Global Honeybee Colony Trend is Positively Related to Crop Yields of Medium Pollination Dependence
Abstract
Recent declination of pollinators especially honeybee population in different geographical locations could threaten agricultural productivity leading to food insecurity. In order to examine the impact of the ‘pollinator crisis’, we hypothesized that the level of pollinator-dependence among the crops would be a significant factor to influence the crop yield. We examined the relationship between honeybee (Apismellifera) population as a major insect pollinator and crop yields with varying pollinator dependence in two different geographical scales, the continental scale and the country. Yield data of 60 crop systems with varying pollinator dependencies and the colony size of honey bee (i.e., the number of bee hives) in 5 continents (Asia, Europe, Africa, Australia and Americas) over the period of 1983-2013 were obtained from FAO database. We emphasised two Asian countries, for instance, Republic of Korea and India, considering socioeconomic development status to examine more closely the pattern in a finer scale. The temporal pattern of honeybee colony was categorized into three levels, increasing, decreasing and stable. Regression analysis showed that honeybee colony pattern was positively correlated with little, modest and great pollinator dependent crops but negatively correlated with essential pollinator dependent crops. In two Asian countries analyzed for this study, Republic of Korea and India showed the same pattern of honeybee colony increase but with different rates and densities. Increased yields observed in crops with medium pollination dependence could have resulted from the increase of honeybee hive numbers, but the yield change of essential pollination dependent crop seemed more related to the socioeconomic condition.
Keywords:
Food security, Pollinators, Yield, Essential crop, Socio-economy, Korea, IndiaINTRODUCTION
Pollination is an essential regulatory ecosystem process transferring male gamete of pollen to the female reproductive organ for reproduction. It depends to a large extent on the symbiotic relationship between the pollinated plant and the pollinator. Pollinators are not required for all crops but they affect significant proportion i.e. 35% of the world’s crop production amount from 87 of the 107 worldleading food crops (Klein et al., 2007). Pollinator diversity could lead the stable pollination and resulting higher yield (Garibaldi et al., 2013). It was claimed that the flowervisitor density is the most important predictor of crop yield for pollinator-dependent crops worldwide. Pollination deficit could be the main determinant of the yield over the diverse agronomic inputs such as fertilizer, pesticides and other fossil fuel energies (Garibaldi et al., 2016). Therefore, it could be reasonable to hypothesize that the loss of pollinators may affect yield of the crops with varying pollinator dependence.
Insect pollinators mainly belong to the orders of Hymenoptera (bees), Lepidoptera (butterflies) and Diptera (syrphid fly). There are other pollinators belonging to higher taxa vertebrates like birds, bat, monkeys etc. Among insect pollinators, honeybee (Apis sp.) dominant in various crop systems plays critical role for pollination (Klein et al., 2007). However, Garibaldi et al. (2013) claimed that wild pollinator diversity could contribute more than honeybee. Even with some controversy on the importance of honeybee among pollinator insects, honeybee is the dominant and major pollinator workhorse (Aizen et al., 2009; Kim et al., 2009). In addition to the honeybee as the major managed pollinator, alternative pollinators such as bumblebees, mason bees and some of the stingless bees are becoming commercially available.
From the perspective of pollination biology, crops are categorized to be pollinator dependent or independent. Pollinator dependent crops require animal pollinators for the production of fruits or seeds, while pollinator independent crops are either pollinated abiotically, mostly wind pollinated or autogamously or cultivated for vegetative parts like leaves, stems or tubers. Degree of pollinator dependency varies.
