Assessment of Sarkanda Stems as Nesting Substrate for Pithitis smaragdula F. (Hymenoptera: Anthophoridae)
Abstract
Small carpenter bees Pithitis smaragdula F, are recognised pollinators of several crops like alfalfa (Medicago sativa L.), pigeon pea (Cajanus cajan Millsp.), cucurbits. Coriander, fennel, okra, onion and sunflower. However, their population fluctuates during different years posing a great limitation in their utilization as effective pollinators. Artificial trap nesting of these bees will help in their conservation in situ and utilizing them for enhancing pollination service in cropping ecosystems. Hollow waste stems of Saccharum sp. cut into small lengths were tried as nesting tunnels for culturing carpenter bees Pithitis smaragdula. The bees accepted these pithy stems for provisioning and nesting activities. The percentage acceptability increased with time and was 17 percent after 34 days of fixing reeds. During the study period, Pithitis bees were found to nest, reproduce and build their populations in these pithy tunnels. The stems of these plants, otherwise going to waste, could be very useful for culturing/keeping these bee pollinators of crops.
Keywords:
Saccharum Spp. Pithitis smaragdula, Pollinators, Biowaste, Bee culture, NestingINTRODUCTION
Insect pollination is crucial for the sustainability of both agricultural and natural ecosystems, since 87% of flowering plants and nearly 35% of crops worldwide depend on insects and other pollinators (Klein et al., 2007; Ollerton et al., 2011). Bees are considered the most efficient insect pollinators due to their unique characteristics (Klein et al., 2007; Potts et al., 2016a; Ollerton, 2017). In their 2016 assessment report on pollinators, pollination, and food production, Potts et al. (2016b) highlight the need for improved policy responses to address declines and deficits in pollination. The report identifies key policy-relevant findings to aid decisionmaking in government, the private sector, and civil society, demonstrating how this vital ecosystem service supports the 2030 Agenda for Sustainable Development.
Both managed and wild bees provide pollination services for a wide variety of fruits, vegetables, forage crops like alfalfa and clovers, oil-producing crops (Breazeale et al., 2008), and wild flowering plants (Klein et al., 2018). Lucerne, also known as alfalfa (Medicago sativa L.), is a cross-pollinated crop valued for its high nutritional content, adaptability, quality characteristics, and herbage yield, making it an important fodder source cultivated in over 80 countries (Russelle, 2001; Iannucci et al., 2002; Hua et al., 2003; Abusuwar and Bakri, 2009; Radović et al., 2009; Keivani et al., 2010; Wang et al., 2011).
Pollination is a major limiting factor in alfalfa seed production. The lucerne flower features a standard petal where bees commonly land, along with two smaller wing petals on each side. These keel petals exert considerable pressure on the female sexual column. Alfalfa flowers are not pollinated unless the keel is forced open, releasing the sexual column, a process known as “tripping” the flower (Pedersen, 2002). Lucerne flowers require bees to visit and trip the sexual column, enabling pod and seed development (Cane, 2002; Brunet et al., 2019; Ambaw and Workiye, 2020). Due to their unique floral structure and specific pollination needs, only bees specialized in tripping can effectively pollinate these flowers. While honeybees are vital pollinators for many crops, they are inefficient in pollinating lucerne due to certain adaptive behavioral traits, their habit of side feeding solely for nectar, and the fact that lucerne pollen lacks the essential amino acid isoleucine (Pitts-Singer and Cane, 2011; Chen and Zuo, 2018; Phillips et al., 2018).
Small carpenter bees, Pithitis smaragdula, are recognized as pollinators of various crops, including alfalfa (Medicago sativa) (Kapil and Kumar, 1969). Owing to their potential as important crop pollinators studies on nesting biology for their conservation attain utmost significance. Ceratina (Pithitis) smaragdula bees are plentiful in nature, with females preferring to build linear nests in dead twigs (Guedot, 2004; Michener, 2007). Female bees construct the nests by selecting twigs, chewing them with their mandibles, and creating tunnels. Nest construction typically takes about a week. It is evident that these bees can be cultured in pithy stems for effective crop pollination. The major problem in their utilization as efficient pollinators is the lack of sufficient population. Efforts are therefore needed to explore the possibility of their harnessing in artificial nesting sites so that sufficient population is available for crop pollination.
