
Foraging Strategies in Honeybees, Apis dorsata F. and Apis florea F. in Relation to Availability of Energy Rewards
Abstract
Foraging behavior of two honeybee species Apis dorsata F. and Apis florea F in relation to sugar composition and energetic of nectar production was studied in a community of plant species where the two bee species forage regularly throughout the year. Qualitative and quantitative analysis of nectars from 51 cultivated and ornamental plants revealed that their nectars contained three types of sugars viz. glucose, fructose and sucrose. Quantitative studies enable categorization of the nectars into three groups a) glucose and fructose dominated, b) sucrose dominated and, c) nectars having nearly equal proportions of glucose, fructose and sucrose. Of the two honeybee species, Apisdorsata preferred relatively large sized sucrose dominated flowers whereas Apis florea small flowers with glucose + fructose dominated sugars. However, energy cost and reward provided a clear picture which indicates that A. dorsata bigger in size and tongue length forage flowers providing high energy rewards compared to A. florea which forage low energy rewarding flowers. Such preferences seem to be associated with the foraging profitability of the pollinators.
Keywords:
Energy rewards, Apis dorsata, Apis florea, Energy costs, Benefits, Foraging behaviorINTRODUCTION
The habitat of most animals provides resources of different types that are essential for the species’ survival, but these may not necessarily be close together. Accordingly, these animals have to forage to bridge some temporal or spatial distance to locate the essential resources (Schoener, 1971). It is one of the most consistent and demanding tasks for any given living organism related to its survivability. The data on foraging ability and behaviour are, therefore, necessary to the understanding of population dynamics and community structure of bees as well as to develop conservation strategies. The foraging behavior of social insects is especially interesting because individuals do not forage to meet their own nutritional needs; rather they forage to meet the needs of the colony (Winston 1987; Robinson 1992; Seeley 1995; Robinson 2002, Seeley 1989; Hunt et al., 1995; Dreller and Page, 1999; Page and Erber, 2002). Flowering plants and honeybees have a special relationship in which both are benefited from each other. Nectar is the carbohydrate compounds mainly sucrose, fructose and glucose portion of the honeybee’s food and is the raw material of honey (Jones and Yates, 1991). Several floral stimuli including colour, shape and odor are responsible to attract the pollinators (Faegri and van der Pijl, 1966; Grant and Grant, 1968). These stimuli seem to work in succession as primary fixity factors and lead pollinators to an end fixity stimuli system comprising the potential energy reward of a flower as nectar and pollen (Sihag, 1982a). It is on account of the interaction of these fixity systems that a pollinator-flower relationship is established which has during the recent years caught the attention of ecologists and some interesting information has thus become available (Heinrich, 1975a,b, 1976a,b; Heinrich and Raven, 1972; Wolf, 1975; Wolf and Hainsworth, 1971, Wolf et al., 1972, 1975; Waddington et al., 1981). Of the several factors, reward system offered by the flowers plays a determinant role besides inter play of other factors which participate in attracting floral visitors. In this connection flower visitors relationship got set heavily between the visitors energy need and the quantity of food it can harvest from flowers (Hocking, 1953; Churchill and Christenensen, 1970; Hainsworth and Wolf, 1972a,b,c; Heinrich and Raven, 1972; Stiles, 1971) which influence the frequency of visits to flowers (Heinrich, 1973; Heinrich, 1975). The social set up of honeybees require since a large quantity of food supply for their brood many more times their own energy requirements is secured by them by repeated visits to flowers (Reddy and Reddy, 1984). This can keep happening only if a flower provides sufficient reward to attract the foragers on one hand and limit the reward on the other hand compelling the visitors to frequent other flowers of the same species (Heinrich, 1975), thus enabling development of an optimum strategy both by flowers and pollinators for maximum cross pollination (Emlen, 1966; Schoenar, 1971; Cody, 1974; Abrol, 2010, 2011). Apis florea F. and A. dorsata F. which stand phylogenetically at the lower level in Apis group of bees make the potential pollinators of several plants in the Indian sub-continent where bee pollinator-flower relationship attains significance (Arias and Sheppard, 2005). This study was made to determine whether the foraging behavior of two sympatric honeybee species which utilize the common resources and differ morphologically from each in terms of energy intake and energy requirements differs from each other. The results obtained are presented in this paper.
MATERIALS AND METHODS
Sampling of bees
The purpose of this study was to describe the energetic mechanism underlying the choice of individual flowers of a plant species by two honeybee species. Material for these studies is comprised of two species of wild nesting honeybees viz. A. dorsata and A. florea which are active throughout the year. In order to determine the response of these species to different nectars, 51 cultivated and ornamental honey plants were studied. Floral attractability and fidelity of these species were adjudged by their relative abundance (measured in terms of number/ha, (Jain and Kapil, 1980; Sihag, 1982b; Abrol, 1992) and in relation to the quality and quantity of nectar. The observations were made throughout the year with the calendar of flowering plants.
Qualitative and quantitative analysis of nectar
