Diversity of Insect Pollinators in Different Agricultural Crops and Wild Flowering Plants in Korea: Literature Review
Abstract
Insect pollination is an important ecosystem process for increased agricultural crop yield as well as for enhancing ecosystem production. We analyzed insect pollinators visiting fruits and flowers in different orchards and wild fields across Korea during the last three decades, published in scientific journals in Korea. A total of 368 species in 115 families of 7 orders were recorded to serve as pollinators in 43 different agricultural crops and wild flowers. The most diverse insect pollinators were the species of Hymenoptera followed by Diptera and Coleoptera. The dominant insect pollinator was the honey bee (Apis melliferas) followed by Eristalis cerealis, Tetralonia nipponensis, Xylocopa appendiculata, Eristalis tenax, Helophilus virgatus and Artogeia rapae. Study methods employed for diversity of pollinators were mostly sweep netting, but trapping was mostly employed for bee diversity and direct observation for lepidopteran diversity. Bee diversity was higher in orchards while insect order level diversity was higher in wild plant pollinators, suggesting the important roles of wild flowers for conservation of insect pollinators.
Keywords:
Apis melliferas, Eristalis cerealis, Tetralonia nipponensis, Xylocopa appendiculata, Eristalis tenax, Helophilus virgatus, Artogeia rapaeINTRODUCTION
Animal-mediated pollination plays an important functional role in most terrestrial ecosystems and provides a key ecosystem service vital to the maintenance of both wild and agricultural plant communities as most angiosperms are pollen-limited and rely on animals for sexual reproduction (Potts et al., 2010; Albrecht et al., 2012). A large proportion of the human diet depends directly or indirectly on animal pollination (Klein et al., 2007; Albrecht et al., 2012; Garibaldi et al., 2013).
Insects are the primary animal-mediated pollinators of 80% of all plant species in Europe, including most fruits, many vegetables and some biofuel crops and include diverse species of Hymenoptera (bees, solitary species, bumblebees, pollen wasps and ants), Diptera (bee flies, houseflies, hoverflies), Lepidoptera (butterflies and moths), Coleoptera (flower beetles), and other insects. Although managed bee hives are increasing worldwide, insect pollinators are considered to be in a state of decline due to a range of recent and projected environmental changes, such as habitat loss and climate change, with unknown consequences for pollination service delivery (Potts et al., 2010).
In Korea, Ko et al. (1977) first examined the diversity and frequency of insect pollinators visiting apple, pear and peach in Seoul and Suwon and reported 26 insect species in three orders and honey bee (Apis mellifera) was the most dominant species to visit fruits. Woo et al. (1986a, b) surveyed visiting insects to apple, pear and peach in 4 areas across Korea and reported different insect diversity depending on fruits: 12 families and 22 species of Hymenoptera and 10 families and 17 species of Diptera visited apple flowers; 13 families and 17 species of Hymenoptera and 10 families and 22 species of Diptera visited pear flowers; and 13 families and 19 species of Hymenoptera and 9 families and 16 species of Diptera visited peach flowers. Lee et al. (2000) reported 68 insect species in 27 families and 6 orders that visited apple flowers in Gyungsangbuk-do province and the Hymenoptera comprised more than half (51.2%) of the total insects and honey bee was the dominant pollinator. Kim and Hwang (2009) recorded insect pollinators visiting peach and pear in Jeonju, Jeollabuk-do province: 11 species in 6 families and 4 orders visiting peach and 17 species in 11 families and 5 orders visiting pear and identified Apis mellifera as the major insect pollinator. Recently Kim et al. (2012, 2013) reported insect pollinator diversity visiting flowers of Asteraceae, Labiatae and Umbelliferae.
We reviewed insect pollinators visiting fruits and flowers in different orchards and wild fields across Korea during the last three decades. The purpose of the study was to see the pattern of diversity of insect pollinators and interactions between insects and plants. We hope that this review can be the baseline data to set up research plans on ecology and conservation of insect pollinators in Korea.