Kapil and Jain (1980) found that among several nesting materials such as dead pithy wood of Albezzia lebbek Benth., Caesalpinia pulcherrima Sw., Sesbania aegyptica Poir. Chenopodium album L., Poinciana regia Boyer., Sophora tomentosa L., Vitis vinifera L., Sorghum vulgare Pers (Jowar), Pennisetum typhoides Rich., and in the hollow stems of Avena sativa L (Oat)., Trticum vulgare L. (Wheat), and Hordeum vulgare L. (Barley). Some of these materials are used for making thatch of hut. Between different nesting materials Saccharum munja Roxb. was found to be highly preferred than other nesting materials. Therefore, studies were focused on further evaluation of Saccharum munja Roxb. as nesting substrate for Pithitis smaragdula.
Pithy stems, such as those of Sarkanda (Saccharum sp.), grow as wild plants along roadsides, railway lines, rivers, canals, and water channels. Sarkanda is used as a raw material for thatching roofs and is preferred for stabilizing erosion-prone slopes, converting them into productive sites of high socio-economic value. It is a tall perennial herb, with stems reaching up to 4 meters in height and leaves up to 3 feet long and 3-10 mm wide. These plants grow long with multiple nodes and internodes. After maturity, the withered stems fall and create a disposal problem, often requiring burning. Historically, these plants have been considered of little use. Therefore, utilizing waste stems of Sarkanda for economically viable purposes is highly desirable. With this goal, the present study was undertaken. This study builds upon previous research on Saccharum munja by quantitatively evaluating its effectiveness.
MATERIALS AND METHODS
1. Study site
The study was carried out from 2012 to 2014 at the Research Farm of Sher-e - Kashmir university of Agricultural Sciences & Technology of Jammu (32.683762°N, 74.824394°E). It has an average elevation of 300 msl. The study area features a humid subtropical climate with extreme summer highs reaching 46℃ (115℉), and temperatures in the winter months occasionally falling below 4℃ (39℉). The study was conducted with the objective to explore the possibility of domiciliation of carpenter bee Pithitis smaragdula. The area is rich in cultivated plants, particularly alfalfa, Egyptian clover, sunflower and several other crops.
Hollow waste stems of Sarkanda (Saccharum sp.) were taken from their wild and cultivated habitats, respectively. The stems of these plants were cut into pieces at their nodes, keeping one end closed and the other open. These pieces formed the hollow nesting tunnels. These stem tunnels were then put in wooden baskets/hives and were placed in shelters near the bloom of Medicago sativa (Figs. 1 & 2). The reeds of S. munja were fixed on April 8 and observations on acceptability of reeds by the bees started after one week and continued at 3 day interval until the crop faded. The nests of Pithitis smaragdula consisted of burrows in pithy stalks each containing a series of brood cells separated by pith partitions and guarded by the mother bee.
2. Observations on acceptability
In total 200 nests were marked for observations on nesting acceptability of Pithitis bees. Twenty marked nests were sampled from 2 locations within 200 m of the study area. Nests were collected at dusk and after all foraging bees had returned to the nest. Entrances were closed with tape and the nest’s height from the soil surface was measured. After collection, the nests were refrigerated for 8 to 10 h to kill the inhabitants. External nests (total length, entrance diameter) were measured before dissection. Nests were opened starting at the entrance and parallel to the length of the branch by slowly and gently splitting the stem with a sharp knife. The kinds of tunnels accepted and their per cent acceptability were determined by recording the total number and the number of tunnels occupied. Measurements were made using a standard digital Caliper and scale, while images of bee stages were taken using Olympus SZX16 stereo zoom microscope (Olympus Corporation, Tokyo, Japan. On the basis of these, usefulness of these bio-wastes was determined.
3. Sampling of bee population
Periods of activity were observed from 5 nests over a period of 5 d during the study period. For this purpose, five nests were marked and the bees were observed when they left for foraging and came after collection of nectar and or pollen. The time spent in each trip was recorded, the time at which bee activity ended in each nest was also recorded. The time from initiation of activity till its complete cessation constituted total time spent by a bee in its foraging activity.
4. Statistical analysis
The recorded data were analysed for their variation between different treatments using Statistical Package for the Social Science (SPSS) and O.P. Stat. (Sheoran et al., 1998, opstat.pythonanywhere.com). A correlation analysis was conducted between time after nest installation and percentage acceptance, and a regression analysis was performed for prediction.
