Honeybee Visiting and Floral Nectar Characteristics of Styrax japonicus Sieb. & Zucc.
Abstract
This study was conducted to prove possibility of honey tree, which analyzed the visited number of honeybees, secreted nectar volume, nectar sugar content and amino acid in addition to estimating honey quantities that can ultimately reap in flowers of Styrax japonicus Sieb. & Zucc.. The surveyed tree’s flowers bloom during 12 days and maximum blooming period of flowers was on 23rd to 25th May in 2013. Honeybee visited flowers in priority and visited number of honeybee per flowering lateral bunch can be assumed 290 honeybees for a day. Honeybee visiting was concentrated at around 10~11 a.m.. On average, nectar volume secreted by nectary was 1.13 ul from one flower and nectar concentration (w/w %) presented 39.6%. Sugar content were calculated at 71.0 ug per flower which was estimated by multiplying secreted nectar volume (ul/flower) by nectar sugar (ug/ul). The minimum estimate of honey harvest for official tree (15 years, Height = 3m, Diameter at breast height = 12cm) in this study was 3,032 mg. Result of amino acid content ratio showed that Proline, Glutamate, Hydroproline, Serine, Asparagine were more abundant than others. Especially, Proline percentages (40.4%) were highest among the other amino acids. Finally, the surveyed tree is considered as possible honey plant because of its nectar characteristics and honeybee visiting. Honey is made of nectar sugar as well as by digestive system of honeybee, therefore a more associated study is necessary to analyze free sugar component in nectar and accurately presume to harvest honey quantity.
Keywords:
Apis mellifera, Honey tree, Honey potential, Sugar contentINTRODUCTION
The path of nectar secretion is that the modified pore tissue is distributed in nectary area and mechanism of secretion starts from Calvin cycle and is originated by the phloem sap and nectar is known to be secreted as sugar is hydrolyzed by accumulation of sugar in parenchyma cell present in nectary and resulting the osmotic phenomenon. This nectar is primarily composed of water and sugar, amino acid, organic acid, protein, fat, vitamin and mineral etc. are dissolved in it (Pacini et al., 2003).
The quantitative collection survey of nectar was started by Beutler (1953) in 1950s and Jablonski and Szklanowska (1979) attempted the method to collect nectar inside the flowers of Robinia pseudoacacia L. for the first time and Kearns and Inouye (1993) reported the nectar sampling method using a centrifugal separator.
In Korea, Chung and Kim (1984) have taken nectar by using Micro-capillary tube in the research on Korean genus Tilia and it was difficult to compare and analyze various species because some studies on blooming process or pollination mechanism have been carried out but no studies on nectar sampling and secreted volume amount estimation for honey plant species have been conducted. Recently, however, studies on honey-related flowering characteristics, visiting of bees and nectar secretion, nectar sugar content are being carried out (Han et al., 2009; Han et al., 2010; Kim et al., 2011; Kim et al., 2012; Kim et al., 2013; Kim et al., 2014). In addition, total amount of nectar, sugar content and content ratio of amino acid etc. have been reported to vary depending on the type of a pollinator and amino acid plays an important role in determining the taste of nectar to a pollinator even if they appear less than the content of sugar in the entire nectar composition (Nicolon et al., 2007).
Nectar is secreted with particular rhythms and can be reabsorbed during the life of flower. The pattern of secretion, cessation and reabsorption define dynamics of nectar production (Nicolson et al., 2007). Generally, flowers pollinated by diurnally pollinator produce nectar during the day but pollinated by nocturnally pollinator expose nectar at night. In addition, the nectar quantity produced per flower demonstrates that flowers pollinated by high energy requiring pollinator such as bats, hawkmoths and birds produce more nectar containing sugar than flowers pollinated by low energy requiring pollinator such as butterflies, bees and flies (Cruden et al., 1983). So, dynamics of nectar production coevolved with the plant pollinators and it is usually linked to the foraging behavior of pollinator activity, together with changing environmental variation.
Of 4,176 kinds of vascular plants in the Korean Peninsula, there are 555 kinds of honey plants (Korea Beekeeping Association, 2014) and about 250 species of trees are being actively used in the domestic beekeeping. Among them, Styrax japonicus Sieb. & Zucc. was presented as a honey plant by Ryu (2003) and especially, Lee (1998) classified it into a major honey plant species with R. pseudoacacia which is a major honey tree in South Korea.
