Evaluation of Total Polyphenol, Flavonoid and Vitamin Content from the Crushed Pollens of Acorn and Actinidia
Abstract
Bee pollen is rich in various nutrients. Bee pollens of acorn (Quercus acutissima) and actinidia (Actinidia arguta) are the most collected in Korea. But stiff pollen wall hinders dissolution of polysaccharides and lowers extraction efficiency. Thus, we evaluated total polyphenol, total flavonoid and vitamin content from the pollen grains crushed by pulverization and freeze-drying method, and pollen extracts obtained by aqueous and ethanolic extraction, respectively. The total polyphenol contents were higher in acorn pollens than in actinidia pollens, in ethanol extracts than in H2O extracts, and highest in freeze_ dried pollen followed by pulverized pollen and pollen load. The same pattern was found in total flavonoid contents. Vitamin B2 contents were very low, but B3 were high regardless of crushing. Vitamin C was only recovered from pollen loads, but negligible in pulverized or freeze-dried pollens.
Keywords:
Acorn, Actinidia, Flavonoid, Freeze-dry, Polyphenol, VitaminINTRODUCTION
Bee pollen is the pollen ball that has been packed by worker honeybees into pellets. Bee pollen added honey and glandular secretions is stored in brood cells (Bogdanov, 2014).
Bee pollen collected from various flowers is the only natural source of proteins, lipids, minerals, vitamins and amino acids for brood rearing and bee growth and development (Human and Nicolson, 2006; Campos et al., 1997). Recent research demonstrates that bee pollen possesses therapeutic benefits like promoting antitumor effects, scavenging free radicals, enhancing the immune function to mention a few (Bogdanov, 2014; Kroyer and Hegedus, 2001), and a good nutritional supplement with the beneficial effect for health (Bogdanov, 2014). Also pollen has high contents of polyphenolic compounds. Recently, many researchers have been focusing on flavonoids and phenolic acids that possess antibacterial, antiinflammatory, anticarcinogenic, immunemodulatory and antioxidant activities (Dini, 2011; Fang et al., 2008; Li et al., 2009; Zhang et al., 2006). This pollen grain wall usually has an outer layer (exine) made of sporopollenin in combination with an inner layer (intine) which is made up largely of cellulose. Sporopollenin elaborated in both surface sculpture and internal structure greatly resists decay and digestion or chemical degradation. The intine compo-sed primarily of cellulose and pectin also resists decay and digestion, and forms the final barrier to the nutrient-rich cytoplasm (Kress et al., 1978; Lee, 1986). Thus, any animal or insect absorbs only some cytoplasmic nutrients (Roulston and Cane, 2000; Blackmore et al., 2010). The research for extracting the cytoplasmic nutrients from pollen grains has been actively carried out recently. Rupture techniques of pollen cell wall were reported using planetary mill (Han et al., 2004; Kim and Son, 1990) or using supercritical carbon dioxide (Xu et al., 2009). Also, extracting nutrients from pollen grains were achieved by treating them with cell-wall-degrading chemical enzymes such as bee larva gut enzyme (Kim, 1989) and hexane or protease (Lee et al., 1997; Choi and Jeong, 2004).
In this study, we evaluated total polyphenol, total flavonoid and vitamin content from acorn and actinidia pollen grains crushed by pulverizing and freeze-drying method, and pollen extracts obtained by aqueous extraction or ethanolic extraction.