Habitat deterioration including habitat degradation and fragmentation of natural habitat (Thomas et al., 2004), higher pathogen prevalence (Colla et al., 2006; Cordes et., 2012; Graystock ., 2013; Furst et al., 2014), competition between native and invasive species (Goulson, 2003), agricultural intensification leading to less plant diversity and climatic change are among main drivers those are responsible for instabilities of pollinator population. In many parts of the world the population of pollinators decline (Lebuhn et al., 2012). The declination of pollinators is likely to cause lower yields of the pollinator dependent crops, leading to agricultural crisis which will be translated to food crises. This situation is referred as ‘pollination crisis’ which becomes a subject of almost all arenas like science, politics and economy (Jung, 2014). Although the current data on pollinator population declination is meagre to conclude in global scale, but the most stringent expectation from the hypothesis of ‘pollinator declination’ is the pollination crisis in the global agriculture. Thus, we could predict lower relative yield among the higher level of pollinator dependent crops than that of pollinator independent crops. Some recent studies including Aizen et al. (2008), Aizen et al. (2009), Garibaldi et al. (2011) are remarkable in this context which evaluated the change in pollinator dependency considering the developed and developing world separately or overall global perspective. Another study by Sinnathamby et al. (2013) evaluated socio economic impacts dur to pollinator decline in US. The present study is of its own kind as we undertake the study to examine the crop yield pattern, temporal trend of honeybee colony and relationship between honeybee (Apis mellifera) population as a major insect pollinator and crop yields with varying pollinator dependence in two different geographical scales, the continental scale and the country.
MATERIALS AND METHODS
Honeybee colony and crop yield data
We adopted honeybee hive number data for the period of 1983-2013 for respective continents and countries from FAO database (FAOSTAT). It includes the total number of commercial hives of the domesticated honeybee, Apis mellifera primarily (Aizen and Harder, 2009). We compiled data for the period of 1983-2013 on average yield of a total 60 crop systems including some aggregation like cereals, pulses, oil crops and oil seeds from FAO dataset(FAOSTAT). Majority of them are taxonomically single species, however cultivar may vary and few crops represent cogeneric species like coffee. Yield might be comparatively reliable parameter as yield is defined as production per unit area harvested. On the other hand production might be limited by other consideration like area of cultivation. The crop systems were categorized into 5 different categories based on their dependency on pollinators as already described in the introduction (Table 1) (Smith et al., 2015). According to Aizen et al. (2009) the degree of pollinator dependence have been classified into five groups: (a) none (production does not increase with animal pollination; class 0), (b) little (0~10% production reduction; class 1), (c) modest (10~40% reduction; class 2), (d) high or great (40~90% reduction; class 3) and (e) essential (>90% reduction without pollinators; class 4).We included 5 continents i.e. Asia, Africa, Europe, Americas and Australia to examine the situation in different geographical regions. Moreover, to understand more specifically India and Republic of Korea were focused as both the countries belong to different socio economic condition. It is true that not all the crops grows in all parts of the world, thus we included only those crops among the 60 systems which grow in the respective region. To avoid the undue influence of some extraordinary yield or loss of one particular crop, we excluded that crop, putative outlier, from our analysis. Like walnut yield was extremely high in Africa and could affect in the statistical analysis. Similarly cashewnut yield loss in Americas was very high, tea in Europe thus we excluded these data form statistical analysis.