The quantitative analysis of nectar was made by measuring the amount of nectar secreted/day. For this purpose, flower buds which may bloom in next 2~3 h were selected and nectar was collected from these opened flowers next day in the fore-noon when temperature fluctuated between 22~24°C. Total dissolved sugars present in nectar were measured with the help of a pocket refractometer model No. 1093, manufactured by M/s Toshniwal Brothers, Pvt. Ltd., New Delhi.
Nectar was collected with micro-capillaries and sugars present in the nectar were analysed by paper chromatography using n-butanol-acetic acid-water (4:1:5) as the solvent (Block et al., 1958). For this purpose, samples collected were immediately loaded on chromatographic sheet (Whatmann No. 1, 55x45cm) for qualitative analysis as described by Partridge (1948) using n-butanol acetic acid and water (4:5:1) as running solvent. The quantitative estimation of different sugars was done according to Johnston et al. (1964). The different components of sugar separated on the chromatograph were eluted by dissolving thespots in 80% ethanol. The extract of the respective sugars, thus obtained was analysed quantiratively by colorimetric method of Yemm and Willis (1954) using 0.2% anthrone dissolved in 70% sulphuric acid and recoerding O.D at 620nm for sucrose and fructose and at 540nm for glucose with Spectronic 20.
Calculation of energy per flower
Total reducing sugars present in a unit amount of nectar were estimated, following Spagyi’s modified method (Nelson, 1944) and their volume in each nectar was then converted into energy (joules) by a method suggested by Heinrich and Raven (1972).
The amount of sugar and energy content per flower per day was calculated using the formula.
Energy per flower per day (joules) = Amount of sugar per flower per day (mg) x 16.74 where, 16.74 is the joule of energy obtained from one 1mg of sugar irrespective of the type of sugar (Heinrich1975; 1mg = 4 cal=4x4.186 joules=16.74 joules).
Body weight and tongue length measurements
Samples of forager bees of both the honeybee species were collected from the flowers. The collected bees taken in a glass jar and then killed under cooling in a deep freezer. After obtaining their body weight on a digital balance, the bees were dissected to separate their tongues. The separated body parts of worker bees were put on glass slides and covered with another glass slides. All measurements were taken by using Leica M 165 C stereo microscope with high speed digital fire wire live camera and LAS measurement module and data transfer. The images were analyzed and the measurements were recorded into a computer. The recorded data were analyzed following Sokal and Rholf (1981).
RESULTS
The data presented in table 1 shows the population dynamics of two honeybee species Apis dorsata and A. florea attracted to different plant species in relation to dominant sugar, Volume of nectar per flower (μl), Percentage of dissolved sugars (%), Total dissolved sugar per flower (mg) and Energy per flower (joules). The qualitative analysis of the nectars from different honeybee plants revealed the existence of primarily three sugars viz. sucrose, glucose and fructose, were categorized in into three categories: Sucrose dominated nectars, Glucose dominated and Equi-proportioned. However, few nectars possessed two sugars wherein either sucrose or fructose was absent. Of the 51 plants 13 were with sucrose dominating nectar such as Hibiscus rosa-sinensis L, Eucalyptus hybrid, Oenothera dromundii Hook, Lens culinaris Medik, Pisum sativum, Cicer arietinum L., Melilotus indica, C. Limettoides Tanaka, C. medica L. var. galgala, Linaria bipartite Willd, Lantana camera L., Cajanus cajan, Helianthus annuus, 7 plants with equal amount of sucrose and glucose+fructose composition in nectar which included Echium vulgare L, Dahlia sp, C. paradisi Macf. C. maxima (Burm) Antirrtinum majus L Luffa acutangula (L) Roxb. Cucumis melo L and 31 with glucose dominating sugars such as Ruelthia tuberosa L., Tecoma stans H.B.& K, Cosmos bipinnata Cav., Brassica compestris L. var. toria, B. napus L., B. carinata L, B. hirta Moench, B. rapa L., B. juncea Czern & Coss, B. oleracea var. botrytis L., Eruca sativa Lam., Raphanus sativas L., Iberis amara L., Cherianthus cheri L., Benincasa hispida Cogn., Momordica charantia L., Citrullus vulgare Schrad, Althaea rosea cav., Prunus persica (L) Batsch, P. domestica (L) Schncid, Citrus sincensis Osbeck, Petunia alba L., L. indica Roxb., Verbena bipinnatifida Schau., Coriandrum sativum, Foeniculum vulgare, Medicago sativa, Trifolium alexandrium, Mangifera indica, Parkinsonia aculeata.
Of 13 plants with sucrose dominating nectar, 7 attracted predominantly A. dorsata and the remaining 6 A. florea. Similarly, of 31 plants having glucose dominated nectars, 27 were visited in large numbers by A. florea, 2 by A. dorsata, C. vulgare showed equal attractability whereas P. alba attracted no A. dorsata bees. Out of 7 plants with equal amount of sucrose and glucose+fructose composition in nectar, 2 plant species received A. dorsata in considerable number,whereas 4 species received A. florea whereas one species of plant received almost equal proportion of the population of two bees species (Table 1, Fig. 1). The bee population-nectar quality pattern apparently indicates general preference of A. dorsata for flowers having nectar with sucrose dominated sugars and A. florea for flowers with glucose dominated nectars. Morphologically both the species of honeybee are quite distinct from each other. Apis dorsata is the largest one whereas A. florea is smallest of all the honeybee species. The body weight and tongue length of two honeybee species, A. dorsata and A. florea are quite different. Apis dorsata is larger in size having much higher body weight (123.00± 5.21, Mean±S.D., N=50) and tongue length (6.45±0.34, Mean±S.D., N=50) and tongue length compared to A. florea (22.6±3.21, Mean±S.D., N=50 ) and 3.31±0.234, Mean±S.D., N=50), respectively.