S. japonicus is a deciduous tree belonging to Styracaceae and it grows up to 10 m and its white flowers bloom in the form of a racemous inflorescence in May and June (Lee, 2006). The tree was famous for landscape and street tree because of high ornamental value and then researches on tree availability and mass propagation were carried out by Kim (1989). Of the bioactivity substance, S. japonicus’s extract showed effectiveness of high anticancer activity and influenced on improvement of immune cell activation (Kwon et al., 2007; Kwon et al., 2014). Especially, S. japonicus’s honey have higher crude protein and ash as well as potassium than R. pseudoacacia’s honey, Paik (2014) is forecasting that S. japonicus’s honey is of benefit to hypertension prevention and immune function.
In a situation where the need for the discovery of new alternative honey tree and creation of honey tree due to the decline of R. pseudoacacia, this study is to identify the possibility as honey tree aimed at S. japonicus.
MATERIALS AND METHODS
Testing material
Targeting two S. japonicus that became 15-year-old in 2013 which is planted in the test forest of Forest Genetic Resources Division of Korea Forest Research Institute located in Suwon, Gyeonggi-do. The average height was 3m and diameter at breast height was 12cm and nectar characteristics were examined when percent flowering is between 60 and 80% in the entire crown.
Flowering rate and characteristics of honeybee visiting
The flowering rate was measured from flower bud stage to falling flower stage in flowering bunch. During the flowering period, the tree showed flowering rate which was surveyed on number of inflorescence aimed at 440 flower buds of two trees. Visited number of honeybee was observed at period of full bloom and surveyed from 06:00 to 19:00 through 1 hour interval. We investigated that three selected flowering lateral bunch was visited on honeybee respectively, visited number was counted during the ten minute and was investigated for thirty minute at total flowering lateral bunch and then multiply visited number by two, which value we estimated visited number for one hour.
Collecting nectar and nectar volume
In order to prevent loss of nectar by bees and insects, three cross-fertilization bags were put per tree and blooming flowers have been collected at the time when there was the most secreted nectar volume of S. japonicus and pistils and stamens were removed and then, nectar secreted from one flower was collected 1 time by using a centrifugal separator (1,000 rpm, 6 min.) and the secreted nectar volume of one flower was estimated. Also, nectar sugar concentration was measured by using a portable saccharometer (GMK-703T). And nectar was filtered in 0.45μM membrane filter until HPLC analysis and then, collected nectar was fixed in Eppendorf vial filled with 80% ethanol and then was stored in a low temperature refrigerator (-70°C).
Sugar content analysis
Collected nectar samples were filtered with 0.45μM syringe filter (Millipore, Billerica, MA, USA) and analyzed by using HPLC (Dionex ultimate 3000, Dionex, USA). Deionized water was used as the mobile phase and the velocity was set to 0.5ml/min and the oven temperature to 80°C Detection was conducted with Shodex Ri-101 detector (Japan) and Aminex 87P column (300mm × 7.8mm / Bio-rad, USA) was used. The content was calculated by the external standard method by integral meter and Sucrose, Glucose and Fructose (Sigma, USA) were used as standard brands. Also, the amount of honey in one flower was estimated through the proportional expression method of Petanidou (2003).
Amino acid content analysis
The amino acids of collected nectar samples were analyzed through O-phthalaldehyd (OPA)-Fluorenylmethyl chloroformate (FMOC) derivatization. The samples were mixed in the Borate buffer, OPA / Mercaptopropionic acid (MPA), FMOC reagent step by step and then, filtered with 0.45μM syringe filter (Millipore, Billerica, MA, USA) and analyzed by using HPLC (1200 series, Aglient, USA.). The mobile phase is A solution: B solution for A solution (pH 8.2) containing 10 mM Na2HPO4, 10mM Na2B4O710H2O and B solution mixed with Water : Acetonitrile : Methanol = 10 : 45 : 45 and the gradient condition was set to 55:45 in 26~28 minutes from initial 100:0 (v/v, %), 0:100 in 28~30.5 minutes and 100:0 from 30.5 minutes. Inno C18 column (Innopia, Korea, 4.6mm×150mm, 5μM) was used by setting the velocity to 1.5ml/min, the amount of injection to 1 ul and the column temperature to 40°C. By connecting UV detector and fluorescence detector, ultraviolet rays were detected at 338 nm, emission wavelength of the OPA derivative at 450nm, excitation wavelength at 340nm, emission wavelength of the FMOC derivative at 305nm and excitation wavelength at 266-nm.