MATERIALS AND METHODS
Bee pollen samples
Acorn and actinidia pollen grains were collected from the National Academy of Agricultural Science (NAAS) apiary located in Wanju province of Korea during April to June 2014. The selected beehives were equipped with bottom-fitted pollen traps. The collected bee pollens were stored at -20°C in freezer for analysis.
Pollen wall rupture
Pollen cell wall was crushed by low-temperature ultrafine pulverizer (HKP-02, Korea Energy Technology, Seoul Korea) and freeze dryer (Freeze dryer, FD5508, Ilshin Lab Co. Ltd, Korea). Pollen grains were ground to a high-speed rotation speed at 80,000rpm by pulverizer which was equipped with a filter of 120 mesh and set at the temperature of -20°C to control the temperature increase of the sample. Pollen grains were frozen to -80°C in the rapid refrigeration (Deep Freezer, DF 9010, Ilshin Lab Co. Ltd, Korea), and then dried in a freeze dryer freeze dryer to -45°C
Pollen extracts preparation
4g of crushed pollens in 20ml of ethanol or distilled water (DW) was shaken in a water bath for 6 hours and centrifuged for 10 min at 10,000rpm. The extracted solution was filtered by Whatman filter paper No. 2 (Whatman, UK).
Total polyphenol content (TPC)
The total polyphenol contents in the pollen extract was estimated according to the Folin-Ciocâlteu method described by Blois (1958), with minor modifications. 0.5ml of the pollen extract was mixed with 1ml of 80% ethanol, 5ml of distilled water and 5ml of 0.2 N Folin-Ciocâlteu reagent. After agitating at room temperature for 5 min, 1ml of 5% sodium carbonate solution (Na2CO3) was added to mixtures and kept in the dark for 1 hour. The absorbance was read at 725 nm in a spectrophotometer. The total quantities of polyphenols in pollen samples were determined from the calibration curve using gallic acid (GA) standard solutions. Total phenol content was expressed as mg GAE/g pollen (mg gallic acid equivalents per gram pollen).
Total flavonoid content (TFC)
The total flavonoid content in the pollen extract was determined in accordance with the method described by Moreno et al. (2000). 25μl of the pollen extract was mixed with 8μl of 5% sodium nitrite (NaNO2) and incubated at room temperature for 5 min. 15μl of 10% aluminium chloride (AlCl3) was added to mixtures and agitated for 6 min and then neutralized with 50μl of 1M sodium hydroxide (NaOH). Absorbance was read at 510 nm in a spectrophotometer. The total quantity of flavonoid was calculated as quercetin equivalents per g pollen (mg QE/g pollen).
Vitamin
Vitamin content of acorn and actinidia pollen grains was determined following the standard method described by Korean Food Standards Codex (2008). The vitamins were measured by Nanospace SI-2 HPLC system (Shiseido, Japan) with a fluorescence (FL) detection for vitamin B2 (Riboflavin) or photodiode array PDA) detector for vitamin B3 (niacin) and vitamin C.
RESULTS AND DISCUSSION
Total polyphenol contents
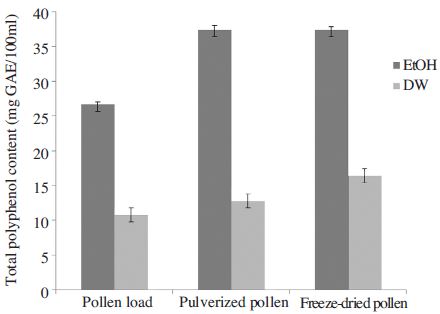
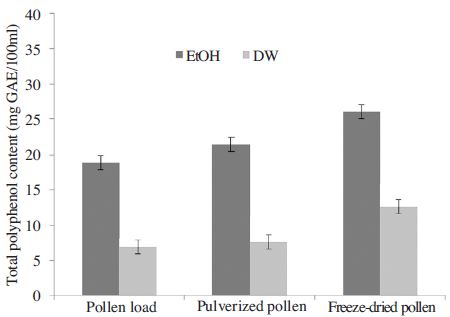
