Efficiency of Artificial Insemination for Breeding Apis cerana in Korea
Abstract
Control mating is very important aspect in bee breeding programs. The technique of artificial insemination is the possible one that can surely control mating of the selected drones with the virgin queen. This is the first time applied this technique with A. cerana in Korea. Altogether 18 queens were artificially inseminated, and 2,000 drones of A. cerana both from Korea and Vietnam were used to evaluate amount of semen collection. Semen from Apis mellifera also was collected as a comparison. Semen of A. cerana is much difficult to separate from mucus in comparing with A. mellifera. The average amount of semen can be collected from one Korean A. cerana drone was 0.09μl and 0.1μl of Vietnamese one, whereas the A. mellifera was more than 6 times (0.58μl semen per A. mellifera drone). Obtaining 1μl of semen have to collect from 11.94 drones that successful semen ejection and have to kill 17 drones of Korean A. cerana. Queens artificially inseminated with 4μl of semen (once insemination) or 8μl of semen (twice insemination, each with 4μl of semen) started laying egg later than naturally mated queens 5.3 and 2.5 days, respectively. The onsets of oviposition of artificially inseminated queens were 12.5 to 15.3 days. Queens received twice inseminations started laying eggs 2.8 days earlier than those received only once insemination. Artificially inseminated queens produced exclusively brood and were similar as the naturally mated ones. The brood production of the queens received once insemination with 4μl of semen was insignificantly different than those received twice inseminations or naturally mated ones, suggesting that one artificial insemination with 4μl of semen is favorable.
Keywords:
Artificial insemination, Insemination, Natural mating, Apis cerana, Apis mellifera, Semen, SpermathecaINTRODUCTION
Apis cerana is one of ten currently recognized honey bee species of the genus Apis (Family Apidae, Subfamily Apinae, Tribe Apini) ( Ruttner, 1988; Arias & Sheppard, 2005). These cavity nesting bees build a series of parallel combs and can be domesticated, and cover huge geographical areas with a large range of ecological and climatic conditions of Asia. A. cerana is an excellent crop pollinator. A. cerana is successfully kept in hives for honey production, and is particularly important for small scale beekeeping. Korean apiculture has a 2000-year history with A. cerana. The exotic honeybee ( Apis mellifera) was firstly imported to Korea since 1900s. Native ( A. cerana) and exotic bees have co-existing but rather in a hostile relationship ( Lee, 2010).
Multiple mating is phenomenon among species of social Hymenoptera ( Strassmann, 2001). Apis genus are copulating multiple times, and they have the high degree of polyandry ( Moritz et al., 1995 ; Oldroyd et al., 1998 ; Rinderer et al., 1998 ). Queens store spermatozoa in their spermathecae and can keep them alive for many years ( Koeniger, 1986; Collins, 2000). Queen mates naturally in many flights ( Woyke, 1973; Koeniger et al., 1986; Ruttner et al., 1972; Jennions and Petrie, 2000), in each mating flight she mates with more than ten drones in the air space of the drone congregation areas (Koeniger, 1979; Ruttner, 1985; Woyke, 1975). Once queens have started egg-laying, they never mate again even if they run out of sperm ( Winston, 1987).
In 1975, Woyke investigated that the queens of A. c. indica fly out between 13:30~15:30 h, and mating occurs between 14:00~15:00 h. The mean duration of a mating flight is 27 minutes and queen mates with an average of 10 drones in one flight ( Woyke, 1975). Queens fly out when they are 3.6 days after emergence ( Sharma, 1960). Yoshida et al. (1994) has reported that the flight times of A. c. japonica queen are 13:15~17:00 h, and those of the drones are 13:15~16:30 h. Successful mating flights of queens occurs between 14:45 and 16:35 h ( Yoshida et al.,1994 ). Natural mated queen has 1.5~3mm 3 (average 1.9mm 3) semen in the oviducts, and a young laying queen has a mean of 1.3 million spermatozoa in the spermatheca. Queen instrumentally inseminated twice with a total of 6 mm 3 semen collected from 20-36 drones has on average 1.4 million spermatozoa in the spermatheca and can lay only fertilized eggs for one year ( Woyke, 1975).