RESULTS AND DISCUSSION
Flowering rate and visited pollinator
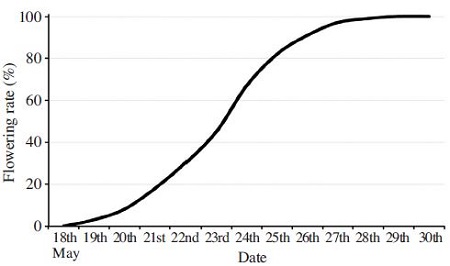
S. japonicus bloomed starting from May 19th and then 6 days later 82% of accumulated ratio of florescence on May 25th. Finally, the florescence was fell off on May 30th. Fig. 2 presents that the peak period of the florescence was appear at 60 to 80% of flowering rate during 24th to 25th of May.
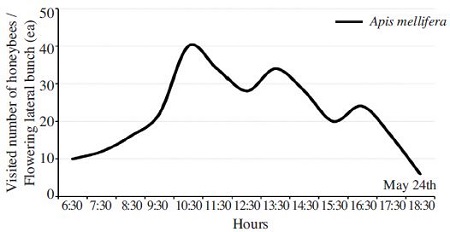
Episyrphus balteatus, Carbula putoni Jacovlev and Hypoderma bovis were visited to flower of S. japonicus. A. mellifera was the main visitor among the pollinators. Fig. 3 presents that a visited number of honeybee on a flowering lateral bunch in the peak period of florescence.
Two hundred ninety honeybees visited on flowering lateral bunch during the one day of maximum blooming period. The most visited time of honeybees was between 10 to 11 am, then the secondly visited time was between 1 to 2 pm. The same study method was used for other trees. 372 honeybees were visited to R. pseudoacacia (Han et al., 2009), 458 honeybees for Tilia amurensis and 488 honeybees for T. manshurica (Han et al., 2010).
The average of 160 honeybees was visited to T. insularis and the visiting of honeybees of S. japonicus was relatively higher than T. insularis (Kim et al., 2013). However, S. japonicus’s visiting of honeybees were less then other trees. The number of visiting of honeybees for all trees is not proper to compare due to the difference of geographical, environment, climatic facts and the period of florescence. However, it is possible to investigate indirectly honeybees preference to find out for honey tree. In order to find out the accurate the number of honeybees visiting for S. japonicus, the other trees which bloom similar period of florescence should be studied together with S. japonicus.
Nectar volume and free sugar content
The analysed results of secreted nectar volume and nectar sugar concentration taken 1 time from one flower by using the centrifuge and HPLC are shown in Table 1. On May 23rd, when surveying the nectar volume and nectar sugar concentration by centrifugal separator and portable saccharometer, 1.30 ul was secreted per flower and 37.5% was measured per flower from 108 flowers. Also, nectar of 1.09 ul and 1.00 ul per flower were secreted and nectar sugar concentrtaion of 39.7% and 41.5% were measured in 24th and 25th. Totally, average 1.13 ul was secreted per flower and average 39.6% was measured per flower.
Of the other results, Evodia daniellii was studied that 2.73 ul of male flower, 0.63 ul of female flower were shown as a result by using a centrifuge. Then it was compare to S. japonicus which was relatively more than nectar volume of female flower, but it was less than nectar volume of male flower of E. daniellii (Kim et al., 2014). In a result, the nectar volume of S. japonicus was relatively less than the total nectar volume was 12.0 ul on 15 years old of T. insularis in Suwon (Kim et al., 2013) and 18.4 ul on 10 years old of T. manshurica (Han et al., 2010) and the total nectar volume was 13.9 ul on 15 years old of Crataegus pinnantifida (Kim et al., 2011). The results were different from those of this result because 3 ul microcapillary tube was used for total nectar volume and nectar has a property of being continually secreted until flowers are fertilized and therefore, total nectar volume secreted per flower was estimated by adding all of the secreted volume of more than 2 times. In addition, nectar concentration and climatic factors were not considered, so it is difficult to compare directly. Nectar volume including water is affected climatic factors. Therefore, it is not proper to use the comparison of the difference (Kim et al., 2012). 1.13 ul of secreted nectar volume per flower by S. japonicus is considered as the least of the total nectar volume, it is the way to find out the sugar content for each flower. Also, S. japonicus was valid as honey tree because S. japonicus includes 15% to 60% sucrose concentration for honeybees (Seely, 1986), Furthermore, S. japonicus has the most preference of nectar concentration (40% to 50%) for honeybees (Waddington, 2001).
