
Antimicrobial Activity of Propolis Extracts against Skin Pathogen
Abstract
Propolis extracts from Korea and Brazil were investigated for their antibacterial and antifungal activities against skin pathogen. For this, we used 80% ethanol extracts of propolis from Korea (KPEE) and Brazil (BPEE). Minimal inhibitory concentration (MIC) for the strains tested was determined using the method of broth dilution with the KPEE and BPEE in serial concentration, respectively. The antibacterial activity of KPEE showed MIC of 0.25μg/ml for Bacillus subtilis and Escherichia coli; 0.5μg/ml for Staphylococcus aureus; 1.0μg/ml for Propionibacterium acnes. However, the MIC of BPEE for B. subtilis, S. aureus, P. acnes, and E. coli was 1.0, 0.5, 4.0, and 0.5μg/ml. The antifungal activity of KPEE using agar diffusion test was 24.34±0.5, 19.19±1.0 and 26.64±1.0 of the inhibitory zone for Trichophyton tonsurans, T. mentagrophytes and T. rubrum. The inhibitory zone of BPEE was 11.48±0.3, 11.0±0.5 and 13.11±0.5 for T. tonsurans, T. mentagrophytes and T. rubrum. All tested skin pathogens were more susceptible to the KPEE than BPEE. It seems that the Korean propolis has antimicrobial activity more than Brazilian propolis.
Keywords:
Korean propolis, Brazilian propolis, Skin pathogens, Antibacterial activity, Antifungal activityINTRODUCTION
Propolis is a natural product of plant resins collected by honeybees (Apis mellifera L.) from various plant sources. Honeybees collect vegetal exudates and form pellets with their mandibles, mixing the exudates with wax and products of their salivary glands (Fearnley, 2001). Its chemical composition is quite complex since more than 300 compounds, such as polypenols, phenolic aldehydes, sequiterpene quinines, coumarins, amino acids, steroids, and inorganic compounds, have been identified in propolis (Fearnley, 2001; Kuropatnicki et al., 2013). Propolis is used to strengthen the nest, provide protection from microorganisms, and as an embalming substance to cover the carcass of a hive invader (Fearnley, 2001). It is used as a remedy in folk medicine since ancient times. The medicinal of propolis have been widely investigated such as antibiotic, antitumor, anti-HIV, cytotoxic and antioxidant activity (Matsuno et al., 1997; Kimoto et al., 1998; Menna-Barreto et al., 2009; Fatahinia et al., 2012). A large number of biological activities of propolis are based on its complex chemical compositions (Bankov, 2005), which are mainly dependent on the plant sources. The propolis extract contains kinds of phenolic compositions and displays good antioxidant property (Dudonne et al., 2011). Moreover, there are growing evidences showing that propolis in market has been partly mixed with ethanol extracts of propolis. Propolis has been used as a constituent health functional food for anti-oral microbes and antioxidant in Korea (KFDA, 2010). These properties of propolis have done their work using propolis from different geographic locations. Nevertheless, propolis has been always active, although it is known that in different geographic areas its chemical compositions vary due to the different plant sources (Markham et al., 1996). In this work we wish to report the results of our study on the antibacterial and antifungal activity of propolis from different locations in Korea and Brazil.
MATERIALS AND METHODS
Preparation of alcohol extracted propolis
Raw propolis samples used in this study were obtained from a manufacturer in Korea 2013. From that manufacturer, we took equal amounts of propolis sample from domestic-collected materials (Korean propolis) and from imported raw propolis from Brazil (Brazilian propolis) during storage for processing in the manufacturer. Raw propolis was cut into small pieces and then extracted with 80% ethanol (1:10 w/v) for 24 h at room temperature (KFDA, 2014). The extracts were evaporated in vacuum to dryness. Yield of extracts is 33.5% of Korea and 32.3 of Brazil propolis. The residue was suspended in H2O and then partitioned with n-hexane, EtOAc and BuOH, respectively. Table 1 showed the extraction yields of propolis.
Chromatography of extracted propolis