MATERIALS AND METHODS
We searched the literatures dealing with the diversity of insect pollinators in domesticated and wild plants in the peer-reviewed scientific journals in Korea (Korean journal of Apiculture, Korean Journal of Environmental Ecology) published since 1977. We recorded the diversity of insect pollinators and their visiting fruits and flowers. Fourteen papers were retrieved mostly from fruit crops and horticultural crops (12) and wild plants (2), respectively (Hong et al., 1989; Kang et al., 2003; Kang et al., 2009, Kim et al., 2005; Kim et al., 2009; Kim et al., 2012, 2013; Ko et al., 1977; Kwon et al., 2011; Lee et al., 2000; Lee et al., 2007; Lee et al., 2008; Woo et al., 1986a, b). Fruit crops include apple, pear, peach, persimmon and wild berry, horticultural crops include strawberry, tomato and sunflower, while the wild plants include 30 wild flowers. Occurrence of each flower visiting insects on each crop was marked as record. Different sampling methods were employed but we grouped those into direct observation, sweep net collection and trapping with pollinator trap or water pan trap.
RESULTS
A total of 368 identified species in 115 families of 7 orders were recorded to serve as pollinators in 43 different fruits and flowers in orchards and wild fields (Fig. 1, Appendix 1 include all listed species including unidentified species). The most diverse insect pollinators were the species of Hymenoptera (110 species, 373 records), followed by Diptera (104 species, 219 records), Coleoptera (88 species, 111 records) and Lepidoptera (47 species, 97 records). The dominant insect pollinator was the honey bee (Apis mellifera, 34 records) followed by Eristalis cerealis (Diptera, 26 records), Tetralonia nipponensis (Hymenoptera, 12 records), Xylocopa appendiculata (Hymenoptera, 12 records), Eristalis tenax (Diptera, 11 records), Helophilus virgatus (Diptera, 11 records), and Artogeia rapae (Lepidoptera, 10 records) (Table 1). Detailed lists were provided with Appendix 1 and 2. The main insect pollinators are bees (Hymenoptera) since bees spend most of their life in collecting pollen, a source of protein that they feed to their developing offspring and nectar, a concentrated energy source (Aizen and Harder, 2009). Honey bee is the best known and widely managed pollinator. In addition there are hundreds of other species of bees, mostly solitary ground nesting species, that contribute some level of pollination services to crops and are very important in natural plant communities (Garibaldi et al., 2013). Among bees, the diversity at the genus level showed that Andrena was the most diverse with more than 21 species, followed by 14 species of Lasioglossum, 8 Bombus, 6 Nomada, and 5 Osmia species.
Flies are considered to be the second most important insect order for both flower visiting and pollination (Larson et al., 2001). A number of dipteran insects are used commercially around the world as pollinators for specific crops, including the use of Eristalis hoverflies on peppers, and calliphorid flies on canola, sunflower, buckwheat, garlic, lettuce and peppers, and other crops (Larson et al., 2001; Defra, 2014). In Korea, the dominant dipteran genus was Drosophila with 5 species followed by 4 species of Paragus and 3 species of Calliphora, Eristalis, Helophilus, Pipiza, Sphaerophoria, and Syrphus, respectively. Drosophilidae have been associated with pollination of Genoplesium (Orchidaceae) in Australia (Bishop 1996; Larson et al., 2001). In addition Drosophilidae and sympatric Nitidulidae (Coleoptera) are known to vector yeasts between flowers of many Convolvulaceae, Hibiscus, and other plants that possess campanulate flowers in the Nearctic, Neotropical, and Australo-Pacific regions (Lachance et al., 2001; Larson et al., 2001; Defra, 2014).
Beetles were the third diverse flower visiting insects group (Fig. 1). Beetles are the largest insect group and pollinate a wide range of plant species with various reproductive characters, pollinating 88% of the 240,000 flowering plants (Endress, 1994). In Australia, 23% of the beetle families (28 families) include the flower visiting insects, while 51% of the dipteran families (44 families) comprise the flower visiting insects (Armstrong, 1979). In Korea about 71 species in 27 families were identified as the flower visiting beetle species.