During mating flights, queen honeybee coupulates with numerous drones. The risk of multiple mating flights of honey bee queens includes increased exposure to predators, risk of injury to the body, and some diseases transmission ( Simmons and Siva-Jothy, 1998). Control mating of queens has been considered as challenge to honey bee breeding. Many attempts to control mating of queens have started on the 1700’s and were unsuccessful. The instrumental insemination technique, developed in 1920’s and got successes in the 1940’s, provides a method of reliable control mating of Apis spp. ( Page and Laidlaw, 1985; Laidlaw, 1987). With instrumental insemination, the queen does not have to leave her hive which could increase the success of mating and survival of the queen. Recently, this technique is well-established and successful for artificial insemination of A. mellifera. Instrumental insemination also enables specific crosses of queens and drones, which do not occur naturally; therefore this specific cross provides advantages in honey bee breeding and its research. With this technique, researchers can be able to pool and homogenized sperm cells from hundreds of drones, and inseminate a group of queens. This allows unique mating system designs, and simplifies stock maintenance ( Page and Laidlaw, 1985; Harbo and Rinderer, 1980). Instrumental insemination has an advantage to store and ship honey bee semen. Short term storage has been improved ( Collins, 2000). The ability of shipping isolated semen of honey bees minimizes risk of spreading pests and diseases.
Over the past 60 years, instrumental insemination has been utilized in bee research institutions, although the adaptation of this technique with A. cerana has been slow because of the poor performance of instrumentally inseminated queens. The objectives of this research are to evaluate the performance of artificially inseminated queens and to compare them with the naturally mated ones.
MATERIALS AND METHODS
The experiments were conducted using colonies of Apis cerana and Apis mellifera at the apiary of National Institute of Agriculture Science, RDA, Rep. of Korea from May to August 2016. Semen of Vietnamese drones was collected at the A. cerana apiary in Hung Yen province of Vietnam in July 2016. Apiaries of A. cerana were setup to rear drones and virgin queens, and to introduce virgin and artificially inseminated queens for two experiments: First experiment had 20 beehives; the second experiment had other 20 beehives in different place. The A. mellifera drones were reared from three different hives in A. mellifera apiary.
Drones of A. cerana and A. mellifera for semen collection were caught on the entrances of different hives and caged in a plastic box (having supplement food and water supplied) with 200 drones in one batches (or tests). There are about 1400 drones of Korean A. cerana (7 tests), 600 drones (3 tests) of Vietnamese A. cerana and 1000 drones of Korean A. mellifera were used to collect semen.
Queen cells of A. cerana were artificially reared twice on May and June 2016. Virgin queens were artificially inseminated 6 days after emergence. The Schley’s instrument was used to do artificial insemination for the virgin queens. CO 2 narcotizing was used during the inseminating process to immobile queens on queen’s holder. The process of artificial insemination was applied following the method of Laidlaw (1987) as in Fig. 2 and 3. To stimulate egglaying of artificially inseminated queen, there were twice CO 2 treatments, once before and once after the date of artificial insemination. Queens received twice insemination didn’t have the second CO 2 treatment.
Semen of drones in one test (200 drones) was collected using glass capillary tube. The amount of collected semen was measured by Harbo syringe ( Fig. 1).
Drones for semen collection were caught in the entrance of the hives between 14:00 h and 15:00 h. Drone cage was kept in cool and dark place about 10 minutes to calm down drones. A researcher squeezed the thorax of the drone to evert the endophallus and semen. Number of drones that endophallus were partly everted or unable to eject semen was considered as failure drones.
On May, 14 virgin queens were introduced into mating hives with medium colony size (about 3,500~4,000 adult bees with 3 brood combs). Four queens were let to naturally mate, the rest 10 queens were artificially inseminated; 5 queens were inseminated once with 4 micro-little (μl) of semen and the other ones were inseminated twice, each time with 4μl of semen.
On June, the experiment was repeated. The artificially inseminated queens and naturally mated ones were let to lay egg in mating hives with medium colony size (about 4,000 adult bees with 3 brood combs) to evaluate queens’ performance by their brood production and colony development. All experiment colonies were similarly supplied with supplemental feedings.