Data analysis
Firstly all the measurements (beehive number) were expressed as the difference from their 30 years (1983-2013) mean and expressed in terms of percentage of changes. The simple linear regression model is as follows:
Y = βX + α
Where X is time and Y is changes of beehives (in %) and α and β are parameters estimated.
We carried out ANOVA followed by Post Hoc LSD (Least Significant Differences) analysis (CI 95%) in order to understand the difference in yield of different pollinator dependent crops. SAS 9.2 (SAS Institute) has been used for statistical analysis.
RESULT AND DISCUSSION
Temporal trend of honey colony size
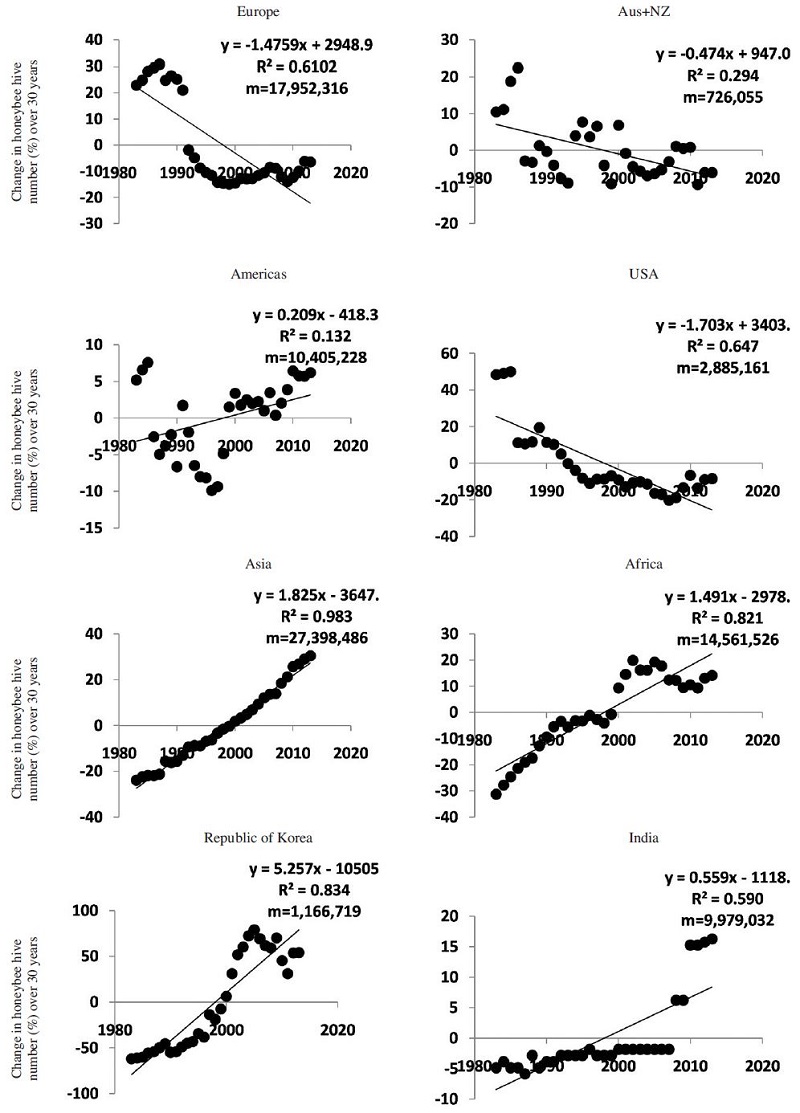
Descriptive statistics of honeybee hive number and density of each continental and country are presented in Table 2. Thirty year average of hive number was highest in Asia followed by Africa. Honeybee hive density per square kilometre over the continents ranged from 0.06 to 0.64 except in Europe where it reached 1.56. Honeybee hive density per agricultural land was between 0.2 and 3. However, the density in Korea was extremely high which was not comparable to any other countries (11.7 and 66 hive per km2 of land or agricultural land, (Table 2). We found three types of honeybee colony patterns; increase, decrease and stable (Fig. 1). It was found that in Europe, Australia & New Zealand, and USA the trend is declining from 1983 to 2013. While in Asia and Africa it was increasing. No consistent pattern was found from whole Americas where the regression coefficient of the slope was not different to zero (Fig. 1) (p<0.001). There was no availability for separate data set for North and South America. So we analysed Americas and USA data separately. For America, beehive numbers had declined sharply during 1992 to 1998 and then rebounded to the 1980s level. While in USA, very sharp declination was found there. The number of beehives counts only European managed honeybee i.e. A. mellifera, although there are other honeybee species like A. dorsata, A. cerena and A. florae. In Korea, the total honey bee population is increasing trend, the population of native honeybee A. cerena is recently sharply declining largely because of the bee disease epidemics (Jung and Cho, 2015).
Crop yield pattern is not consistent with pollinator dependence
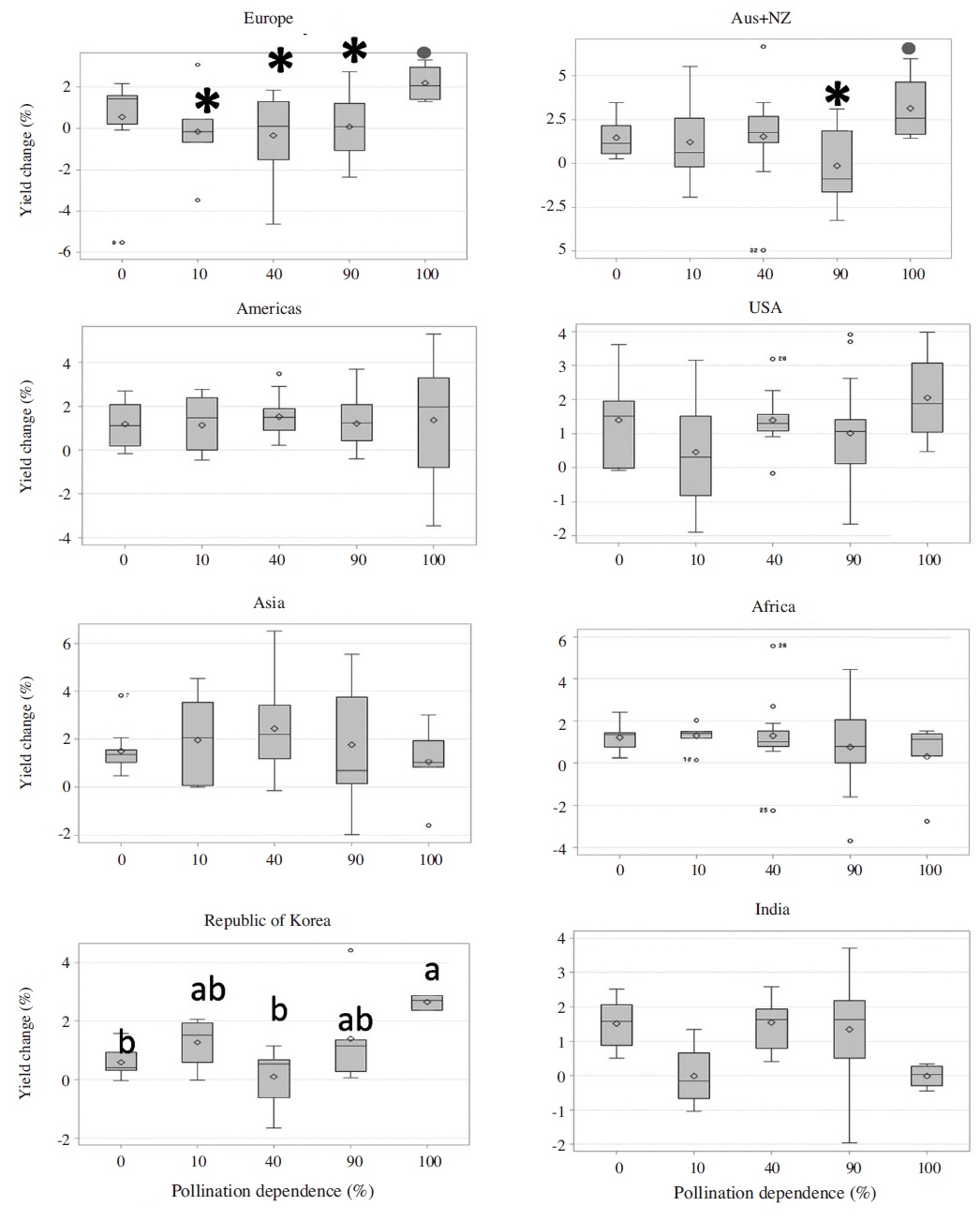
In the contrary to our expectation we found no significant differences in the average yield changes among the crops of different pollinator dependences in the continents of pollinator declining (Europe: df=4, 45, F=0.68, p=0.6; Aus & NZ: df=4, 37, F= 0.87, p=0.48; USA: df=4, 37, F=0.88, p=0.48), or pollinator increasing (Asia: df=4, 51, F=0.55, p=0.70; Africa: df=4, 47, F=0.99, p=0.42), or pollinator stable in Americas (df=4, 50, F=0.43, p=0.78). The same trend was found from India (df=4, 35, F=1.03, p=0.40). However, average yield changes among the crops with different pollination dependence categories were significantly different from Korea (df=4, 26, F=3.93, p=0.01) where yield change of essential pollinator dependence was highest. Similar pattern (high yield change of essential crops relative to less or no pollinator dependent crops) was found from Europe, Australia & New Zealand and USA (Fig. 2).
Correlation between yield and honeybee population is significant
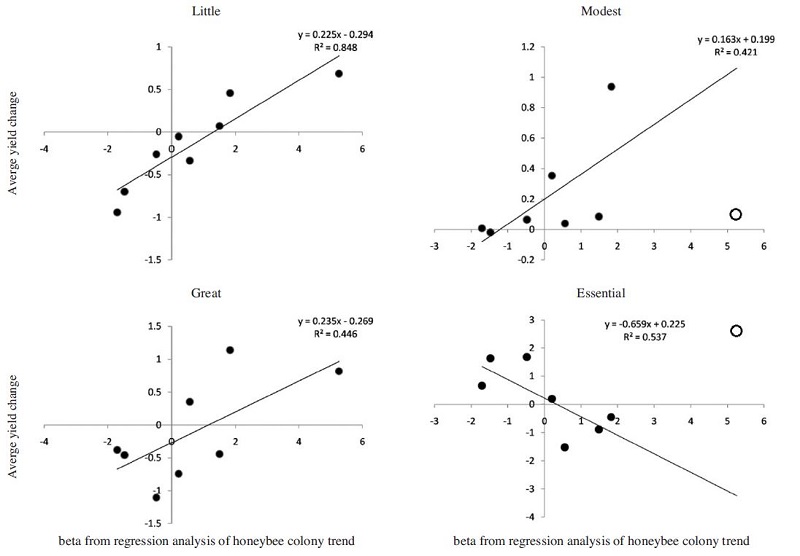
Fig. 3 showed the relationship between the honeybee hive pattern and yield changes of crops with different pollination dependence. Coefficient β (slope or gradient) from the regression analysis of honeybee colony pattern positively correlated with the yield change of little, modest and great pollination dependent crops (P<0.05, R2=0.85, 0.42, 0.45 respectively). Also β was negatively associated with the yield change of essential pollination dependent crops (P<0.05, R2=0.54). These results clearly demonstrate that honeybee population trend could explain yield change of some crops but not all over the continental scale or even to country level. During the analysis of the regression, we excluded some outlier data points (e.g. value of 2.72% for yield change of essential crop, and 0.07% for modest crop from Korea).