Differential attractability of honeybee Apis dorsata and A. florea to nectar production characteristics (per flower) of different plants
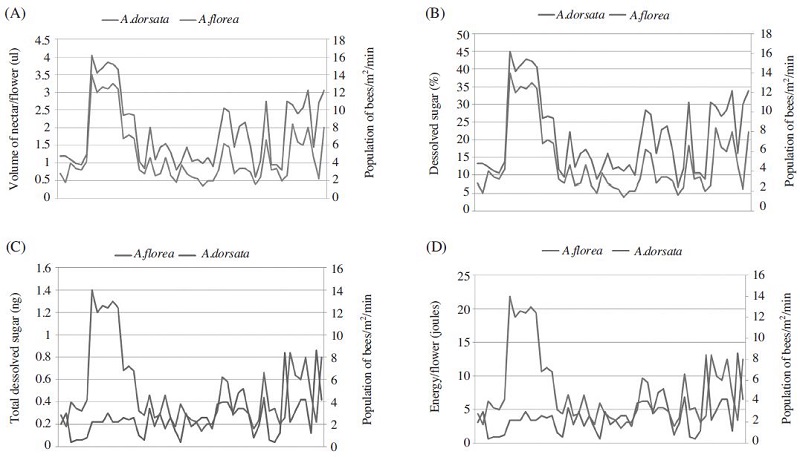
Graphical representation of attractabilty of Apis dorsata and Apis florea to A) nectar volume, B) percentage of dissolved solids, C) total dissolved sugar and D) energy per flower. X- Axis represent nectar volume, percentage of dissolved solids, total dissolved sugar and energy per flower whereas Y- Axis represent dependent variables such as A. dorsata and A. florea.
Correlation analysis of different parameters (Table 2) such as volume of nectar per flower (μl), percentage of dissolved sugars (%), total dissolved sugar per flower (mg) and energy per flower (joules) in relation to A. florea and A. dorsata revealed that all these parameters strongly influenced the visits of A. dorsata as relationship with all the factors was highly significant and positive whereas the relationship with A. florea was either non-significant or negative except nectar sugar concentration which strongly influenced the foraging population. Interrelationships among different nectar characteristics revealed that nectar volume was highly significant and positively correlated sugar content and energy rewards whereas negatively with sugar concentration. Nectar sugar concentration decreases as the volume increases in flowers. Similarly, sugar content was highly significant and positively correlated with energy per flower, as both these are dependent parameters as the sugar content increases energy value increases viceversa. Relationship between nectar sugar concentration and sugar content was found to be non-significant.
Multiple correlation analysis of the data also revealed the similar trend where R2 for A. florea and A. dorsata was 27 and 51 percent, respectively; indicating that 73% in case of former and 49% in case of later remained unexplained (Table 3). The studies clearly reveal that in case of A. dorsata all the studied parameters could shape the foraging behavior and selection of flowers compared to A. florea which because of low energy requirements could forage on any plant species available.

