535-538.
[https://doi.org/10.3406/bsef.2006.16372]

-
Wen, P., Y. N. Cheng, S. H. Dong, Z. W. Wang, K. Tan and J. C. Nieh. 2017. The sex pheromone of a globally invasive honey bee predator, the Asian eusocial hornet Vespa velutina. Sci. Rep. 7(1): 12956.
[https://doi.org/10.1038/s41598-017-13509-7]

-
Winfrey, C. and O. M. Fincke. 2017. Role of visual and non-visual cues in damselfly mate recognition. Int. J. Odo- natol. 20(1): 43-52.
[https://doi.org/10.1080/13887890.2017.1297259]

-
Witzgall, P., P. Kirsch and A. Cork. 2010. Sex pheromones and their impact on pest management. J. Chem. Ecol. 36(1): 80-100.
[https://doi.org/10.1007/s10886-009-9737-y]

-
Wu, Y., S. M. Bogdanowicz, J. A. Andres, K. A. Vieira, B. Wang, A. Cossé and S. E. Pfister. 2020. Tracking inva- sions of a destructive defoliator, the gypsy moth (Ere- bidae: Lymantria dispar): Population structure, origin of intercepted specimens, and Asian introgression into North America. Evol. Appl. 13(8): 2056-2070.
[https://doi.org/10.1111/eva.12962]

-
Wyatt, T. D. 2003. Pheromones and Animal Behavior: Com- munication by Smell and Taste. Cambridge University Press.
[https://doi.org/10.1017/CBO9780511615061]

-
Zhang, D. D. and C. Löfstedt. 2015. Moth pheromone recep- tors: gene sequences, function, and evolution. Front. Ecol. Evol. 3: 105.
[https://doi.org/10.3389/fevo.2015.00105]