Protected agriculture and pollination
A significant fraction of the crop products like tomatoes, cucumber, strawberries, melons comes from the green houses. In South Korea, the production area under protective cover including single plastic tunnel, multi-span plastic green houses and glass houses is about 52000 ha (~30% of total horticultural area) (Stellen and van Uffelen, 2006). In many parts of the world especially developed regions, systems of protected agriculture are being used and contribute to the gross agricultural production (Jensen and Malter, 1995). Globally the principle green house crops include cucumber, eggplant, melon, strawberries, squash, watermelon, pepper, tomato and other vegetable crops and there is a significant increasing trend in percent of green house area (Jensen and Malter, 1995). In most cases, managed pollinators like honeybee or bumblebees are used as pollinators in the green house products. Thus, there is little scope to assess the current population status of pollinators from these green house yields.
On the other hand, there are many regions in the world where it is difficult for any system of protected agriculture to compete with open field agriculture presumable because of lower economic status. The total area covered under protected cultivation in India is approximately 30,000 ha (Sweta et al., 2014), only 0.23% of the total area under the horticulture cultivation in India in 2012. However, there are initiatives to adopt protective cultivation methods. In Africa, continent the contribution of green house agriculture is comparatively less in comparison to other developed areas of the world. Eventually this condition provides symmetry to assess the pollinators comparing different pollinator dependent crop yield. It is also true that these low economic regions are generally fall in the range of tropics and undoubtedly have higher biological diversity including insects and pollinators. May be this is the reason there is no significant declination in the yield of crop highly dependent on pollinators.
Pollination is not the sole factor for high or low yield of crops
Pollinator dependent crops include fruits, vegetables, seeds, nuts and oils. Many of them provide important dietary source including protein, minerals and vitamins and undoubtedly they are indispensible part of nutritional security. With the increasing demand of food as a resultant of increasing population of the developing countries expansion of many moderately pollinator dependent crops occurs like soybean in Argentina, Paraguay, Uruguay and Bolivia, Canola in Canada etc (Brookes and Barfoot, 2015). The higher yield of these moderate pollinator dependent crops can be attributed to factors like genetically modification, favourable climatic conditions, absence of dearth season for pollinators. At the same time several pollinator dependent crops also represent important sources of economy especially for developing country. For example, decline in the production of coffee or cocoa has limited effect on global agriculture production or human health but definitely would significantly affect those countries whose economy depends on exportation of these products.. Thus it would be unreasonable to assess the pollination population dynamics based only yield analysis of crops. Less yield or production does not necessarily imply the crisis of pollinators. We have already discussed the high yield of essential pollinator dependent crops in comparatively developed regions like Europe, USA, Australia and New Zealand in the context of protected agriculture. Any negative trend of yield of pollinator dependence crops of those places does not correlate with declination of managed honeybee (Apis mellifera) populations in North America and some parts of Europe (Wantabe, 1994; Kluser and Peduzzi, 2007; Oldroyd, 2007), as well as more recent reports of declination even extinction of some native bees like bumblebees (Martins and Melo, 2010). Moreover, the status of present pollinator population is restricted with geographical regions. Thorough review clearly indicates that most of the recent studies represent few regions of the world, accounting only 4% of the total volume of data from Africa (Archer et al., 2014). Another concern is methodology involved to study pollinator population, insect pollinators in particular. Almost every method has its own limitations and supplies different information. Netting flower visitors provides the information about the potential of the visitor as pollinator, whereas pan traps provide limited information on species pollinating abilities (Popic et al., 2013).
