Evaluation of Nutritional Potential of Amorpha fruticosa Pollen Collected by Honey bees
Abstract
We investigated the nutritional composition including proximate, amino acid, vitamin and minerals content of Amorpha fruticosa pollen collected by Apis mellifera bees to use as sources for feeding bee colonies and as healthy human food supplements with Q. acutissima and A. arguta pollen. Crude protein and fat content were found 23.05% and 3.12%, respectively. Eighteen amino acids from A. fruticosa pollen were found, including 8 essential amino acids and 9 non-essential amino acids. The predominant amino acids were glutamic acid, aspartic acid and proline, accounting for about 33.5% of total free amino acids. The concentration of vitamin C was the highest value of 57.8mg/100g, followed by niacin and B2 among the detected vitamins. The predominant minerals were potassium (410.32mg/100g), followed by calcium and magnesium, whereas sodium (Na), iron (Fe) and zinc (Zn) were found as minor elements.
Keywords:
Amino acid, Mineral, Pollen, VitaminINTRODUCTION
Honey production from approximately 1.7 million colonies owned by around 21 thousand Korean beekeepers was almost 36 thousand M/T in 2014 (Korea statistical yearbook, 2014). Beekeepers mainly harvest commodities such as honey, beeswax, pollen and royal jelly. Honeybees gather the nectars and pollens from flowers of plant. Honey is mainly gathered from the flowers of acacia tree (Robinia pseudoacacia), whereas bee pollens are mainly collected from acorn (Quercus acutissima) and actinidia (Actinidia arguta) tree in Koreas (Korea beekeeping industry information, 2015). Bee pollen is the pollen ball that has been packed by worker honeybees into pellets. Bee pollen grains added honey and are stored in brood cells (Bogdanov, 2014). Bee pollen is the only natural source of proteins, lipids, minerals, vitamins and amino acids for brood rearing and bee growth and development (Human and Nicolson, 2006; Campos et al., 1997). Bee pollen grains possess therapeutic benefits like promoting antitumor effects, scavenging free radicals, enhancing the immune function to mention a few (Bogdanov, 2014; Kroyer and Hegedus, 2001), and a good nutritional supplement with the beneficial effect for health (Bogdanov, 2014). Pine pollen has used as a food and medicine even before the Joseon Dynasty in Korea. As pollen grains demand in recent years have increased exponentially due to their biological activities or function, production of pollen is not sufficient to meet its demand currently. Acorn and actinidia pollen grains are a major and popular in the markets in Korea. Amorpha fruticosa which belong to the legume family (Fabaceae), tree came to Korea in the 1930s and planted in railroad and highway sides, embankments, ridge all over the country (Kim et al., 2005). These trees bloom from May to June and their pollen grains are usually found at beehives, with orange color. In this study, we evaluated the nutritional potential of Amorpha fruticosa pollen grains collected by Apis mellifera bees as sources for feeding bee colonies and as healthy human food supplements.
MATERIALS AND METHODS
Bee pollen samples
Pollen grains were collected directly from Apis mellifera bees returning to the beehives with pollen traps. Pollen traps were fitted on the entrances of hives in the National Academy of Agricultural Science (NAAS) apiary located in Wanju province of Korea during May to June 2015. The collected pollens were hand-sorted by color and appearance and identified by SEM examination. The selected pollens were stored at -20°C in freezer for chemical analysis.
Proximate chemical analysis
Amorpha fruticosa pollen was analyzed for chemical composition following official methods. Moisture, crude protein, fat and ash were determined following the standard method described by Korean Food Standards Codex (2008): Water and ash content were determined by drying of pollen to constant weight at 105°C and at 550°C, respectively. Crude protein content was obtained by the semi-micro Kjeldahl method using the 6.25 factor for conversion into protein. Fat content was estimated using a Soxhlet extractor with diethyl ether as solvent. All analyses were performed in duplicate.
Amino acid
Amino acid content of A. fruticosa pollen was determined following the standard method described by Korean Food Standards Codex (2008). Pollen grains were analyzed by the PicoTagⓇ method (3.9mm×15cm column) using a Waters HPLC amino acid analyzer (Waters, Millipore Corp., Milford, MA). The sample was hydrolyzed with 6 N HCl in a sealed tube at 105°C in an oven for 20 h and derivatised with phenylisothiocyanate (PITC) to produce phenylthiocarbamyl (PTC) amino acids, and then analyzed by reverse-phase HPLC. Buffers used in this analysis were 0.14 M sodium acetate trihydrate and water-acetonitrile (60:40). An absorbance was detected at 254nm using UV spectrophotometer. For tryptophan, pollen grains were hydrolysed with saturated barium hydroxide at 110°C for 16 h, then analysed by reverse-phase HPLC using a Symmetry column (4.6×150mm) with detection at 285nm.
Vitamin
Vitamin content of A. fruticosa pollen was determined following the standard method described by Korean Food Standards Codex (2008). The vitamins were measured by Nanospace SI-2 HPLC system (Shiseido, Japan) with a fluorescence (FL) detection for vitamin B2 (Riboflavin) or photodiode array PDA) detector for vitamin B3 (niacin) and vitamin C.
Minerals
Mineral content of A. fruticosa pollen was determined following the standard method described by Korean Food Standards Codex (2008). The minerals were measured by ICP-OES Perkin Elmyer 8300 (USA).
RESULTS AND DISCUSSION
Proximate chemical composition