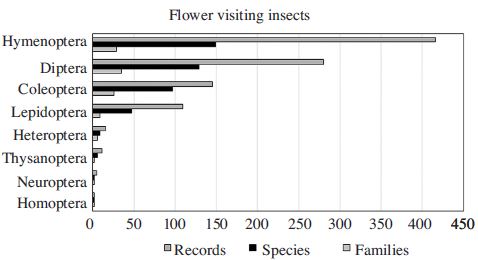
Distribution of pollinating insect order in family, species (identified and unidentified species) and record levels from the literature review.
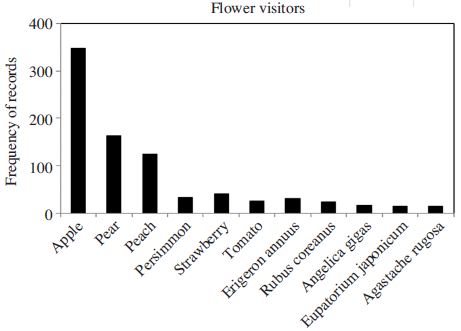
Frequency distribution of flower visiting insects along with different crops and wild plants. Plants with lower frequency were excluded.
In the present review 43 butterflies visited flowers of diverse crops and wild plants. In addition only four species of moths (Adelidae, Geometridae and Zygaenidae) were recorded (Appendix 1). Many sphingid and noctuid moths that visit flowers of wild plants during daytime were, however not included in the studies that we surveyed.
Among the plants, most pollinators were observed as visiting major fruits such as apple, pear, and peach, followed by some horticultural crops (Fig. 2). Meanwhile the insect pollinators on wildflowers were relatively uncommon, except an exotic herb, Erigeron annuus (Fig. 2). This does not exactly reflect the field condition, but more the study intensity because this review was confined to the record counting rather than density estimation. Based on plant types, record frequency of Hymenoptera was highest in fruit crops (Fig. 3) indicating that diversity of bee pollinators on fruit crops is higher than horticultural crops or wild plants. From wild plants, higher frequencies of Hymenoptera, Diptera, and Lepidoptera as well as Coleopteran insects were relatively higher (Fig. 3). This is the important finding in terms of pollinator conservation because diverse plant flower morphology and nutritional availability provoke the wild pollinator diversity. In contrast, pollinator diversity from horticultural crops were low (Fig. 3), partially because of the nature of cultivation of those crops inside the greenhouse.
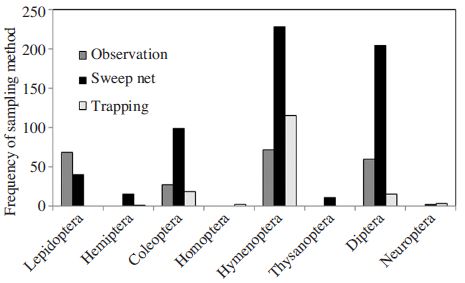
There are basically three types of sampling; direct observation, sweep net collection and trapping with pollinator trap or water pan trap (Fig. 4). Depending on the insect order, different methodologies were empl oyed. For lepidopteran insects, visual observation was dominant, while the other taxa were collected by sweep netting. It is interesting to see that trapping with water pan or pollinator trap was mostly employed to study bee diversity (Fig. 4).
The role of insect pollinators in diverse ecological systems including agriculture is important (Garibaldi et al., 2013). About 75% of global food crops are at least partially dependent on animal pollinators and majority of them are insects. Here we reviewed the diversity of insect pollinators, and found that bees, bumblebees and syrphid flies are most important pollinator groups. However, we found that wild plants harbored low frequency of insect pollinators but, with high taxonomic diversity, which may imply some important context for conservation and enhancement of wild pollinators. Further, we need more detailed research on diversity of pollinators and biological interactions between plants and pollinators in diverse ecosystems.