Correlation coefficient matrix exhibiting the relationship of bee activity with floral nectar characteristics
DISCUSSION
The studies revealed the existence of primarily three sugars viz. sucrose, glucose and fructose, were categorized in into three categories: Sucrose dominated nectars, Glucose dominated and Equi-proportioned (Table 1). However, few nectars possessed two sugars wherein either sucrose or fructose was absenta condition typical of nectars from most flowers (Percival, 1961). The two honeybee species exhibited a marked preference for either sucrose dominated or glucose dominated sugars. The behavior pattern of these two honeybee species, A. dorsata and A. florea is contrary to the behaviour of A. mellifera which preferred a sugar solution containing equal mixtures of sucrose, glucose and fructose over other test solutions (Wykes, 1952a,b) and sweet clover with nectar having balanced sucrose glucose-fructose composition over alfalfa, alsike or red clover in which sucrose was dominant (Furgala et al., 1958). The foraging behaviour of A. florea is nevertheless remarkably compatible with its natural habitat niche demanding low energy requirements. It is hence indicative of variable ecological distribution of the two indigenous wild honeybee species i.e. A. dorsata and A. florea (Kapil et al., 1971). This aspect demands a detailed study on energy budget-niche determination interactions. It is further interesting to find that A. dorsata preferred nectar yielding plants high energy rewards regardless of the kind of sugars present. By contrast, A. florea preferred flowers with low energy rewards. Seemingly, energy reward makes a suitable parameter to determine the foraging strategies of these bees. Hibiscus rosa sinensis is an exception, however. Here less population of A. dorsata on this species does not appear to be related to the energy reward. Rather because the flower is red coloured, is perhaps less conspicuous to bees/insects (Heinrich and Raven, 1972).
Waddington et al. (1981) opined that the total energy reward and the intermittent energy flow are the two parameters which interact with each other to determine the foraging strategies of bees. In this connection, the studies made on bumblebees (Waddington et al., 1981; Heinrich, 1976b) are of interest. They observed that bumblebees visited in large numbers and were in general distributed over such flowers producing the largest amount of nectar per day. The foraging strategy exhibited by A. dorsata seem to follow this pattern. However, A. florea falls apart and the difference relates to its differential energy requirements.
The occurrence of sucrose or glucose doiminated nectars indicate that total caloric reward is an important factor for foragers and determines their population on a particular crop. Abrol (1985) found that large sized bees such as Xylocopa fenestrata, Megachile lanata, M cephalotes and Apis dorsata visited sucrose dominated flowers such as Cajanus cajan and Pongamia glabra providing large amounts of nectar with more caloric rewards than did Apis florea and Pithtis smaragdula with their low energy requirements relied on glucose dominated flowers of Coriandrum sativum, Trifolium alexandrium, Medicago sativa and Foeniculum vulgare. Wyke (1952) suggested that bees preferred equi-proportioned sugars, viz glucose, fructose and sucrose than any other combination but neither Waller (1972) using behavioural responses, nor Whitehead and Larsen (1976) with their electrophysiological studies support Wyke’s results. Bachman and Waller (1977) also did not support her contention.