The proximate composition including moisture, crude protein, fat and ash presented in Table 1. The quantity of carbohydrate was obtained by difference. Data show that crude protein content was abundant in pollen after carbohydrate. This nutrient is often considered as an indicator of pollen value for human food (Martins et al., 2011). The amounts of crude protein and fat were 23.05% and 3.12%, respectively. The crude protein and carbohydrate levels of A. fruticosa were slightly higher than that of Quercus acutissima and pine pollen, but lower than that of Actinidia arguta pollen (Choi and Jeong, 2004; Lee et al., 1997). The fat content of A. fruticosa was higher than that of Actinidia arguta pollen, similar to that of pine pollen and lower than that of Quercus acutissima (Choi and Jeong, 2004; Lee et al., 1997). Variations in the chemical composition of pollen grains reflect differences in species, environmental conditions during maturation and age and vigor of the plants (Solberg and Remedios, 1980; Stanley and Linskens, 1974).
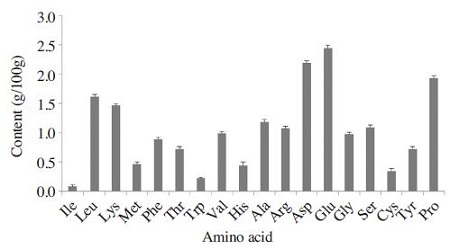
Amino acid
The content of amino acids in A. fruticosa pollen is shown in Fig. 1. Data present that eighteen amino acids from A. fruticosa pollen were found, including eight essential amino acids (lysine, isoleucine, leucine, methionine, phenylalanine, valine, threonine and tryptophan). Of all of the detected amino acids, glutamic acid, aspartic acid and proline were found in the high concentrations. However, ten amino acids (tryptophane, cystine, histidine, methionine, tyrosine, threonine, isoleucine, phenylalanine, glycine, valine) were found in the concentration of below 1g/100g in A. fruticosa pollen. Leucine and lysine was also found in relatively high concentrations. Glutamic acid, aspartic acid and lysine have important roles in maintaining the allergenic acitivities of the allergen molecule (King et al., 1974). The predominant amino acids of glutamic acid, aspartic acid and proline in A. fruticosa pollen accounted for about 33.5% of total free amino acids. Glutamic acid, aspartic acid and leucine were the predominant amino acids in Q. acutissima and A. arguta pollen grains, constituting approximately 30.1% and 31.5%, respectively (Hong et al., 2013; Lee et al., 1997). Also pine pollen showed a high content of aspartic acid, alanine and glutamic acid, accounting to roughly 36.7% of total free amino acids (Han et al., 2004; Lee et al., 1997). One interesting finding was that proline was the third most predominant amino acid. The third predominant amino acid was leucine in Q. acutissima and A. arguta pollen grains (Hong et al., 2013). In generally, pollen contains all essential amino acids but the amounts may vary between plant species (Leblanc et al., 2009).
Vitamin
The vitamin content from A. fruticosa pollen presented in Table 2. Data show that the amount of vitamin C was the highest value of 57.8mg/100g, followed by niacin and B2. The content of vitamin C obtained in this study was lower than that of Q. acutissima pollen, but higher than that of A. arguta pollen (Hong et al., 2015) and pine pollen (Han et al., 2004), whose values of vitamin C are 48.4 and 2.25mg/100g, respectively. While the concentration of niacin was 8.16mg/100g, which was largely lower than Q. acutissima (48.2mg/100g) and A. arguta (21.0mg/100g) pollen grains, 48.2 and 21.0mg/100g (Hong et al., 2015). The vitamin B2 concentration found in this study is similar to those described by Hong et al. (2015), which showed values of vitamin B2 of 1.2 and 1.0mg/100g in Q. acutissima and A. arguta pollen grains of bee pollen, respectively. Han et al. (2004) reported superior result from pine pollen which was observed value of vitamin B2 of 7.93mg/100g.
Mineral composition
Table 3 shows data on the mineral composition of A. fruticosa pollen. The predominant minerals were potassium (410.32mg/100g), followed by calcium (112.21mg/100g) and magnesium (84.13mg/100g), whereas sodium (Na), iron (Fe) and zinc (Zn) were present in trace amount, which are considered as antioxidants due to their structural components of the antioxidant enzymes. The results are similar to those described by Han et al. (2004), who observed the mineral composition in pine pollen. Similarly, potassium (K) was found at the highest level followed by calcium (Ca) in pollen collected from Romania (Stanciu et al., 2012). Stanley and Linskens (1974) reported that the mineral content of pollen were variable due to factors such as bee species and plant species. The high ratio of K/Na makes bee pollen an interesting food for diets with a defined electrolytic balance (Wesh and Marston, 1983). Ratio of potassium/sodium of A. fruticosa pollen was 44.03, which was higher than that of Q. acutissima (12.59) and A. arguta pollen (10.88) grains (Lee et al., 1997).
In this study, A. fruticosa pollen grains collected by bee was characterized by a high content of crude protein and carbohydrates, and high levels of amino acids, vitamins and minerals. Thus, it can be suggested to be used as excellent sources for feeding bee colonies and as healthy human food supplements with the pollen grains of Q. acutissima and A. arguta in Korea.
Acknowledgments
This work was supported by “Research program for Agricultural Science & Technology Development (Project No. PJ010837)” Rural Development Administration, Republic of Korea.
References
- Bogdanov, S., (2014), Pollen. Production. Nutrition and Health. A Review, p3, Bee Product Science.
-
Choi, S.J., and Y.H. Jeong, (2004), Effect of proteases on the extraction of crude protein and reducing sugar in pollen, J. Korean Soc. Food Sci. Nutr, 33, p1353-1358.
[https://doi.org/10.3746/jkfn.2004.33.8.1353]