According to Nepi and Stpiczynsk (2007), plants try to strive to maintain homeostasis of nectar concentration depending on changes in the environment to attract a pollinator ecologically and maintain the energy balance of the plant by adjusting the sugar concentration. Therefore, for the selection of honey trees or individuals, it is determined that sugar content per flower that can be calculated with sugar content per unit volume and secreted nectar volume or dry nectar volume (dry nectar) should be a major factor than nectar volume and nectar sugar concentration affected by meteorological factors (Kim et al., 2012).
According to HPLC analysis, free sugar content was found to be 56.2 ug/ul in 23rd, 54.2 ug/ul in 24th, 81.0 ug/ul in 25th. It turned out that the most free sugar content was calculated in 25th. The sugar content per flower was calculated by multiplying sugar content per unit volume by HPLC and nectar volume per flower estimated by the centrifugal separator and as a result, the free sugar content per flower could be calculated to be 72.9 ug, 59.3 ug and 81.0 ug in 23rd, 24th and 25th, respectively. Totally, average 1.13 ul was secreted per flower and nectar concentration of 39.6% was calculated in nectar and free sugar content of 63.8 ug/ul was analyzed and then sugar content per flower could be calculated to be 71.0 ug averagely in S. japonicus nectar.
In addition, compared with the results showing average 48.0 ug and 37.8 ug, calculated sugar content per flower of E. daniellii male and female tree reported by Kim et al. (2014) was less than S. japonicus’s sugar content per flower. However, the results could vary from environmental factor. Actually, because S. japonicus’s calyx is bigger than E. daniellii calyx, we thought that S. japonicus’s nectar characteristics were more values than E. daniellii. Nectar is secreted as sugar stored at nectary parenchyma cell present under the calyx is hydrolyzed and the width of the calyx influences the temporary storage space of nectar so if the calyx is wide, pollination of pollinator is considered to be easy and more nectar volume to be secreted under the same conditions. Based on the results of these studies, ongoing research should be carried out by associating the structural and physiological characteristics of flowers in addition to external conditions.
Nectar sugar content and estimated honey production
Table 2 show that nectar and honey potential were estimated by sugar content per flower and flower potential in S. japonicus (Heigh=3m, Diameter at breast height=12cm).

Estimated sugar and honey production based on sugar content of flower and flower potential measurements
S. japonicus’s flowers blossomed from 19th May to 30th May averagely. When the flowering rate was 100 percentage, number of flowers estimated 36,300 which was investigated by Han et al. (2010) method. Mean of sugar content multiplied by 36,300, which was the estimated number of flowers per tree was 2,577.3mg sugars per tree. Petanidou (2003) said that nectar sugar content and amount of honey can be calculated at a rate of 85:100 to estimate the amount of honey in the nectar sugar content and if nectar sugar content is concentrated by more than 85%, it can be reached to the level of honey. According to the results of this study, the honey harvest per flower could be estimated to be 81.4 ug in one flower of S. japonicus. It is determined that the result shows the minimum honey yield because they are sugar content taken once from flowers and more honey can be harvested than the results of this study because feeding of bees is done at least once until fertilization is made. The honey making process is associated with digestion and reproduction of bees but in order to develop an estimation equation to calculate the amount of honey that can be harvested, more ongoing research is needed targeting species of trees likely to be honey plant trees.
Analysis of amino acid
The results of analyzing the amino acid content and ratio of S. japonicus’s nectar analyzed by HPLC are as shown in Table 3.