The other explanation of differential behavior of two honeybee species is related to their body size (Harder, 1985). The body weight and tongue length of two honeybee species, A. dorsata and A. florea are quite different. Apis dorsata is larger in size having much higher body weight and tongue length as compared to A. florea. Evidently, Apis dorsata had higher energy requirements and foraging rate than A. florea. Abrol (2006) studied the foraging behavior of honeybee A. florea F. on carrot (Dacus carota) flowers and found that of all the insects, the dwarf honeybee A. florea F. was the most abundant. This was mainly due to the fact that A. florea had much suitability of its tongue length to the corolla length of carrot bloom compared to A. dorsata. Besides, difference in the body weight of two species is also responsible to determine in the energy cost of an individual (Wolf, 1975; Wolf et al., 1975). By this logic, A. dorsata spends more energy during foraging and hence its foraging cost stands higher than A. florea. This makes A. dorsata to become selective in obtaining an energy reward.
Nectar enrichment and intermittent energy reward are the other factors to determine the foraging strategies of the bees (Waddington et al., 1981). Differential preference of A. dorsata shown in table 1 can probably be explained well by this point. Rewarding system developed by the flowers enable pollinators to discriminate between the closely related plant species or ecotypes. This has resulted in a copartnership between the flowers and their pollen vectors. Co-evolution has brought a close correlation between pollinator needs and floral energy expenditures (Heinrich, 1975a).
Species provide more intermittent energy reward compared with the lower nectar yielding species. And if the floral reward is coupled with the foraging cost of the bees, only foraging on those species will be profitable whose energy reward exceeds the foraging cost of a pollinator. Probably, this is the reasons that A. dorsata preferred Tecoma stans over Brassica compestris var. toria, Eucalyptus sp. over Brassica juncea, T. stans over Eucalyptus sp., Citrus paradise over Eucalyptus sp. and C. limettoides over Eucalyptus sp. On the other hand, A. florea did not exhibit a specialized foraging pattern. The reason obviously seems to be the low foraging cost which may be compensated by the small quantity of nectar. Correlation analysis and multiple regression analysis of the data also confirmed the above observations.
The study examined parameters such as nectar sugar composition of flowers, availability of rewards, nectar volume, nectar concentration, energy per flower coupled with body size and tongue length of bees which determine foraging strategies of these bees. However, this gives rise to many more questions for future research. These are: what is the energy budget pattern of these bees? What is the energy economy? How these bees respond to the persistent versus intermittent energy rewards?, and what should be the optimum floral reward which is profitable to a species? The exploration of these questions is likely to generate better understanding of plant strategies to attract the pollinators, pollinator strategies to discriminate ‘major’ and ‘minor’ energy sources and ultimately the co evolutionary basis of flower pollinator relationship. Hence, these may prove fruitful in evolving high energy rewarding varieties with reference to pollination of crops and development of Apiculture.
References
- AAbrol, D.P., (2006), Factors influencing flight activity of Apis florea F, an important pollinator of Daucus carota L, Journal of Apicultural Research and Bee World, 45(2), p2-6.
- Abrol, D.P. Foraging, (2011), Honeybees of Asia, In: Howard R. Hepburn, and Sarah E. Radloff (ed), Honeybees of Asia, Springer, Germany, p257-292.
- Abrol, D.P., (1992), Bioenergetics in bee flowers interrelationships- an analysis of foraging behaviour, Korean J. Apic, 7(I), p39-66.
- Abrol, D.P., (2010), Foraging behaviour of Apis florea F., an important pollinator of Allium cepa L, Journal of Apicultural Research and Bee World, 49(4), p318-325.
- Abrol, D.P., (1985), Analysis of biophysical interactions in causing foraging behaviour of some bees- A study in bioenergetics, Ph.D Thesis, Haryana Agricultural University, Hisar, p287.
-
Arias, M.C., and W.S. Sheppard, (2005), Phylogenetic relationships of Honeybees (Hymenoptera: Apinae: Apini) inferred from nuclear and mitochondrial DNA sequence data, Mol Phylogenet Evol, 37(1), p25-35.
[https://doi.org/10.1016/j.ympev.2005.02.017]