- Han, M.R., S.J. Lee, and M.H. Kim, (2004), Development of pine pollen cell wall rupture technique using a high impact planetary milling process, Dankook Journal of the New Material Technology, 12, p43-54.
- Hong, I.P., M.Y. Lee, S.O. Woo, H.S. Sim, Y.S. Choi, S.M. Han, H.K. Kim, K.H. Byeon, M.L. Lee, and N.G. Ha, (2013), Change of chemical composition in Acorn pollen after physical treatment, Korean J. Apiculture, 28, p217-221.
-
Hong, I.P., S.O. Woo, S.M. Han, S.G. Kim, H.R. Jang, M.Y. Lee, Y.S. Choi, H.K. Kim, and M.L. Lee, (2015), Evaluation of total polyphenol, flavonoid and vitamin content from the crushed pollens of Acorn and Actinidia, Korean J. Apiculture, 30, p225-229.
[https://doi.org/10.17519/apiculture.2015.09.30.3.225]

-
Human, H., and S.W. Nicolson, (2006), Nutritional content of fresh, bee-collected and stored pollen of Aloe grestheadii var. davyana (Asphodelaceae), Phytochem, 67, p1486-1492.
[https://doi.org/10.1016/j.phytochem.2006.05.023]

- Kim, J.H., D.H. Kim, J.H. You, M.C. Kwon, H.J. Lee, H.J. Lee, and H.Y. Lee, (2005), Anticancer and immune activities of extracts from Amorpha fruticosa L, Korean J. Medicine Crop Sci, 13, p41-47.
-
King, T.P., P.S. Norman, and N. Tao, (1974), Chemical modification of the major allergen of ragweed pollen, antigen E, Immunochemistry, 11, p83-92.
[https://doi.org/10.1016/0019-2791(74)90321-8]

- Korea beekeeping industry information, (2015), Korea beekeeping association.
- Korea statistical yearbook, (2014), Statistics Korea.
- Korean Food Standards Codex, (2008), Ministry of food and drug safety.
-
Kroyer, G., and N. Hegedus, (2001), Evaluation of bioactive properties of pollen extracts as functional dietary food supplement, Innov. Food Sci. Emerg. Technol, 2, p171-174.
[https://doi.org/10.1016/S1466-8564(01)00039-X]

-
LeBlanc, B.W., O.K. Davis, S. Boue, A. Delucca, and T. Deeby, (2009), Antioxidant activity of Sonoran Desert bee pollen, Food Chem, 115, p1299-1305.
[https://doi.org/10.1016/j.foodchem.2009.01.055]

- Lee, B.Y., H.D. Choi, and J.B. Hwang, (1997), Component analysis of Korean pollens and pollen extracts, Korean J. Food Sci. Technol, 29, p869-875.
- Martins, M.C.T., M.A. Morgano, E. Vicente, S.R. Baggio, and D.B. Rodriguez-Amaya, (2011), Physicochemical composition of bee pollen from eleven Brazilian states, Journal of Apicultural Science, 55, p107-116.
- Solberg, Y., and G. Remedios, (1980), Chemical composition of pure and bee collected pollen, Scientific Reports Agricultural University, Norway, 59, p2-12.
- Stanciu, O.G., L.A. Marghitas, D. Dezmirean, and M.G. Campos, (2012), Specific distribution of minerals in selected unifloral bee pollen, Food Science and Technology Letters, 3, p27-31.
- Stanley, R.G., and H.F. Linskens, (1974), Pollen Biology, Chemistry and Management, Springer Verlag, New York.
- Wesh, S.O.., and R.M. Marston, (1983), Nutritional Bioavailability of Zin, Washington, D.C, American Chemical Society.