Honeybee is not only the pollinator, time to acknowledge other
The idea of pollinator declination is relatively recent (Kevan, 1999; Raw, 2001; Spira, 2001) but as because they are important contributor to world food production and nutritional security there is growing concern among both scientific community and general public (e.g. IPBES, 2016). Although there are about 20000 estimated bee species, the most common domesticated honeybee Apismellifera is often considered as a main pollinator workhorse, synonymously represents the pollinator populations and certainly misleads to understand actuality. Several crops need pollinators for successful reproduction but the pollinator should not necessarily be honeybee, for example bumblebee for tomato (Morandine et al., 2001). Recent studies evidence the importance including flower visitation, pollen deposition of wild pollinators in pollinating 41 crop systems worldwide while role of honeybee was limited to only 14% of the surveyed crops, suggesting a new practice for integrated management of both honeybees and diverse wild insect assemblages to have the synergistic effect in order to enhance global crop yield (Garibaldi etal., 2013). The practice of pollination by wild pollinators could be achieved through reducing application of insecticides, enhancing richness of flowering plants (Garibaldi et al., 2014). However initiative should be taken in order to study individual pollinator and their pollination potentiality. The future use of the many undiscovered pollinators depends on the establishment of methods to breed them in the necessary quantities (Velthuis, 2002).
The declination of pollinators ideally could simply equate to pollination crisis but in reality the relation is complex. Yield oriented intensive agriculture through large scale monocropping ultimately leads to loss of plant diversity which in turn could be a potential threat for majority of pollinators. On the other hand unwise use of insecticides, antibiotics often create to undesired pressure on the biotic components of agro-ecosystems including pollinator populations. Although there is lack of information on pollinator population worldwide, the trend of pollinator decline in several parts of world definitely advocate undertaking preventive measure. Undoubtedly the present study has many limitations to represent the realistic scenario of pollinator population. Moreover the agricultural production data irrespective of yield or productivity is not a completely realistic measurement of the question concerned pollinator status and other factors like nutrition, irrigation, climatic conditions etc. are not be overlooked. The regional initiatives taken for pollinator monitoring hopefully come up with more vivid understanding about pollinator’s population structure and functionality.
Acknowledgments
This work was supported by the 2015 Research Grant of Andong National University to CJ.
References
- Abrol, D.P., (1989), Studies on ecology and behaviour of insect pollinators frequenting strawberry blossoms and their impact on yield and fruit quality, Trop. Ecol, 30, p96-100.
- Abrol, D.P., and A. Kumar, (2009), Foraging activity of Apis species on strawberry blossoms as influenced by pesticides, Pak Entomol, 31, p57-65.
- Ahn, S.B., I.S. Kim, W.S. Choi, and K.M. Choi, (1989), Survey on the use of honeybee pollination pollination of strawberries grown in plastic green house, Korean J. Apic, 4, p1-8.
-
Aizen, M.A., L.A. Garibaldi, S.A. Cunningham, and A.M. Klein, (2008), Long-term global trends in crop yield and production reveal no current pollination shortage but increasing pollinator dependency, Curr. Biol, 18, p1572-1575.
[https://doi.org/10.1016/j.cub.2008.08.066]