The performance of queens was evaluated as her egg laying capacity and the colony development. Observations on the brood production were carried out twice in July and August: After the queens laid eggs for 25~30 days, on 20 July, all colonies were evaluated for the number of worker bees and broods (larvae and pupae); the second evaluation was done after the another 20 days (on 8 August). A frame (with size similar to bee comb provided with wire-grid of 5x5cm), was used to estimate number of worker bees and number of broods (larvae and pupae) ( Delaplane, 2013).
Data analysis
Experiments were set up as complete randomized design. Data were analyzed using one-way analysis of variance (ANOVA), and Fisher least significant difference (LSD) test was performed to make pairwise comparisons among treatment means (at a significance level of α=0.05 and 0.01). Data of onset oviposition of queens in experiment 1 and 2 were pooled to evaluate differences of groups. The volume of drones’ semen production was analyzed separately between two different honey bee species, Apis cerana and Apis mellifera. The means and standard deviations of each variable were calculated. All calculations are performed using the MS Exel and Statistica software.
RESULT AND DISCUSSION
Semen production of drones
In Apis cerana, there were high percentages of drones that cannot eject semen even the endophallus is completely everted. Some with the endophallus was everted but broken or ruptured at the tip, and consequently the semen was mixed with hemolymph. Such semen was contaminated and could not be collected and used. The drones that can not evert endophallus or contaminated semen were considered as failure drones.
The records in table 1 compare semen collection from A. cerana drone in Korea and Vietnam. In 7 batches (tests) with 1400 A. cerana drones in Korean were collected to artificially inseminate queens. Each test was done with 200 drones to collect around 4μl semen. The average of 0.09μl semen produced from one Korean drone was significant lower than that of Vietnamese one (0.1μl semen per drone). In comparison with A. mellifera, it was 0.58μl semen collected from one drone, about 6.5 times higher than that of Korean A. cerana drone. 1.42 drones of Korean A. cerana were killed to get one drone with successful semen ejection, this data is little lower than that of A. cerana drones yielded in Vietnam and higher than A. mellifera (1.54 and 1.15 killed drones to have one semen ejection, respectively). To collect 1μl of semen, we had to gather this amount from 11.94 successful semen ejected drones, and the data of Vietnamese drones were lower than that by 9.74 drones, however, this difference was not significant.
Number of drones to collect 1μl of semen was about 17 drones (11~12 drones successfully ejected semen), it is higher number than the data reported by Woyke (1975) with 12~13 drones of A. c. indica, because the amount of semen collected from one drone of Korean A. cerana (0.09 μl) is less than that of A. c. indica (0.16μl) as found by Woyke (1975) or 0.2μl by Ruttler et al.(1972) . The volume of semen per drone of A. mellifera was 0.58μl, that is similar to the study of Harbo (1986). To avoid stuck of glass tip by mucus during semen collection, we did not try to harvest all the semen that spread in a very thin layer above the mucus. This can explain why the amount of semen collected from one drone in our data was lower than the results of Woyke (1973 and 1975).
Onset of the oviposition
Eighteen queens were successfully inseminated using artificial insemination in the two experiments. Fourteen queens in the first experiment of May 2016; of them 4 queens were mated naturally and 10 queens were artificially inseminated once or twice. In this trial, 1 natural mated queen and 2 artificial inseminated ones were absconded, 1 artificially inseminated queen died with unknown reason (it might be injured by instrumental insemination). Only the queens laid egg were taken into the account. Due to the robbing behavior of A. mellifera from other colonies after mating two weeks, some colonies could not develop or abscond. Because of this, we did not research on egg-laying and colony development of first experiment, and the other experiment was carried out in different place to avoid robbing. Twelve queens were used in the second experiment of June 2016, 4 naturally mated queens and 4 queens were artificially inseminated for one time, and other 4 queens were inseminated twice.
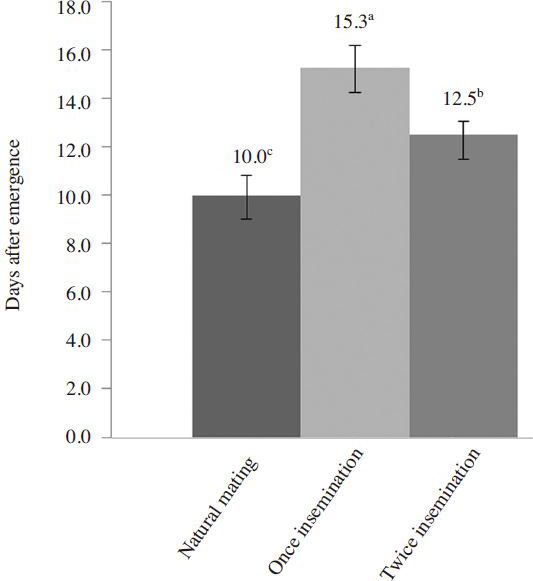