RESULTS
1. Commencement and cessation of foraging activities
Pithitis smaragdula is a polylectic bee and therefore may be found visiting numerous and diverse floral resources. Near the nesting area, individuals were found foraging on a variety of cultivated crops, including alfalfa, sunflowers, coriander, fennel onion flowers. They were also found visiting other crops such as okra, sponge gourd, and pumpkin. The bees in different nests initiated foraging in the early morning between 6.40 a.m to 7.00 a.m and continued their foraging activities up to 4.40 p.m to 4.55 p.m. Thereafter activities ceased completely and no bee was observed. On an average, bees spent 589.00 to 675.00 min per day with an average of 618.20 min per day in field activities. The time spent in foraging trip for nectar and pollen varied 18 to 30 min with an average of 22.4 min (Table 1).
2. Acceptability of nesting materials
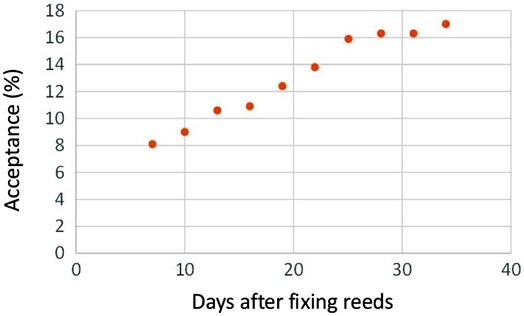
The data presented in Table 2 shows that after one week. 8 percent reeds were already occupied by P. smaragdula. Acceptance went on increasing and by May 12 (at the maturity of crop) 17 per cent reeds were accepted. The nests of Pithitis smaragdula consisted of burrows in pithy stalks each containing a series of brood cells separated by pith partitions and guarded by the mother bee.
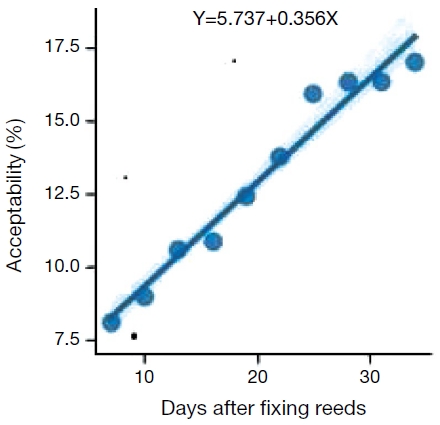
The data presented in Fig. 3 revealed that percentage of acceptability of reed stems increased with time. It was 8.1 per cent after 7 days of fixing which increased continuously and was 17.0 per cent after 28 days of fixing. Correlation analysis of the data (Fig. 4) also revealed that it followed a linear relationship with increase in number of days. The coefficient of correlation was found to be highly significant (r=0.961, n-2=48, P≤0.01).
Scatter diagram showing percentage acceptance of reed stems of Saccharum munja in relation to number of days of fixing.
3. Nest architecture
The accepted twigs had an average (±SD) length of 7.07±0.47 cm and twig thickness 8.18±0.22 mm (Table 3). The nest entrance was on average 2.96±0.07 mm wide. Nest thickness was 3.14±0.06 mm. Each nest had on average 4.94±0.25 cells (ranging from 1-7 cells), and cell lengths averaged 6.05±0.53 mm (n=54 cells). Cells were separated by pithy partitions constructed by the bees, the depth of which ranged from 0.3 to 0.6 mm. Cell septum thickness (mm) was 3.2±0.10. The number of adults per nest was 1.01±0.05. The height of nest from ground level (cm) was 59.55±3.34. In some cases, empty cells were found between otherwise fully constructed cells. Tunnel choice seemed to be influenced by the size (thoracic diameter) of the bee pollinators. In some cases, empty cells were found between otherwise fully constructed cells.
DISCUSSION
Much less is known about the situation of wild insect pollinators with respect to their conservation. The studies revealed that P. smaragdula would be an ideal candidate for managed pollination services. The acceptability of nesting materials easily available in nature which otherwise go waste is an important step for harnessing and multiplication of small carpenter bee Pithitis smaragdula. The multiplication of these bees shall help in boosting crop production in the context of alarming decline in managed pollinators. There is clear evidence of recent declines in both wild and domesticated pollinators, and parallel declines in the plants that rely upon them (Potts et al., 2010).
The results are in agreement with earlier studies (Kapil and Kumar, 1969; Daly et al., 1971; Batra, 1976a, 1976b) who reported pithy stalks of Saccharum bengalense Retz. (Poales: Poaceae) under the synonymous name of Erianthus munja (Roxb.) Jeswiet as suitable nesting substrate for Pithitis smaragdula. Yogi and Khan (2014) recorded nesting success of small carpenter bees Ceratina propinqua and Ceratina simillima on pithy stalks of Ravenna grasses. The high nest acceptance rate observed in this study suggests that this plant is an ideal nesting substrate for Pithitis smaragdula. In a similar study, Ali et al. (2016) during a quick sampling of bees around Ismaila, Pakistan found numerous nesting sites of small carpenter bees P. smaragdula preferentially nesting in dead stalks of Ravenna grass (Saccharum ravennae L.; Poales: Poaceae). However, dimensions of average length of nest and diameter were low in this study as reported by Ali et al. (2016). This difference may be due to variation in size of the bees in different geographic locations. Evidently, preference of nesting materials and nesting dimensions need to be explored in different areas for efficient utilization of bees for crop pollination. The present investigation, however, differ from earlier studies made by Malaipan, 1992 who observed Pithitis