-
Aizen, M.A., L.A. Garibaldi, S.A. Cunningham, and A.M. Klein, (2009), How much does agriculture depend on pollinators? Lessons from long term trends in crop production, Ann. Bot, 103, p1579-1588.
[https://doi.org/10.1093/aob/mcp076]

-
Aizen, M.A., and L.D. Harder, (2009), The global stock of domesticated honey bees is growing slower than agricultural demand for pollination, Curr. Biol, 19, p915-918.
[https://doi.org/10.1016/j.cub.2009.03.071]

-
Archer, C.R., C.W.W. Pirk, L.G. Carvalheiro, and S.W. Nicolson, (2014), Economic and ecological implications of geographic bias in pollinator ecology in the light of pollinator declines, Olkos, 123, p401-407.
[https://doi.org/10.1111/j.1600-0706.2013.00949.x]

- Brookes, G., and PP. Barfoot, (2015), GGM crops: global socioeconomic and environmental impacts 1999-2013, PG Economics Ltd, UK, Dorchester, UK, [available at: www.appg-agscience.org.uk/linkedfiles/GMcropsglobalimpactstudyfinalMay2015.pdf].
-
Colla, S.R., M.C. Otterstatter, R.J. Gegear, and J.D. Thomson, (2006), Plight of the buble bee: Pathogen spillover from commercial to wild populations, Biol. Cons, 129, p461-467.
[https://doi.org/10.1016/j.biocon.2005.11.013]