A thin-layer chromatography was performed on C18 plates (Merck 15683, USA), which were cut from original plates into 5×10cm before use. TLC Spotting Capillary Tube (90μg) was used for samples application. The plates were developed at room temperature in a twin trough chromatographic chamber with the use of several mobile phase systems: methanol - water - chloroform in volume composition 80:20:0.1. The chamber was previously saturated with vapour of 50ml of mobile phase for 30 min. After development, the plates were dried in air dryer. Densitometric and spectrodensitometric analyses were carried out by UV detector, 10% sulfuric acid (H2SO4) and 1% iron chloride (FeCl3).
Minimal inhibitory concentrations (MIC) for antibacterial activity
MIC were performed using the following ATCC strains from the Korean Culture Center of Microorganisms, Seoul, Korea : Staphylococcus aureus (6538), Escherichia coli (21277), Bacillus subtilis (9372), and Propionibacterium acnes (6919). S. aureus and E. coli inoculated in Brain Heart Infusion agar (Difco) and B. subtilis inoculated in Nutrient Agar (Difco) were incubated aerobically at 37°C for 2 days. P. acnes was cultured at 37°C on Reinforced Clostridium Medium (BD, MD, USA) under anaerobic conditions before the assay. MIC for propolis against the tested strains were determined using the propolis extracts in serial concentrations. Control wells with serial concentrations of ethanolic alcohol solution were also tested. All tests were performed in quadruplicate.
Agar diffusion test for antifungal activity
Screening for antifungal activity was performed agar diffusion test using the following ATCC strains from the Korean Culture Center of Microorganisms, Seoul, Korea: Trichophyton tonsurans (18020), Trichophyton mentagrophytes (28185) and Trichophyton rubrum (28188). T. tonsurans, T. mentagrophytes and T. rubrum were grown in Saboraud agar (Difco) and incubated at 26°C for 4 days. For the investigation of the antifungal activity the agar cup method was used (Spooner and Sykes, 1972). Samples of 50μg/well of each propolis extract were seeded in each well . EtOH is a negative control. The antifungal activity was measured as a diameter of the inhibitory zones. An inhibitory zone with diameter less than 10mm corresponds to lack of activity.
RESULTS
TLC analysis
TLC analysis of 80% ethanol extracts, hexane fraction, ethyl acetate fraction, butanol fraction and H2O fraction of propolis of different origin on the TLC plate showed different chromatogram suggesting different chemical constituents (Fig. 1). When C18 plate was sprayed with 10% H2SO4, several spots were detected at Rf value around 0.5. More the Korea propolis than the Brazilian propolis has yellow spot that could be flavonoids. 80% ethanol extracts and ethyl acetate fractions of propolis had similar patterns compared to other fractions one. TLC plate treated with 1% FeCl3 revealed that the Koran propolis and Brazilian propolis extracts had a different pattern. The 80% ethanol extracts and ethyl acetate fraction of propolis had the gray spots that could be phenolic compounds. There are no spots on hexane, butanol, and water fraction of propolis. The spots of phenolic compounds are more the Korean propolis than Brazilian propolis. Most of components existed in propolis samples were detected at 254 nm of UV wavelength.
For the investigation of the antimicrobial activity we used 80% ethanol extracts of propolis from Korea (KPEE) and Brazile (BPEE).
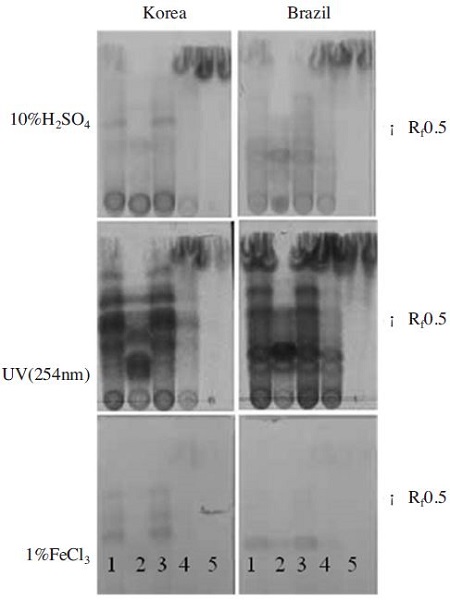
Comparison of the thin layer chromatograms profiles of propolis extracts from Korea and Brazils. C18 plates developing solvent 1: 80% EtOH fraction, 2: Hexane fraction, 3: Ethyl acetate, 4: Butanol fraction, 5: H2O. Samples of 5μl of 1/10 dilution of each propolis extract were seeded in each position. Rf: retardation factor.
Antibacterial activity
MIC was determined as the lowest concentration of the propolis extract, which inhibited the growth of the tested bacteria. The KPEE and BPEE showed antimicrobial activity against all tested strains. Table 2 presents the MIC obtained for each strain tested. The MIC values of KPEE were 0.25~1μg/ml against all tested strains. Antibacterial effects of BPEE were shown by against B. subtilis, S. aureus, P. acnes, and E. coli with MIC values ranged from 0.5 to 4μg/ml. According to these data, the antibacterial activity of the KPEE is significantly higher than that of the BPEE one. The MIC values of the KPEE against B. subtilis, S. aureus, P. acnes, and E.coli are lower than those of the BPEE, which indicate higher antibacterial activity.