smaragdula nesting in mulberry (Morus indica L.) 2.7%, sesbania (Sesbania javanica Mg.) 2.2%, eupatorium (Eupatorium odoratum L.) 2.4% and lantana (Lantana camara L.) 0.8%. The present studies also differ from the results obtained by Anusree and Chellappan (2022) who found nesting sites of Small carpenter bees Ceratina smaragdula (F.) and C. hieroglyphica Smith (Xylocopinae: Apidae) in dried twigs of peacock flower tree Caesalpinia pulcherrima at Vellanikkara Kerala, India. The results obtained in present study also differ from the studies made by Udayakumar and Shivalingaswamy (2019) who observed that small carpenter bees constructed nests in the pruned pithy stems of Caesalpinia pulcherrima in Bangalore, India. The observed differences in nesting materials may be due to different geographic locations and climatic conditions of different areas. Tunnel choice seemed to be influenced by the size (thoracic diameter) of the bee pollinators. Farmers could be encouraged to place such stalks horizontally above the surface of the ground in accordance with the natural nests observed, and to conserve natural habitats for wild bees surrounding crop environments.
Arakaki et al. (2001) reported that Pithitis smaragdula (Fabricius), an Asiatic bee (Hymenoptera: Apidae) is now apparently established on Oahu. Although there is a remarkable diversity of species of Pithitis (Ceratina) in Asia (Michener, 2007), there have been comparatively few studies on their nesting biology and floral associations outside of Japan, most having been undertaken on a subset of relatively common taxa (Batra, 1976b; Malaipan, 1992; Rehan et al., 2009; Yogi and Khan, 2014; Anusree and Chellappan, 2022).
This is in stark contrast to the more extensive literature on the co-occurring, Asiatic, large carpenter bees, genus Xylocopa Latreille, obviously reflecting a researcher bias toward conspicuous and robust species that are easily observed and often nest in human constructions (Kapil and Dhaliwal, 1968, 1969; Raju and Rao, 2006; Boontop et al., 2008; Punekar et al., 2010; Hannan et al., 2012; Hongjamrassilp and Warrit, 2014).
Pithitis smaragdula initiated foraging early in the morning and activities completely ceased at around 4.55 p.m. On an average bees spent 618.20 min in field activities. Commencement and cessation of field activities is under the key control of physical environment and occurs when specific conditions are obtained. Abrol and Kapil (1986) found that commencement of flight activities of Megachile species occurred when minimum threshold of temperature was attained while cessation was mainly by decline in values of light intensity and solar radiation.
CONCLUSIONS
The present study, therefore, provides a simple but new and useful technology for culturing useful and specialist pollinators of crops and opens ways for such ventures for the utilization of other similar materials which otherwise are going to waste. The process can be used in countries with poor economy and poorly developed technology. No material cost is involved (the hives can be fabricated by the crop growers themselves) and the expected benefits due to managed pollination are enormous (Sihag, 1982, 1986; Abrol, 1991; Shebl et al., 2018). Local educational and outreach programs are needed to educate farmers, who often operate at a small scale within isolated villages, of the need to conserve natural areas, particularly those bordering crop fields. Relying on honeybees for crop pollination may lead to the decrease of seed production. Evidently, conservation of wild bees will solve this problem. Using and conserving alfalfa pollinating bees will increase alfalfa seed production. Pithy stems of the Saccharum munja can be used to conserve these bees by serving as the artificial nesting substrates for the bees for their valuable ecosystem service in terms of crop pollination. Farmers can be encouraged to provide artificial nesting substrates using the pithy stems of S. munja to conserve these bees. Using artificial hives are recommended to farmers for many reasons: easy to handle, storage and reuse. There is great potential for nesting and pollination studies throughout the varied habitats and elevations of the country. In spite of technology developed for domestication of Pithitis smaragdula build up of sufficient population of bees required for pollination still remains a limitation. Large number of Saccharum needs to fixed in advance in areas requiring pollination services by Pithitis smaragdula.
References
- Abrol, D. P. 1991. Conservation of pollinators for promotion of agricultural production in India. J. Anim. Morphol. Physiol. 38(1x2): 123-139.
-
Abrol, D. P. and R. P. Kapil. 1986. Factors affecting pollination activity of Megachile lanata Lepel. Proc. Indian Acad. Sci. (Anim. Sci.) 95: 757-769.
[https://doi.org/10.1007/BF03179493]