Total polyphenol content of pollen extracts of acorn and actinidia was obtained using the standard curve of gallic acid (R2=0.99). Fig. 1 and 2 show that the total polyphenol contents varied with the extract solvents or plants. The total polyphenol content was found to be highest in freeze-dried pollen, followed by pulverized pollen and pollen grain in actinidia pollen. The total polyphenol content from actinidia pollen was increased 10% in pulverized pollen and 27.4% in freeze-dried pollen than pollen grain, respectively. We obtained similar result from acorn pollen. The total polyphenol content from freeze-dried or pulverized acorn pollen was increased 28.7% than pollen grain. Also the ethanol pollen extracts showed higher levels of total polyphenol content than water extracts in both acorn and actinidia pollens in this study, which are comparable to the results of other research. The total polyphenol content from freeze-dried pollen extracted with ethanol was 2-fold higher than that from acorn and actinidia pollen extracted with water. The freeze-dried acorn pollen extracted with ethanol showed the highest values of total polyphenol in comparison to the uncrushed acorn pollen or actinidia pollen. The total polyphenol content from freeze-dried or pulverized acorn pollen was higher than reported by Hong et al. (2014), who measured the total phenolic compound from acorn pollen treated with medicinal mushrooms.
Total flavonoid content
Using the standard curve generated by quercetin (R2=0.9806), the total flavonoid content from acorn and actinidia pollen extracts are presented in Table 1 and 2. The freeze-dried acorn pollen extracted with ethanol showed the highest values of 26.7±0.47mg, followed by pulverized pollen and pollen grain in total flavonoid content. The total flavonoid content from acorn pollen was increased 17.7% in pulverized pollen and 27.4% in freezedried pollen compared to pollen grain, respectively. Similar results were obtained from actinidia pollen. Also higher total flavonoid levels were detected in ethanol extract than in water extract from both acorn and actinidia pollen. The total flavonoid content from freeze-dried acorn pollen extracted with ethanol was 6.8 times higher than that from pollen extracted with water. There are no reports of the flavonoid content in acorn and actinidia pollen.
Vitamin
The content of vitamins B2, B3 and C in acorn and actinidia pollens is shown in Tables 3 and 4. Data shows that the amount of vitamin C was the highest value of 98.9mg/100g, followed by vitamin B3 and B2 in acorn and actinidia pollen grains. The content of vitamin C was largely decreased in acorn pollen after pulverizing or freeze-drying, whereas that of vitamin B3 was slightly decreased. The vitamins B2 and B3 content in actinidia pollen was slightly increased after pulverizing or freezedrying, but that of vitamin C was largely decreased as acorn pollen. The vitamin B2 concentration of acorn pollen grain found in the present study was slightly higher than that reported by Lee et al. (1997). The vitamin B2 concentrations of acorn and actinidia pollen grains obtained was slightly higher than that of pine pollen grain reported by Lee et al. (1997), but lower than reported from Han et al. (2004).
Acknowledgments
This work was supported by “Research program for Agricultural Science & Technology Development (Project No. PJ010837)” Rural Development Administration, Republic of Korea.
LITERATURE CITED
-
Blackmore, S., Wortley, A. H., Skvarla, J. J., Gabarayeva, N. I., and J. R. Rowley, (2010), Developmental origins of structural diversity in pollen walls of Compositae, Plant Syst Evol, 284, p17-32.
[https://doi.org/10.1007/s00606-009-0232-2]