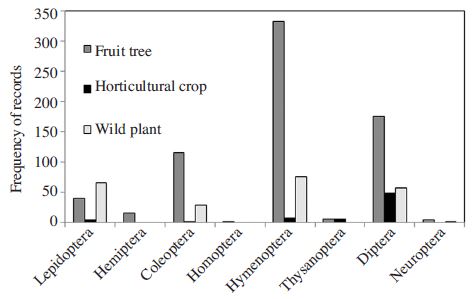
Frequency distribution of insect orders on different types of plants: fruit tree, horticultural crops and wild plants.
Acknowledgments
This research was partly supported from NIE research grant on evaluation of pollination service and RDA Agenda research project (PJ010487).
LITERATURE CITED
-
Aizen, M. A., L. D. Harder, (2009), The global stock of domesticated honey bees is growing slower than agricultural demand for pollination, Curr. Biol, 19, p915-918.
[https://doi.org/10.1016/j.cub.2009.03.071]

-
Albrecht, M., B. Schmid, Y. Hautier, and C.B. Müller, (2012), Diverse pollinator communities enhance plant reproductive success, Proc Biol Sci, 279(1748), p4845-4852.
[https://doi.org/10.1098/rspb.2012.1621]

-
Armstrong, J. A., (1979), Biotic pollination mechanisms in the Australian flora- a review, N. Z. Bot, 17, p467-508.
[https://doi.org/10.1080/0028825x.1979.10432565]

- Bishop, A., (1996), Field guide to the orchids of New South Wales and Victoria, University of New South Wales Press, Sydney.
- Department for Environment, Food and Rural Affairs (Defra), (2014), The national pollinator strategy: for bees and other pollinators in England, Defra, Bristol.
- Endress, P. K., (1994), Diversity and evolutionary biology of tropical flowers, Cambridge University press, Cambridge.
-
Garibaldi, L. A., I. Steffan-Dewenter, R. Winfree, M.A. Aizen, R. Bommarco, S. A. Cunningham,, C. Kremen, L. G. Carvalheiro, L. D. Harder, O. Afik, I. Bartomeus, F. Benjamin, V. Boreux, D. Cariveau, N. P. Chacoff, J. H. Dudenhöffer, B. M. Freitas, J. Ghazoul, S. Greenleaf, J. Hipólito, A. Holzschuh, B. Howlett, R. Isaacs, S. K. Javorek, C. M. Kennedy, K. M. Krewenka, S. Krishnan, Y. Mandelik, M. M. Mayfield, I. Motzke, T. Munyuli, B. A. Nault, M. Otieno, J. Petersen, G. Pisanty, S. G. Potts, R. Rader, T. H. Ricketts, M. Rundlöf, C. L. Seymour, C. Schüepp, H. Szentgyörgyi, H. Taki, T. Tscharntke, C. H. Vergara, B. F. Viana, T.C. Wanger, C. Westphal, N. Williams, and A. M. Klein, (2013), Wild pollinators enhance fruit set of crops regardless of honey bee abundance, Science, 339, p1608-1611.
[https://doi.org/10.1126/science.1230200]

- Hong, K. J., S. H. Lee, and K. M. Choi, (1989), The flower visiting insects on the blossoms of pear, peach and apple trees in Suweon, Korean J. Apiculture, 4(2), p16-24.
- Kang, C. H., H. S. Huh, and C. G. Park, (2003), Insect visitors of sweet persimmon blossoms and their daily activity, J. Agriculture & Life Sciences, 37, p1-4.
- Kang, M. S., H, J. Kim, , S. H., K. Y. Lee, and E. S. Baik, (2009), Effects of pollinators on the fruit production of Korean black rasspberry, Korean J. Apiculture, 24, p153-158.
- Kim, D., H. S. Lee, and C. Jung, (2009), Comparison of flower visiting hymenopteran communities from apple, pear, peach and persimmons blossoms, Korean J. Apiculture, 24, p227-235.
- Kim, G-T., D. P. Lyu, and H. J. Kim, (2012), Floral Characteristics of Asteraceae Flowers and Insect Pollinators in Korea, Korean J. Environ. Ecol, 26, p200-209.