- Abusuwar, A. O. and E. Bakri. 2009. Effect of Water Quality and Weeding on Yield and Quality of Three Alfalfa (Medicago sativa L.) Cultivars. Aust. J. Crop Sci. 3: 315-321.
-
Ali, H., A. S. Alqarni, M. Shebl and M. S. Engel. 2016. Notes on the nesting biology of the small carpenter bee Ceratina smaragdula (Hymenoptera: Apidae) in northwestern Pakistan. Fla. Entomol. 99(1): 89-93.
[https://doi.org/10.1653/024.099.0116]

- Ambaw, M. and M. Workiye. 2020. Evaluation and demonstration of the roll of honey bees on seed yield of alfalfa (Medicago sativa FL77) in Kulumsa, Ethiopia. J. Entomol. Zool. Stud. 8: 2269-2272.
-
Anusree, P. P. S. and M. Chellappan. 2022. Comparative study on nest architecture and lifecycle of two small carpenter bees Ceratina smaragdula (f.) and Ceratina hieroglyphica Smith. Indian J. Entomol. 84(1): 38-43.
[https://doi.org/10.55446/IJE.2021.339]

- Arakaki, K. T., W. D. Perreira, D. J. Preston and J. W. Beardsley. 2001. Pithitis smaragdula (Fabricius), an Asiatic bee (Hymenoptera: Apidae) now apparently established on Oahu. Proc. Hawaiian Entomol. Soc. 35: 151.
- Batra, S. W. T. 1976a. Comparative efficiency of alfalfa pollination by Nomia melanderi, Megachile rotundata, Anthidium florentinum and Pithitis smaragdula (Hymenoptera: Apoidea). J. Kans. Entomol. Soc. 49: 18-22.
-
Batra, S. W. T. 1976b. Nests of Ceratina, Pithitis and Braunsapis from India (Hymenoptera: Anthophoridae). Orient. Insects 10: 1-9.
[https://doi.org/10.1080/00305316.1976.10432314]