In S. japonicus’s nectar, more content were detected in the order of Proline, Glutamate, Hydroproline, Serine, Asparagine and they accounted for 75.2% of the total nectar amino acid. Of dominated amino acids, Proline showed the content of 40.4% in nectar and then prolinerich nectar is highly preferred by bees because Proline is an essential amino acid in the egg-laying of a queen bee (Hrassnigg et al., 2003), required for the development of an insect and oxidation of the wing muscles, metabolized faster than other amino acids and can release more ATP (Adenosine triphosphate) without complete metabolism (Carter et al., 2006). Asparagine known as an amino acid repellent to honeybees, Andrenidae and flies showed the 17.1% content ratio in nectar (Petanidou et al., 2006). Kim et al. (2014) reported that proline showed the content of 12.2% in E. daniellii’s male flower nectar and 7.5% in female flower nectar. It compared the results that proline in S. japonicus’s nectar showed the more content ratio than proline ratio of E. daniellii’s nectar.
GABA (Gamma-aminobutyric acid) and Phenylalanine among the nectar amino acids of most plants in the Mediterranean region are known to account for a large percentage in the preference of honeybees and Megachilidae so this study analyzed GABA 3.0%, Phenylalanine 1.0% in nectar. These amino acid ratios are considered to show differences due to tree species characteristics and climatic differences. These amino acids play an important role in determining the taste of nectar for pollinators so differences in amino acid composition are considered to influence the bee visiting. Studies on the feeding behavior of bees depending on the amino acid content should be continuously carried out in order to accurately determine the potential of honey trees because the difference in amino acid content is influenced climatically and environmentally.
CONCLUSION
The surveyed tree’s flowers bloom during 12 days and maximum blooming period of flowers was on 23rd to 25th May in 2013. Honeybee visited flowers in priority and visited number of honeybee per flowering lateral bunch can be assumed 290 honeybees for a day. On average, nectar volume secreted by nectary was 1.13 ul from one flower and nectar concentration presented 39.6%. Sugar content were calculated at 71.0 ug per flower which was estimated by multiplying secreted nectar volume by nectar sugar content. The minimum estimate of honey harvest for official tree (15 years, Height = 3m, Diameter at breast height = 12cm) in this study was 3,032mg. Result of amino acid content ratio showed that Proline, Glutamate, Hydroproline, Serine, Asparagine were more abundant than others. Especially, Proline percentages (40.4%) were highest among the other amino acids. Finally, the surveyed tree is considered as possible honey plant because of its nectar characteristics and honeybee visiting.
LITERATURE CITED
- Beutler, R., (1953), Nectar, Bee World, 34, 106-116, 128-136, 156-162.
-
Carter, C., S. Shafir, L. Yehonatan, R.G. Palmer, and R. Thornburg, (2006), A novel role for proline in plant floral nectars, Naturwissenchaften, 93, p72-79.
[https://doi.org/10.1007/s00114-005-0062-1]

- Chung, Y.H., and K.J. Kim, (1984), Flowering process and pollination mechanism of Genus Tilia in Korea, Korean J. Botany, 27, p107-127.
- Cruden, R. W., S. M. Hermann, and S. Peterson, (1983), Patterns of nectar production and plant-pollinator coevolution, In B. Bentley, and T. Elias (Eds.), New York, Columbia University Press, The Biology of Nectaries, p80-125.
- Han, J., M.S. Kang, S.H. Kim, K.Y. Lee, and E.S. Baik, (2009), Flowering, Honeybee visiting and nectar secretion characteristics of Robinia pseudoacacia L. in Suwon, Gyeonggi province, Korean J. Apiculture, 24(3), p147-152.
- Han, J., S.H. Kim, M.S. Kang, C.S. Kim, and E.S. Baik, (2010), Flowering and nectar characteristics of Tilia amurensis Rupr. and Tilia manshurica Rupr. et Max, Korean J. Apiculture, 25(3), p216-221.
- Hrassnigg, N., B. Leongard, and K. Crailsheim, (2003), Free amino acids in the hemolymph of honey bee queens (Apis mellifera L.), Amino Acids, 24, p205-212.
- Jablonski, B., and K. Szklanowska, (1979), The proposal of changing the method of plant nectar secretion investigation. zesz, Nauk, 23, p105-113.
- Kearns, C.A., and D.W. Inouye, (1993), Techniques for pollination biologists, University Press of Colorado, Niwot, Colorado.