Fig. 4 shows that the naturally mated queens started in laying eggs after about 10 days from emergence. The artificially inseminated queens started in laying eggs after about 15.3 and 12.5 days since insemination for once and twice inseminated queens, respectively. Naturally mated queens started in laying eggs significantly earlier than artificially inseminated ones by about 2.5 to 5.3 days. Queens inseminated artificially twice laid eggs significantly earlier than those inseminated once.
The criteria for the success of instrumental insemination are based on the onset of oviposition and worker brood production. Mating flight (s) and the act of mating may stimulate physiological development that may be slower in artificially inseminated queens than naturally mated ones. Queens received inadequate number of sperms in first mating flight would take other mating flight (s), and then the onset of oviposition is delayed ( Koeniger et al., 1990 ; Oldroyd and Wongsiri, 2006). The number of spermatozoa in 1μl of semen of A. cerana drone is about 1.9~2.9 million ( Yoshida et al., 1994 ; Ruttner et al., 1973). Only about 10% of spermatozoa from one drone can reach to the spermatheca of the queen whereas more than 90% of spermatozoa, injected into the oviduct and spermaduct, is rejected ( Koeniger et al., 2004 ). In the report of Ruttner (1985), up to 1.9 million living spermatozoa were counted in the spermatheca of A. cerana. Woyke (1975) also reported that naturally mated queens had 0.66~1.99 million spermatozoa in the spermatheca (with average: 1.33 million), whereas the queens artificially inseminated with 6μl semen had 1.44 million spermatozoa in the spermatheca. Naturally mated queens start laying egg sooner than artificially inseminated ones. This can be explained by the different time of insemination. A. cerana bees do the mating flight (s) about 3.6 days after emergence ( Sharma, 1960), but the queens were artificially inseminated 6 days after emergence with about 3 days difference. Moreover, the artificially inseminated queens were exposed to CO 2 treatment to stimulate egg laying, and this may influence the physiological development and the onset of oviposition.
Colony development and brood production
Table 2 shows that the number of worker bee and larvae in the colonies headed with both artificially inseminated queens and naturally mated ones were insignificantly different while the pupae numbers were significantly different in the first observation. The naturally mated queens laid eggs earlier than artificially mated ones, thus they had higher number of pupae after the onset of oviposition by one month. Number of larvae after one month in all colonies headed with naturally mated queens or artificially inseminated ones were insignificantly different. Therefore, numbers of pupae in the second observation in August were not significantly different.
In August, numbers of worker bee, pupae and larvae were insignificantly different in all the colonies. The numbers of worker bee and larvae were slightly increased, and were higher than that of July but the number of pupae was decreased. After the second evaluation on August, some colonies had symptoms of Sacbrood virus infection, and the queen of infected colonies was caged in about 7 days to treat this disease. Due to the Sacbrood virus infection, we did the colonies evaluation in only 2 months.
Our result suggested that artificially inseminated queens laid egg and produced brood as well as natural mated queen did. Woyke (1975) reported that instrumentally inseminated queens produced exclusively worker brood during the first year. The other observations on the artificially inseminated queens of A. c. indica in Thailand and A. c. cerana in China (reviewed by Wongsiri, 2006) shown that the artificially inseminated queens produced insignificant different number of brood in comparison with naturally mated ones ( Oldroyd and Wongsiri, 2006).
ACKNOWLEDGEMENTS
This research was supported by an Agenda project (PJ011201022016) from NAAS, Rural Development Administration Republic of Korea. We would like to thank technicians (Mrs. Choi Jae Hyeon with beekeeping activities, Mrs. Yoon Mi Young for Sacbrood virus sampling and molecular detection) in the honey bee laboratory for their kind helps to our research.
LITERATURE CITED
- Arias, M. C., and W. S. Sheppard, (2005), Phylogenetic relationships of honey bees (Hymenoptera : Apinae: Apini) inferred from nuclear and mitochondrial DNA sequence data , Molecular Phylogenetics and Evolution, 37(1), p25-35.
-
Collins, A. M., (2000), Relationship between semen quality and performance of instrumentally inseminated
honey bee queens
, Apidologie, 31, p421-429.
[https://doi.org/10.1051/apido:2000132]