- Boontop, Y., S. Malaipan and K. Chareansom. 2008. Large carpenter bees in Thailand and biology of Xylocopa nasalis (Westwood). Thailand Nat. Hist. Museum J. 3: 5-15.
- Breazeale, D., G. Fernandez and R. Narayanan. 2008. Modeling pollination factors that influence alfalfa seed yield in north-central Nevada. J. Cent. Eur. Agric. 9: 107-116.
-
Brunet, J., Y. Zhao and M. K. Clayton. 2019. Linking the foraging behavior of three bee species to pollen dispersal and gene flow. PLoS ONE 14: e0212561.
[https://doi.org/10.1371/journal.pone.0212561]

-
Cane, J. H. 2002. Pollinating bees (Hymenoptera: Apiformes) of U.S. alfalfa compared for rates of pod and seed set. J. Econ. Entomol. 95: 22-27.
[https://doi.org/10.1603/0022-0493-95.1.22]

-
Chen, M. and X.-A. Zuo. 2018. Pollen limitation and resource limitation affect the reproductive success of Medicago sativa L. BMC Ecol. 18: 28.
[https://doi.org/10.1186/s12898-018-0184-x]

-
Daly, H. V., G. E. Bohart and R. W. Thorp. 1971. Introduction of small carpenter bees into California for pollination. 1. Release of Pithitis smaragdula. J. Econ. Entomol. 64: 1145-1150.
[https://doi.org/10.1093/jee/64.5.1145]

- Guedot, C. 2004. Nest location and nest recognition in two solitary bee species Osmia lignaria Say and Megachile rotundata (F.) (Hymenoptera: Megachilidae). (PhD thesis), Utah State University, Utah, USA.
-
Hannan, M. A., A. S. Alqarni, A. A. Owayss and M. S. Engel. 2012. The large carpenter bees of central Saudi Arabia, with notes on the biology of Xylocopa sulcatipes Maa (Hymenoptera, Apidae, Xylocopinae). Zookeys 201: 1-14.
[https://doi.org/10.3897/zookeys.201.3246]

-
Hongjamrassilp, W. and N. Warrit. 2014. Nesting biology of an Oriental carpenter bee, Xylocopa (Biluna) nasalis Westwood, 1838, in Thailand (Hymenoptera, Apidae, Xylocopinae). J. Hymenoptera Res. 41: 75-94.
[https://doi.org/10.3897/JHR.41.7869]

- Hua, J., B. Yufen and Z. Jun. 2003. A study on alfalfa pollinating mechanism and relationship of pollinating insects. Cao Ye Ke Xue Pratacultural Sci. Caoye Kexue 20: 1-6.
-
Iannucci, A., N. Di Fonzo and P. Martiniello. 2002. Alfalfa (Medicago sativa L.) seed yield and quality under different forage management systems and irrigation treatments in a Mediterranean environment. Field Crops Res. 78: 65-74.
[https://doi.org/10.1016/S0378-4290(02)00094-1]