-
Cordes, N., W-F. Huang, J.P. Strange, S.A. Cameron, T.L. Griswold, J.D. Lozier, and L.F. Solter, (2012), Interspecific geographic distribution and variation of the pathogens Nosemabombi and Crithidia species in United States bumble bee populations, J. Invertebr. Pathol, 109, p209-216.
[https://doi.org/10.1016/j.jip.2011.11.005]

- FAOSTAT database, Available at: http://faostat3.fao.org/ accessed on 15th March, 2016.
-
Furst, M.A., D.P. McMahon, J.L. Osborne, R.J. Paxton, and M.J.F. Brown, (2014), Disease associations between honeybees and bumblebees as a threat to wild pollinators, Nature, 506, p364-366.
[https://doi.org/10.1038/nature12977]

-
Garibaldi, L.A., M.A. Aizen, A.M. Klein, S.A. Cunningham, and L.D. Harder, (2011), Global growth and stability of agricultural yield decrease with pollinator dependence, Proc. Natl. Acad. Sci. USA, 108, p5909-5914.
[https://doi.org/10.1073/pnas.1012431108]

-
Garibaldi, L.A., I. Steffan-Dewenter, R. Winfree, M.A. Aizen, R. Bommarco, S.A. Cunningham, C. Kremen, L.G. Carvalheiro, L.D. Harder, O. Afik, I. Bartomeus, F. Benjamin, V. Boreux, DD. Cariveau, N.P. Chacoff, J.H. Dudenhoffer, B.M. Fretas, J. Ghazoul, S. Greenleaf, J. Hipolito, A. Holzschuh, B. Howlett, R. Isaacs, S.K. Javorek, C.M. Kennedy, K.M. Krewenka, S. Krishnana, Y. Mandelik, M.M. Mayfield, I. Motzke, T. Munyuli, B.A. Nault, M. Otieno, J. Petersen, G. Pisanty, S.G. Potts, R. Rader, T.H. Ricketts, M. Rundlof, C.L. Seymour, C. Schuepp, H. Szentgyorgyi, H. Taki, T. Tscharntke, C. H. Vergara, B.F. Viana, T.C. Wanger, C. Westphal, N. Williams, and A.M. Klein, (2013), Wild pollinators enhance fruit set of crops regardless of honey bee abundance, Science, 339, p1608-1611.
[https://doi.org/10.1126/science.1230200]

-
Garibaldi, L.A., L.G. Carvalheiro, S.D. Leonhardt, M.A. Aizen, B.R. Blaauw, R. Isaacs, M. Kuhlmann, D. Kleijn, A. M. Klein, C. Kremen, L. Morandin, J. Scheper, and R. Winfree, (2014), From research to action: enhancing crop yield through wild pollinators, Front. Ecol. Environ, 12, p439-447.
[https://doi.org/10.1890/130330]

-
Garibaldi, L.A., L.G. Carvalheiro, B.E. Vaissiere, B. Gemmil-Herren, J. Hipolito, B.M. Freitas, H.T. Ngo, N. Azzu, A. Saez, J. Astrom, J. An, B. Blochtein, D. Buchori, F.J.C. Garcia, F.O. da Silva, K. Devkota, M.F. Rebeiro, L. Freitas, M.C. Gagliannone, M. Goss, M. Irshad, M. Kasina, A.J.S.P. Filho, L.H.P. Kiill, P. Kwapong, G.N. Parra, C. Pires, V. Pires, R.S. Rawal, A. Rizali, A.M. Saraiva, R. Veldtman, B.F. Viana, S. Witter, and H. Zhang, (2016), Mutually beneficial pollinator diversity and crop yield outcomes in small and large farms, Science, 351, p388-391.
[https://doi.org/10.1126/science.aac7287]

-
Goulson, D., (2003), Effects of introduced bees on native ecosystems, Annu. Rev. Ecol.Evol. Syst, 34, p1-26.
[https://doi.org/10.1146/annurev.ecolsys.34.011802.132355]

-
Graystock, P., K. Yates, S.E.F. Evison, B. Darvill, D. Goulson, and W.O.H. Hughes, (2013), The Trojan hives: pollinator pathogens,imported and distributed in bumblebee colonies, J. Appl. Ecol, 50, p1207-1215.
[https://doi.org/10.1111/1365-2664.12134]

- Jensen, M.H., and A.J. Malter, (1995), Protected Agriculture: A Global Review.World Bank Technical Paper Number 253, The World Bank, Washington, D.C, (1995), p101-112.
- Jung, C., (2008), Economic value of honeybee pollination on major fruit and vegetable crops in Korea, Korean J. Apic, 23, p147-152.
-
Jung, C., (2014), Global attention on pollinator diversity and ecosystem service: IPBES and Honeybee, J. Apic, 29, p213-215.
[https://doi.org/10.17519/apiculture.2014.09.29.3.213]

-
Jung, C., and S-K. Cho, (2015), Relationship between honeybee population and honey production in Korea: A historical trend analysis, J. Apic, 30, p7-12.
[https://doi.org/10.17519/apiculture.2015.04.30.1.7]

- Katayama, E., (1987), Utilization of honeybees as pollinators for strawberries in plastic green house in Tochigi Prefecture, Honeybee Sci, 8, p147-150.
-
Kevan, P.G., (1999), Pollinators as bioindators of the state of environment: species, activity and diversity, Agriculture Ecosystem and Environment, 74, p373-393.
[https://doi.org/10.1016/S0167-8809(99)00044-4]