- Bachmann, W.W., and G.D. Waller, (1977), Honeybee responses to sugar solutions of different compositions, Journal of Apicultural Research, 16, p165-169.
- Block, R.J., E.L. Durrum, and G. Zweig, (1958), A manual of Paper Chromotography and Paper Electrophoresis, Academic Press. INC Publishers, New York, p170-214.
-
Churchill, D.M., and P. Christenenson, (1970), Observations on pollen harvesting by brush tongued lorikeets, Austr J. Zool, 18, p427-437.
[https://doi.org/10.1071/ZO9700427]

-
Cody, M., (1974), Optimization in ecology, Science, 183, p1156-64.
[https://doi.org/10.1126/science.183.4130.1156]

-
Dreller, C., and R.E. Page, (1999), Genetic, developmental and environmental determinants of honey bee foraging behavior, In: DetrainC.DeneubourgJ.L.and, PasteelsJ.M.(eds)Information Processing in Social Insects, Birkhauser Verlag, Basel, Switzerland, p187-202.
[https://doi.org/10.1007/978-3-0348-8739-7_10]

-
Emlen, J.M., (1966), The role of time and energy in food preferences, Am. Natur, 100, p611-617.
[https://doi.org/10.1086/282455]

- Faegri, K., and L. van der Pijl, (1966), Principles of Pollination Ecology, Pergamon, New York.
-
Furgala, B., T.A. Gochnauer, and F.G. Holdaway, (1958), Constituent sugars of some northern legume nectars, Bee World, 39, p203-205.
[https://doi.org/10.1080/0005772X.1958.11095065]

- Grant, V., and K.A. Grant, (1968), Hummingbirds and their flowers:, Columbia University Press, New York.
- Gupta, J.K., and M.C.M. Reddy, (1992), Foraging intensity of insects and nectar of wild cherry Prunus puddum Roxb, Entomon, 17, p91-94.
-
Hainsworth, F.R., and L.L. Wolf, (1972a), Energetics of nectar extraction in a small, high altitude, tropical hummingbird, Selasphorus flammula, J. comp. Physiol, 80, p377-387.
[https://doi.org/10.1007/BF00696435]

-
Hainsworth, F.R., and L.L. Wolf, (1972b), Power for hovering flight in relation to body size in hummingbirds, Amer. Naturalist, 106, p589-596.
[https://doi.org/10.1086/282799]

-
Hainsworth, F.R., and L.L. Wolf, (1972c), Crop volume, nectar concentration, and hummingbird energetics, Comp. Biochem. Physiol, 42, p359-366.
[https://doi.org/10.1016/0300-9629(72)90117-X]

-
Harder, L.D., (1985), Morphology as a predictor of flower choice by bumble bees, Ecology, 66, p198-210.
[https://doi.org/10.2307/1941320]

-
Heinrich, B., (1975), Energetics of pollination, Ann Rev. Ecol. Syst, 6, p137-171.
[https://doi.org/10.1146/annurev.es.06.110175.001035]

-
Heinrich, B., and P.H. Raven, (1972), Energetics and pollination ecology, Science, 176, p597-602.
[https://doi.org/10.1126/science.176.4035.597]

-
Heinrich, B., (1973), The energetics of bumblebees, Sci, Amer, 228(4), p96-102.
[https://doi.org/10.1038/scientificamerican0473-96]

- Heinrich, B., (1975a), The role of energetics in bumblebee-flower interrelationships, In: L.E. Gilbert, and P.H. Raven (Eds.), Coevolution of Animals and Plants, Univ, Texas Press, Austin, p141-158.
-
Heinrich, B., (1975b), Energetics of pollination, Ann. Rev. Ecol. Syst, 6, p139-170.
[https://doi.org/10.1146/annurev.es.06.110175.001035]

-
Heinrich, B., (1976a), Resource partitioning among some eusocial insects: Bumblebees, Ecology, 57, p874-889.
[https://doi.org/10.2307/1941054]

-
Heinrich, B., (1976b), The foraging specialization of individual bumblebees, Ecol. Monogr, 46(2), p105-128.
[https://doi.org/10.2307/1942246]

-
Heinrich, B., Raven, P.H., (1972), Energetics and pollination ecology, Science, 176, p597-602.
[https://doi.org/10.1126/science.176.4035.597]

- Hocking, B., (1953), The intrinsic range and speed of insect flight, Trans R Entomol Soc Lond, 104, p223-345.
- Hunt, G.E., R.E. Page, M.K. Fondrk, and C.J. Dullum, (1995), Major quantitative trait loci affecting honey bee foraging behavior, Genetics, 141, p1537-1545.
- Jain, K.L., and R.P. Kapil, (1980), Foraging rhythm of megachilid bees in relation to the flowering of Medicago sativa and Parkinsonia aculeate L, Indian Bee J, 42(2), p35-38.
- Jones, D., and B.D. Yates, (1991), Beekeeping study notes, 2nd Edition, Bee Books New and Old Tapping Wall Farm, Burrowbridge, Bridgwater TA7 ORY, Somerset, U.K.
- Kapil, R.P., G.S. Grewal, S. Kumar, and A.S. Atwal, (1971), Insect Pollinators of rapeseed and mustard, Indian J. Ent, 33(1), p61-66.
- Nelson, N., (1944), A photometric adaptation of the Somogyi method for the determination of glucose, J. Biol. Chem, 153, p375.
-
Page, R.E., and J. Erber, (2002), Levels of behavioral organization and the evolution of division of labor, Naturwissenschaften, 89, p91-106.
[https://doi.org/10.1007/s00114-002-0299-x]