Antifungal activity
The KPEE and BPEE were all tested at 50μg per well. The antifungal activity of KPEE and BPEE on the growth T. tonsurans, T. mentagrophytes and T. rubrum on Saboraud agar is presented in Fig. 2 and Table 3. The KPEE showed the highest antifungal activity against all tested fungi after incubation of 3 days as compared to BPEE. It was found that KPEE showed a strong and wide spectrum of activity against T. tonsurans, T. mentagrophytes and T. rubrum with a zone of inhibition ranging from 19.19±1.0 to 26.64±1.0mm. However, BPEE showed the least antifungal activity from 11.0±0.5 to 13.11±0.5mm against all tested fungi.
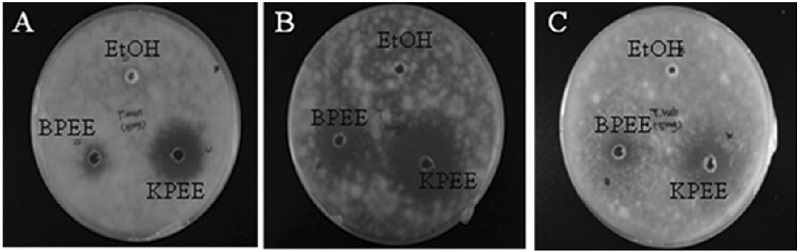
Comparative results of antifungal activity against Trichophyton mentagrophytes (A), T. tonsurans (B), and T. rubrum (C) of KPEE and BPEE by the agar diffusion method. Samples of 50μg/well of each propolis extract were seeded in each well. EtOH is negative control.
DISCUSSION
Propolis, a resinous substance produced by honeybees, has been used by humans as a remedy in traditional medicine for its health properties since ancient times, and it is still used for treatment of wounds, burns, sore throat, and so forth (Ghisalberti, 1979). Propolis contains various chemical components, which exhibit a broad spectrum of biological activities (Fearnley, 2001). The composition of propolis is complex and largely depends on the geographical origin and specific flora on the site of its collection (Sforcin et al., 2000). Numerous researches have been carried out to identify and characterize the antibacterial and antifungal compounds of propolis. Phenolic substances, flavonoids, and cinnamic acids derivatives compose the major bioactive components of propolis (Grange and Davey, 1990; Marcucci et al., 2001; Silici and Kutluca, 2005). The antimicrobial proprieties of propolis are related to the synergistic effect of its components. It has been demonstrated that ethanol extracts exhibit a wide range of biological activities, including bacteriostatic activity against many strains with a significant effect on Grampositive and a limited action on Gram-negative bacteria propolis (Gebara et al., 2002). However, there are only few study reports published where the effects of the Korean propolis against biofilms pathogens were investigated. Poplar-type propolis is a resinous substance collected by honey bees from buds of poplar trees. The skin is the largest organ of the human body. The activities against skin pathogen bacterial and fungal strains were tested. As the skin forms a barrier against harmful chemical and physical impact of the environment, it also protects the organism from infection by pathogens, parasites, fungi, bacteria and viruses (Hipler and Elsner, 2006). The bacterial and fungal flora of the skin has been a problem of interested to investigated for many years, yet it is difficult to find a satisfactory description.
This study has shown the Korean propolis ethanolic extracts has antimicrobial activity more than the Brazilian propolis against the following skin pathogens: B. subtilis, S. aureus, P. acnes, E. coli, T. tonsurans, T. mentagrophytes and T. rubrum.
Two kinds of materials, derived from propolis were investigated that the extracts of propolis samples with 80% ethanol used in ‘health food’(KFDA, 2014). We find that 80% ethanol extracts of the Korean propolis had many flavonoids and phenolic compounds compared with the Brazilian propolis one. Our results present unambiguous proof that there is the great differences in the antimicrobial activity of propolis from Korea and Brazil. It is important to result that the Korean propolis has the antibacterial and antifungal activity greater than that of the Brazilian. It seems that the Korean propolis are not only healthy foods, but have general pharmacological value as a natural mixture and not as a source of new powerful antibacterial and antifungal compounds against skin pathogen.
Acknowledgments
This work was carried out with the support of "Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ01091202)" Rural Development Administration, Republic of Korea.
LITERATURE CITED
-
Bankova, V., (2005), Recent trends and important developments in propolis research, Evidence-based Complementary and Alternative Medicine, 2, p29-32.
[https://doi.org/10.1093/ecam/neh059]