- Kim, D.W., H.S. Lee, and C. Jung, (2009), Comparison of flower-visiting Hymenopteran communities from Apple, Pear, Peach and Persimmons blossoms, Kor. J. Apic, 24, p227-235.
-
Klein, A-M., B.E. Vaissiere, J.H. Cane, I. Steffan-Dewenter, S.A. Cunnigham, C. Kremen, and TT. Tscharntke, (2007), Importance of pollinators in changing landscapes for world crops, Proc. R. Soc. B, 274, p303-313.
[https://doi.org/10.1098/rspb.2006.3721]

- Kluser, S., and P. Peduzzi, (2007), Global pollinator decline: a literature review, Geneva, UNEP/GRID.
-
Lebuhn, G., S. Droege, E.F. Connor, B. Gemmil-Herren, S.G. Potts, R.L. Minckley, T. Griswold, R. Jean, E. Kula, D.W. Roubik, J. Cane, K.W. Wright, G. Frankie, and F. Parker, (2012), Detecting insect pollinator declines on regional and global scale, Conserv. Biol, 27, p113-120.
[https://doi.org/10.1111/j.1523-1739.2012.01962.x]

-
Martins, A.C., and G.A.R. Melo, (2010), Has the bumblebee Bombusbellicosus gone extinct in the northern portion of its distribution range in Brazil?, J. Insect Conserv, 14, p207-210.
[https://doi.org/10.1007/s10841-009-9237-y]

-
Morandin, L.A., T.M. Laverty, and P.G. Kevan, (2001), Bumblebee (Hymenoptera: Apidae) activity and pollination levels in commercial tomato greenhouses, J. Eco. Entomol, 94, p462-467.
[https://doi.org/10.1603/0022-0493-94.2.462]

-
Oldroyd, B.P., (2007), What’s killing American honey bees, Plos Biol, 5, pe168.
[https://doi.org/10.1371/journal.pbio.0050168]

-
Popic, T.J., Y.C. Davila, and G.M. Wardle, (2013), Evaluation of common methods for sampling invertebrate pollinator assemblages: net sampling out-perform pan traps, PLoS ONE, 8, e66665.
[https://doi.org/10.1371/journal.pone.0066665]

-
Raw, A., (2001), The risks ofpollinator decline and the global pollinator initiative, Acta Hort, 561, p327-330.
[https://doi.org/10.17660/ActaHortic.2001.561.49]

- Sakai, T., and M. Matsuka, (1988), Honeybee pollination in Japan, with special reference to strawberry production, Honeybee Sci, 9, p97-101.
- Singh, Y., (1979), Pollination activity of on strawberry at Jeolikot, District Nainital, India, Indian Bee J, 41, p17-19.
- Sinnathamby, S., Y. Assefa, A. Granger, L. Tabor, and K. Douglas-Mankin, (2013), Pollinator decline: US agro-socioeconomic impacts and responses, Nat. Env. Sci, 4, p1-13.
-
Smith, M.R., G.M. Singh, D. Mozaffarian, and S.S. Myers, (2015), Effects of decrease of animal pollinators on human nutrition and global health: a modelling analysis, Lancet, 386, p1964-1972.
[https://doi.org/10.1016/S0140-6736(15)61085-6]

- Spira, T.P., (2001), Plant -pollinator interactions: A threatened mutualism with implications for the ecology and management of rare plants, Nat. Area J, 21, p78-88.
- Stallen, M., and R. Van Uffelen, (2006), Green house sector study South Korea, Final report. LEI, Agriculture Economics Research Institute and Consortium, The Hague, 2nd May, 2006, [Available at: www.freshplaza.com/2010/0319/southkorea.pdf ].
- Sweta, S.K., Bhatia, and M. Malik, (2014), Protected farming, Popular Kheti, 2, p74-79.
-
Thomas, J.A., M.G. Telfer, D.B. Roy, C.D. Preston, J.J. Green-wood, J. Asher, R. Fox, R.T. Clarke, and J.H. Lawton, (2004), Comparative losses of British butterflies, brds and plants and the global extinction crisis, Sciences, 303, p1879-1881.
[https://doi.org/10.1126/science.1095046]

- Velthuis, H.H.W., (2002), The historical background of the domestication of the bumblebee, Bombus terrestris and its introduction in agriculture, p177-184, In Pollinating Bees- The conservationlink between agriculture and nature, eds. By P. Kevan, and V.L. Imperatriz Fonseca, Ministry of Environment/ Brasilia.
-
Wantabe, M.E., (1994), Pollination worries rise as honey bees decline, Sciences, 265, p1170-1170.
[https://doi.org/10.1126/science.265.5176.1170]