- Kapil, R. P. and J. S. Dhaliwal. 1968. Biology of Xylocopa species. I. Seasonal activity, nesting behavior and life cycle. Journal of Research, Punjab Agricultural University, Ludhiana 5: 406-419.
- Kapil, R. P. and J. S. Dhaliwal. 1969. Biology of Xylocopa species. II. Field activities, flight range and trials on transportation of nests. Journal of Research, Punjab Agricultural University, Ludhiana 6: 262-271.
- Kapil, R. P. and K. L. Jain. 1980. Biology and utilization of insect pollinators for crop production. Final Technical Report PL 480 PROJECT. Department of Zoology, Haryana Agricultural University, Hisar, India, 81pp.
- Kapil, R. P. and S. Kumar. 1969. Biology of Ceratina binghami Cockerell (Ceratinini: Hymenoptera). Journal of Research, Punjab Agricultural University, Ludhiana 6: 359-371.
- Keivani, M., S. S. Ramezanpour, H. Soltanloo, R. Choukan, M. Naghavi and M. Ranjbar. 2010. Genetic Diversity Assessment of Alfalfa (‘Medicago sativa’ L.) Populations Using AFLP Markers. Aust. J. Crop Sci. 4: 491.
-
Klein, A. M., B. E. Vaissiere, J. H. Cane, I. Steffan-Dewenter, S. A. Cunningham, C. Kremen and T. Tscharntke. 2007. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B Biol. Sci. 274: 303-313.
[https://doi.org/10.1098/rspb.2006.3721]

-
Klein, A. M., V. Boreux, F. Fornoff, A. C. Mupepele and G. Pufal. 2018. Relevance of wild and managed bees for human well-being. Curr. Opin. Insect Sci. 26: 82-88.
[https://doi.org/10.1016/j.cois.2018.02.011]

- Malaipan, S. 1992. Biology and nesting plant preference of small carpenter bees Ceratina spp. and Pithitis smaragdula (F.) (Anthophoridae). Kasetsart Journal: Natural Science 26: 126-130.
- Michener, C. D. 2007. The Bees of the World (2nd ed.). Johns Hopkins University Press, Baltimore, Maryland, 992 p.
-
Ollerton, J. 2017. Pollinator Diversity: Distribution, Ecological Function, and Conservation. Annu. Rev. Ecol. Evol. Syst. 48: 353-376.
[https://doi.org/10.1146/annurev-ecolsys-110316-022919]

-
Ollerton, J., R. Winfree and S. Tarrant 2011. How many flowering plants are pollinated by animals? Oikos 120: 321-326.
[https://doi.org/10.1111/j.1600-0706.2010.18644.x]

-
Pedersen, M. W. 2002. Lucerne Pollination. Bee World 42: 145-149.
[https://doi.org/10.1080/0005772X.1961.11096861]

-
Phillips, B. B., A. Williams, J. L. Osborne and R. F. Shaw. 2018. Shared traits make flies and bees effective pollinators of oilseed rape (Brassica napus L.). Basic Appl. Ecol. 32: 66-76.
[https://doi.org/10.1016/j.baae.2018.06.004]

-
Pitts-Singer, T. L. and J. H. Cane. 2011. The alfalfa leaf cutting bee, Megachile rotundata: The world’s most intensively managed solitary bee. Annu. Rev. Entomol. 56: 221-237.
[https://doi.org/10.1146/annurev-ento-120709-144836]

-
Potts, S. G., J. C. Biesmeijer, C. Kremen, P. Neumann, O. Schweiger and W. E. Kunin. 2010. Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25(6): 345-353.
[https://doi.org/10.1016/j.tree.2010.01.007]