-
Blois, M. S., (1958), Antioxidants determination by the use of a stable free radical, Nature, 181, p1199-1200.
[https://doi.org/10.1038/1811199a0]

- Bogdanov, S., (2014), Pollen. Production. Nutrition and Health, A Review p3, Bee Product Science.
-
Campos, M. G., Markham, K. R., Mitchell, K. A., and A. P. Cunha, (1997), An approach to the characterization of bee pollens via their flavonoid/phenolic profiles, Phytochem. Anal, 8, p181-185.
[https://doi.org/10.1002/(SICI)1099-1565(199707)8:4<181::AID-PCA359>3.0.CO;2-A]

-
Choi, S. J., and Y. H. Jeong, (2004), Effect of proteases on the extraction of crude protein and reducing sugar in pollen, J Korean Soc Food Sci Nutr, 33, p1353-1358.
[https://doi.org/10.3746/jkfn.2004.33.8.1353]

-
Dini, I., (2011), Flavonoid glycosides from Pouteria obovata (R. Br.) fruit flour, Food Chem, 124, p884-888.
[https://doi.org/10.1016/j.foodchem.2010.07.013]

-
Fang, K. F., Wang, Y. N., Yu, T. Q., Zhang, L. Y., Baluska, F., Samaj, J., and J. X. Lin, (2008), Isolation of de-exined pollen and cytological studies of the pollen intines of Pinus bungeana Zucc. Ex Endl. and Picea wilsonii Mast, Flora, 203, p332-340.
[https://doi.org/10.1016/j.flora.2007.04.007]

- Han, M. R., S. J. Lee, and M. H. Kim, (2004), Development of pine pollen cell wall rupture technique using a high impact planetary milling process, Dankook Journal of the New Material Technology, 12, p43-54.
-
Hong, I. P., S. O. Woo, S. M. Han, J. H. Yeo, and M. L. Cho, (2014), Germination and antioxidant activity of Korean oak pollen treated with medicinal mushrooms, Kor J Myco, 42, p165-169.
[https://doi.org/10.4489/KJM.2014.42.2.165]

-
Human, H., and Nicolson, S. W., (2006), Nutritional content of fresh, bee-collected and stored pollen of Aloe grestheadii var. davyana (Asphodelaceae), Phytochem, 67, p1486-1492.
[https://doi.org/10.1016/j.phytochem.2006.05.023]

- Kim, D. S., (1989), Effect of larva gut enzyme on pollen, Korean J Food Sci Technol, 21, p404-408.
- Kim, J. G., and J. H. Son, (1990), Progress of chemical composition on pulverization of pollen load, Korean J. Apiculture, 5, p23-30.
- Korean Food Standards Codex, (2008), Ministry of food and drug safety.
-
Kress, W. J., Stone, D. E., and S. C. Sellers, (1978), Ultrastructure of exine-less pollen: Heliconia (Heliconiaceae), Amer. J. Bot, 65, p1064-1076.
[https://doi.org/10.2307/2442323]

-
Kroyer, G., and Hegedus, N., (2001), Evaluation of bioactive properties of pollen extracts as functional dietary food supplement, Innov. Food Sci. Emerg. Technol, 2, p171-174.
[https://doi.org/10.1016/S1466-8564(01)00039-X]

- Lee, B. Y., H. D. Choi, and J. B. Hwang, (1997), Component analysis of Korean pollens and pollen extracts, Korean J Food Sci Technol, 29, p869-875.
- Lee, S. T., (1986), Palynology and plant systematics, Korean J. Apiculture, 1, p46-53.
-
Li, F., Q. P. Yuan, and F. Rashid, (2009), Isolation, purification and immunobiological activity of a new water-soluble bee pollen polysaccharide from Crataegus pinnatifida Bge, Carbohydrate Polymers, 78, p80-88.
[https://doi.org/10.1016/j.carbpol.2009.04.005]

-
Moreno, M. I. N., Isla, M. I., Sampietro, A. R., and M.A. Vattuone, (2000), Comparison of the free radical-scavenging activity of propolis from several regions of Argentina, J Ethnopharmacol, 71, p109-114.
[https://doi.org/10.1016/S0378-8741(99)00189-0]

-
Roulston, T. H., and J. H. Cane, (2000), Pollen nutritional content and digestibility for animals, Plant Syst. Evol, 222, p187-209.
[https://doi.org/10.1007/BF00984102]

-
Xu, X., Sun, L. P., Dong, J., and H. C. Zhang, (2009), Breaking the cells of rape bee pollen and consecutive extraction of functional oil with supercritical carbon dioxide, Innovative Food Science and Emerging Technologies, 10, p42-46.
[https://doi.org/10.1016/j.ifset.2008.08.004]

-
Zhang, J., Stanley, R. A., Adaim, A., Melton, L. D., and M. A. Skinner, (2006), Free radical scavenging and cytoprotective activities of phenolic antioxidants, Mol Nutr Food Res, 50, p996-1005.
[https://doi.org/10.1002/mnfr.200600072]