- Kim, G.T., (1989), Effects of pretreatment on the field germination rate of some tree seed, J. Korean For. Soc, 78(1), p26-29.
- Kim, M.S., S.H. Kim, J. Han, M.S. Kang, and Y.K. Park, (2011), Honeybee Visit and Netar Secretion Characteristics of the Chinese Hawthorn, Crataegus pinnatifida Bunge, Korean J. Apiculture, 26(1), p11-14.
- Kim, M.S., S.H. Kim, J. Han, and J.S. Kim, (2012), Analysis of secretion quantity and sugar composition of nectar from Tilia amurensis Rupr, Korean J. Apiculture, 27(1), p79-85.
- Kim, M.S., S.H. Kim, J.H. Song, and H. Kim, (2013), Honeybee visiting and secreted nectar characteristics of Tilia insularis Nakai and relation with meteorologic traits, Korean J. Apiculture, 28(5), p331-337.
-
Kim, M.S., S.H. Kim, J.H. Song, and H. Kim, (2014), Analysis of secreted nectar volume, sugar and amino acid content in male and female flower of Evodia daniellii Hemsl, J. Korean For. Soc, 103(1), p43-50.
[https://doi.org/10.14578/jkfs.2014.103.1.43]

- Korea Beekeping Association, (2014), http://www.korapis.or.kr.
- Kwon, O.W., C.H. Kim, H.S. Kim, M.C. Kwon, J.H. Ahn, H.J. Lee, H.Y. Kang, and H.Y. Lee, (2007), Comparison of immuno modulatary and anticancer activities according to the parts of the Styrax japonica Sieb. et Zucc, Korean J. Medicinal Crop Sci, 15(3), p170-176.
-
Kwon, O.W., W.J. Kim, and H.J. Lee, (2014), Anti-cancer activity of Styrax japonica Bark Extracts, J. Korean Wood Sci. & Tech, 42(1), p68-77.
[https://doi.org/10.5658/WOOD.2014.42.1.68]

- Lee, C.B., (2006), Coloured flora of Korea, Hyangmoonsa, p914.
- Lee, K.J., (1998), Seasonal distribution of flowering and classification of 198 woody species into honey-producing and pollen-collecting plants in Korea, Korean J. Apiculture, 13(2), p121-132.
-
Nepi, M., and M. Stpiczynska, (2007), Nectar resorption and translocation in Cucurbita pepo L. and Platanthera chlorantha Custer (Rchb.), Plant Biology, 9, p93-100.
[https://doi.org/10.1055/s-2006-924287]

- Nicolson, S.W., M. Nepi, and E. Pacini, (2007), Nectar production and presentation, Nectaries and Nectar, p167-214.
- Pacini, E., M. Nepi, and J.L. Vesprini, (2003), Nectar biodiversity: a short review, Plant Syst. Evol, 238, p7-21.
- Paik, W.K., A.K. Kwak, M.L. Lee, and H.S. Sim, (2014), Studies on the chemical characteristics of Jujube (Zizyphus jujube var. inermis) and Snowbell (Styax japonica) honey produced in Korea, Korean J. Apiculture, 29(2), p125-135.
- Petanidou, T., (2003), Introducing plants for bee-keeping at any cost ? - Assessment of Phacelia tanacetifolia as nectar source plant under xeric Mediterranean conditions, Plant Syst. Evol, 238, p155-168.
-
Petanidou, T., A.J. Van Laere, W.N. Ellis, and E. Smets, (2006), What shapes amino acid and sugar composition in Mediterranean floral nectars?, Oikos, 115, p155-169.
[https://doi.org/10.1111/j.2006.0030-1299.14487.x]

- Ryu, J.B., (2003), Classification of honey plants in Korea, Korean J. Apiculture, 18(1), p5-22.
-
Seely, T.D., (1986), Social foraging by honeybees: how colonies allocate foragers among patches of flowers, Behav. Ecol. Sociobiol, 19, p343-354.
[https://doi.org/10.1007/BF00295707]

-
Waddington, K.D., (2001), Subjective evaluation and choice behavior by nectar- and pollen- collecting bees, In: L. Chittka, and J. D. Thomson (Eds.), Cambridge University Press, Cognitive ecology of pollination, p41-60.
[https://doi.org/10.1017/CBO9780511542268.004]