-
Dudonné, S., Poupard, P., Coutiére, P., Woillez, M., Richard, T., Mérillon, J. M., Vitrac, X., (2011), Phenolic composition and antioxidant properties of poplar bud (Populus nigra) extract: individual antioxidant contribution of phenolics and transcriptional effect on skin aging, Journal of Agricultural and Food Chemistry, 59, p4527-4536.
[https://doi.org/10.1021/jf104791t]

- Fearnley, J., (2001), Bee propolis, >Souvenir Press, London.
-
Fatahinia, M., Khosravi, A.R., Shokri, H., (2012), Propolis efficacy on TNF-α IFN-γ and IL2 cytokines production in old mice with and without systemic candidiasis, Journal of Medical Mycology, 22, p237-242.
[https://doi.org/10.1016/j.mycmed.2012.05.004]

- Genara, E. C. E., Lima, L. A., Mayer, M. P. A., (2002), Propolis antimicrobial activity against periodontopathic bacteria, 33, p365-369.
- Ghisalberti, E. L., (1979), Propolis: A Review, Bee World, 60, p59-84.
- Grange, J. M., Davey, R. W., (1990), Antibacterial properties of propolis, Journal of the Royal Society of Medicine, 83, p159-160.
-
Hipler, U.C., Elsner, P., (2006), Biofunctional Textiles and the Skin, “Current Problems in Dermatology”, by Burg, G., KARGER, Zurich.
[https://doi.org/10.1159/isbn.978-3-318-01349-8]

- Kimoto, T., Arai, S., Kohguchi, M., Nomura, Y., Micallef, M.J., Kurimoto, M., Mito, K., (1998), Apoptosis and suppression of tumor growth by artepillin C extracted from Brazilian propolis, Cancer Detection and Prevention, 22, p506-515.
-
Kuropatnicki, A. K., Szliszka, E., Krol, W., (2013), Historical aspects of propolis research in modern times, Evidence- Based Complementary and Alternative Medicine, 2013, p1-12.
[https://doi.org/10.1155/2013/964149]

-
Markham, K. E., Mitchel, K. A., Wilkin, A. L., Daldy, J. A., Lu, Y., (1996), HPLC and GC-MS identification of the major organic constituents in New Zealand propolis, Phytochemistry, 42, p205-211.
[https://doi.org/10.1016/0031-9422(96)83286-9]

- Matsuno, T., Matsumoto, Y., Saito, N., Morikawa, J., (1997), Isolation and characterization of cytotocic diterpenoid isomers from propolis, Zeitschrift fur Naturforschung. C, 52, p702-704.
-
Marcucci, M. C., Ferreres, F., Garcia-Viguera, C., Bankova, V. S., De Castro, S. L., Dantoas, A. P., Valente, P. H. M., Paulino, N., (2001), Phenolic compounds from Brazilian propolis with pharmacological activities, Journal of Ethnopharmacology, 74, p105-122.
[https://doi.org/10.1016/S0378-8741(00)00326-3]

-
Menna-Barreto, R.F., Salomao, K., Dantas, A.P., Santa-Rita, R.M., Soares, M.J., Barbosa, H.S., de Castro, S.L., (2009), Different cell death pathways induced by drugs in Trypanosoma cruzi: an ultrastructural study, Micron, 40, p157-168.
[https://doi.org/10.1016/j.micron.2008.08.003]

-
Sforcin, J. M., Fernandes, A., Lopes, C. A. M., Bankova, V., Funan, S. R. C., (2000), Seasonal effect on Brazilian propolis antibacterial activity, Journal of Ethnopharmacology, 73, p243-249.
[https://doi.org/10.1016/S0378-8741(00)00320-2]

-
Silici, S., Kutluca, S., (2005), Chemical composition and antibacterial activity of propolis collected by three different races of honeybees in the same region, Journal of Ethnopharmacology, 99, p69-73.
[https://doi.org/10.1016/j.jep.2005.01.046]

- Spooner, F. D., Sykes, G., (1972), Laboratory assessment of antibacterial activity, In Norris, J. R., Ribbons, D. W., Methods in Microbiology, 7B, Academic Press, London.