-
Partridge, S.M., (1948), Filter paper partition chromatography of sugars, Biochem J, 42, p238.
[https://doi.org/10.1042/bj0420238]

-
Percival, M.S., (1961), Types of nectar in Angiosperms, New Phytol, 60, p235-281.
[https://doi.org/10.1111/j.1469-8137.1961.tb06255.x]

-
Robinson, G.E., (1992), Regulation of division of labor in insect societies, Annu Rev Entomol, 37, p637-665.
[https://doi.org/10.1146/annurev.en.37.010192.003225]

-
Robinson, G.E., (2002), Genomics and integrative analyses of division of labor in honeybee colonies, Am Nat, 160, p5160-5172.
[https://doi.org/10.1086/342901]

-
Schoener, T.W., (1971), Theory of feeding strategies, Ann. Rev. Ecol. Syst, 2, p369-404.
[https://doi.org/10.1146/annurev.es.02.110171.002101]

-
Seeley, T.D., (1989), Social foraging in honey bees: how nectar foragers assess their colony's nutritional status, Behav Ecol Sociobiol, 24, p181-199.
[https://doi.org/10.1007/BF00292101]

- Sihag, R.C., (1982a), Environmental regulation of pollination process in cultivated crops, Indian Bee J, 44(2), p42-45.
- Sihag, R.C., (1982b), Role of competition with Parkinsonia aculeate L. on pollination and seed production in Medicago sativa L, Indian Bee J, 44(4), p89-90.
- Sokal, R.R., and F.J. Rholf, (1981), Biometry, W.H Freeman & Co, Sanfrancisco.
-
Stiles, F.G., (1971), Time, energy, and territorially of the Anna Hummingbird (Calypte anna), Science, 173, p818-821.
[https://doi.org/10.1126/science.173.3999.818]

-
Waddington, K.D., Allen, T., and Heinrich, B., (1981), Floral preferences of bumblebees (Bombus edwardsii) in relation to intermittent versus continuous rewards, Anim. Behav, 29, p779-784.
[https://doi.org/10.1016/S0003-3472(81)80011-5]

-
Waller, G.D., (1972), Evaluating responses of honey bees to sugar solutions using an artificial-flower feeder, Annual Review of Entomology, 65, p857-862.
[https://doi.org/10.1093/aesa/65.4.857]

-
Whitehead, A.T., J.R. Larsen, (1976), Electrophysiological responses of galeal contact chemoreceptors of Apis mellifera to selected sugars and electrolytes, J Insect Physiol, 22, p1609-1616.
[https://doi.org/10.1016/0022-1910(76)90052-4]

- Winston, M.L., (1987), The Biology of the Honey Bee, Harvard University Press, Cambridge, MA.
-
Wolf, L.L., Hainsworth, F.R., and Gill, F.B., (1975), Foraging efficiency and time budgets in nectar feeding birds, Ecology, 56, p117-128.
[https://doi.org/10.2307/1935304]

-
Wolf, L.L., (1975), Energy intake and expenditure in a nectar feeding sunbird, Ecology, 56, p92-104.
[https://doi.org/10.2307/1935302]

-
Wolf, L.L., and F.R. Hainsworth, (1971), Time and energy budgets of territorial hummingbirds, Ecology, 52, p980-988.
[https://doi.org/10.2307/1933803]

-
Wolf, L.L., F.R. Hainsworth, and F.G. Stiles, (1972), Energetics of foraging: Rate and efficiency of nectar extraction by hummingbirds, Science, 176, p1351-1352.
[https://doi.org/10.1126/science.176.4041.1351]

-
Wykes, G.R., (1952a), An investigation of the sugars present in the nectar of flowers of various species, New Phytol, 51, p210-215.
[https://doi.org/10.1111/j.1469-8137.1952.tb06127.x]

- Wykes, G.R., (1952b), The preferences of honeybees for solutions of various sugars which occurs in nectar, Jour. Exp. Biol, 29, p511-519.
-
Yemm, E.W., and A.J. Willis, (1954), The estimation of carbohydrates in plant extracts by anthrone, Biochem J, 57, p508.
[https://doi.org/10.1042/bj0570508]