- Kim, G-T., D. P. Lyu, and H. J. Kim, (2013), Floral characteristics of Labiatae and Umbelliferae Flowers and insect pollinators in Korea, Korean J. Environ. Ecol, 27, p22-29.
- Kim, H-H., and C. Y. Hwang, (2009), Species and diurnal activities of the insect pollinators on peach and pear orchards in Jeonju, Korean J. Apiculture, 24, p75-81.
- Kim, Y. S., S. B. Lee, Y. S. Jo, M. L. Lee, H. J. Yoon, M. Y. Lee, and S. H. Nam, (2005), The comparison of pollinating effects between honey bees (Apis mellifera) and Bumblebee (Bombus terrestris) on the kiwifruit raised in greenhouse, Korean J. Apiculture, 20, p47-52.
-
Klein, A. M., B. E. Vaissiere, J. H. Cane, I. Steffan-Dewenter, S. A. Cunningham, C. Kremen, and T. Tscharntke, (2007), Importance of pollinators in changing landscapes for world crops, Proc. R. Soc. B, 274, p303-313.
[https://doi.org/10.1098/rspb.2006.3721]

- Ko, K. C., K. S. Woo, and W. C. Kim, (1977), A study on the activities of insect pollinators frequenting various fruit trees near Seoul area, Seoul Natl. Univ., Coll. of Agric, 2, p407-421.
- Kwon, H. J., Y. P. Kim, K. R. Choe, and H. K. Song, (2011), Pollination mechanism of Stewaria koreana Nakai (Theaceae) in Korea, Korean J. Apiculture, 26, p157-161.
-
Lachance, M. A., W. T. Starmer, C. A. Rosa, J. M. Bowles, J. S. F. Barker, and D. H. Janzen, (2001), Biogeography of the yeasts of ephemeral flowers and their insects, FEMS Yeast Res, 1, p1-8.
[https://doi.org/10.1111/j.1567-1364.2001.tb00007.x]

-
Larson, B. M. H., P. G. Kevan, and D. W. Inouye, (2001), Flies and flowers: taxonomic diversity of anthophiles and pollinators, Can. Entomol, 133, p439-465.
[https://doi.org/10.4039/ent133439-4]

- Lee, H. S., S. W. Lee, and H. K. Ryu, (2000), The insects foraging on apple orchards in Kyungpook Province, Korean J. Apiculture, 15, p9-20.
- Lee, S. B., D. K. Seo, K. H. Choi, S. W. Lee, H. J. Yoon, H. C. Park, and Y. D. Lee, (2008), The visited insects on apple flowers, and the characteristics on pollinating activity of pollinators released for pollination of apple orchard, Korean J. Apiculture, 23, p272-282.
- Lee, S. B., D. K. Seo, Y. S. Kim, N. I. Gwak, H. J. Yoon, H. C. Park, and S. J. Hwang, (2007), The pear flower-visiting insects, and the characteristics on pollinating activity of honeybee (Apis mellifera L.) and bumblebee (Bombus terrestris L.) at pear orchard, Korean J. Apiculture, 22, p125-132.
-
Potts, S. G., J. C. Biesmeijer, C. Kremen, P. Neu,amm, O. Scjweiger, and W. E. Kunin, (2010), Global pollinator declines: trends, impacts and drivers, Trends. Ecol. Evol, 25, p345-353.
[https://doi.org/10.1016/j.tree.2010.01.007]

- Woo, K. S., H. Y. Choo, and K. R. Choi, (1986a), Studies on the ecology and utilization of pollinating insects, Korean J. Apiculture, 1, p54-61.
- Woo, K. S., H. Y. Choo, and K. R. Choi, (1986b), Studies on the ecology and utilization of pollinating insects (II), Korean J. Apiculture, 1, p119-125.
Appendix
Appendix 1.
List of flower visiting insects of Hymenoptera and Diptera on different types of plants (F, H, and W denote fruit crop, horticultural crop and wild plants)
Appendix 2.
List of flower visiting insects of Lepidoptera, Coleoptera and other orders on different types of plants (F, H, and W denote fruit crop, horticultural crop and wild plants)