-
Potts, S. G., V. L. Imperatriz-Fonseca and H. T. Ngo (eds). 2016a. The assessment report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES) on pollinators, pollination and food production. Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services, Bonn, Germany. 552 pages.
[https://doi.org/10.5281/zenodo.3402856]

-
Potts, S. G., V. Imperatriz-Fonseca, H. T. Ngo, M. A. Aizen, J. C. Biesmeijer, T. D. Breeze, L. V. Dicks, L. A. Garibaldi, R. Hill and J. Settele. 2016b. Safeguarding pollinators and their values to human well-being. Nature 540: 220-229.
[https://doi.org/10.1038/nature20588]

-
Punekar, S. A., N. K. P. Kumaran and H. R. Bhat. 2010. Observations on an unusual behaviour in the carpenter bee Xylocopa aestuans (Latreille, 1802) (Hymenoptera: Apidae) of the Western Ghats, India. J. Threatened Taxa 2: 1232-1233.
[https://doi.org/10.11609/JoTT.o2271.1232-3]

-
Radović, J., D. Sokolović and J. Marković. 2009. Alfalfa-most important perennial forage legume in animal husbandry. Biotechnol. Anim. Husb. 25: 465-475.
[https://doi.org/10.2298/BAH0906465R]

- Raju, A. J. S. and S. P. Rao. 2006. Nesting habits, floral resources and foraging ecology of large carpenter bees (Xylocopa latipes and Xylocopa pubescens) in India. Curr. Sci. 90: 1210-1217.
-
Rehan, S. M., M. H. Richards and M. P. Schwarz. 2009. Evidence of social nesting in the Ceratina of Borneo (Hymenoptera: Apidae). J. Kans. Entomol. Soc. 82: 194-209.
[https://doi.org/10.2317/JKES809.22.1]

-
Russelle, M. P. 2001. Alfalfa: After an 8000-year journey, the “Queen of Forages” stands poised to enjoy renewed popularity. Am. Sci. 89: 252-261.
[https://doi.org/10.1511/2001.3.252]

-
Shebl, M. A., H. A. Hassan, S. M. Kamel, M. A. Osman and M. S. Engel. 2018. Biology of the mason bee Osmia latreillei (Hymenoptera: Megachilidae) under artificial nesting conditions in Egypt. J. Asia-Pacific Entomol. 21(3): 754-759.
[https://doi.org/10.1016/j.aspen.2018.05.008]

- Sheoran, O. P., D. S. Tonk, L. S. Kaushik, R. C. Hasija and R. S. Pannu. 1998. Statistical Software Package for Agricultural Research Workers. Department of Mathematics Statistics, CCS HAU, Hisar, 139-143.
- Sihag, R. C. 1982. Effect of competition with Parkinsonia aculeata L. on pollination and seed production in Medicago sativa L. Indian Bee J. 44(4): 89-90.
-
Sihag, R. C. 1986. Insect pollination increases seed production in cruciferous and umbelliferous crops. J. Apic. Res. 25(2): 121-126.
[https://doi.org/10.1080/00218839.1986.11100704]

-
Udayakumar, A. and T. M. Shivalingaswamy. 2019. Nest architecture and life cycle of Small Carpenter bee, Ceratina binghami Cockerell (Xylocopinae: Apidae: Hymenoptera). Sociobiology 66(1): 61-65.
[https://doi.org/10.13102/sociobiology.v66i1.3558]

-
Wang, X., X. Yang, L. Chen, G. Feng, J. Zhang and L. Jin. 2011. Genetic diversity among alfalfa (Medicago sativa L.) cultivars in Northwest China. Acta Agric. Scand. Sect. B Soil Plant Sci. 61: 60-66.
[https://doi.org/10.1080/09064710903496519]

-
Yogi, M. K. and M. S. Khan. 2014. Nesting biology of the small carpenter bees Ceratina propinqua and Ceratina simillima (Hymenoptera: Apidae). Anim. Biol. 64: 207-212.
[https://doi.org/10.1163/15707563-00002442]