-
Delaplane, K. S., J. Van Der Steen, and E. Guzman, (2013), Standard methods for estimating strength parameters of
Apis mellifera
colonies
, V. Dietemann, J.D. Ellis, P. Neumann Eds, Journal of Apicultural Research, 52(1).
[https://doi.org/10.3896/IBRA/1.52.1.03]

- Harbo, J. R., and T. E. Rinderer, (1980), Breeding and genetics of honey bees, Beekeeping in the United States Agriculture handbook, 335, p49-57.
-
Harbo, J. R., (1986a), Oviposition rates of instrumentally inseminated and naturally mated queen honey bees
(Hymenoptera: Apidae)
, Ann. Entomol. Soc. Am, 79(1), p112-115.
[https://doi.org/10.1093/aesa/79.1.112]

- Harbo, J. R., (1986b), Propagation and instrumental insemination, In: Bee Genetics and Breeding (Rinderer T. E., Ed.), Academic Press, Orlando, p361-389.
-
Jennions, M. D., and M. Petrie, (2000), Why do females mate multiply? A review of the genetic benefits, Biol. Rev. Camb. Phil. Soc, 75, p21-64.
[https://doi.org/10.1111/j.1469-185X.1999.tb00040.x]

-
Koeniger, G., N. Koeniger, and M. Fabritius, (1979), Some detailed observations of mating in the honeybee, Bee World, 60, p53-57.
[https://doi.org/10.1080/0005772X.1979.11097736]

- Koeniger, G., (1986), Reproduction and mating behavior, In: Bee Genetics and Breeding (Rinderer T. E., Ed.), Academic Press, Orlando, p255-280.
-
Koeniger, G., N. Koeniger, M. Mardan, G.W. Otis, and R. P. K. Punchihewa, (1990), Number of spermatozoa in queens and drone indicate multiple mating in
Apis andreniformis
and
Apis dorsata, Apidologie, 22, p181-186.
[https://doi.org/10.1051/apido:19900402]

- Koeniger, G., and N. Koeniger, (2004), Mating behavior in honey bees (Genus Apis) , Tropical Agr. Re. and Ext, 7, p13-28.
- Laidlaw, H. H., (1987), Instrumental Insemination of honey bee queens: Its origin and development , Bee World, 68, p17-38.
- Lee, M.Y., (2010), Present Status of Korean Beekeeping Industry, Korean Journal of Apiculture, 25(2), p137-144.
-
Moritz, R. F. A., P. Kryger, N. Koeniger, A. Estoup, and S. Tingek, (1995), High degree of polyandry in Apis dorsata queens detected by DNA microsatellite
variability
, Behavioral Ecology and Sociobiology, 37, p357-363.
[https://doi.org/10.1007/BF00174141]

-
Oldroyd, B. P., M. J. Clifton, K. Parker, S. Wongsiri, T. E. Rinderer, and R. H. Crozier, (1998), Evolution of mating behavior in the genus Apis and an estimate of mating frequency in
A. cerana
(Hymenoptera: Apidae)
, Annals of the Entomological Society of America, 91, p700-709.
[https://doi.org/10.1093/aesa/91.5.700]

- Oldroyd, B. P., and S. Wongsiri, (2006), Asian honey bees: biology, conservation and human interactions, Cambridge MA, Harvard University Press.
-
Page, R.E., and H. H. Laidlaw, (1985), Closed Population Honeybee Breeding, Bee World, 66, p63-72.
[https://doi.org/10.1080/0005772X.1985.11098826]

- Rinderer, T. E., J. A. Stelzer, B. P. Oldroyd, and S. S., (1998), Levels of polyandry and intracolonial genetic relationships in Apis koschevnikovi, Journal of Apicultural Research, 37, p281-287.
- Ruttler, F., J. Woyke, and N. Koeniger, (1972), Reproduction in Apis cerana. 1. Mating behavior , J. Apic.. Res, 11(3), p141-146.
- Ruttler, F., J. Woyke, and N. Koeniger, (1973), Reproduction in Apis cerana. 2. Reproductive organs and natural insemination , J. Apic. Res, 12(1), p21-34.
- Ruttner, F., (1985), Reproductive behavior in honeybees, In: Experimental behavioral ecology and sociobiology. (Fortschritte der Zoologie, no. 31, Hölldobler B and Lindauer M, Eds.) , Sinauer Associates, Sunderland, p225-236.
- Ruttner, F., (1988), Biogeography and taxonomy of honeybees, Heidelberg, Springer-Verlag, Berlin, Germany.
-
Sharma, P. L., (1960), Observation on the swarming and mating habits of the Indian honeybee, Bee world, 41(5), p121-125.
[https://doi.org/10.1080/0005772X.1960.11096778]

- Simmons, L. W., and M. T. Siva-Jothy, (1998), Sperm competition in insects: mechanisms and the potential for selection , In: Birkhead T. R., M©™ller A. P. (Eds.) Sperm Competition and Sexual Selection , Academic Press, San Diego, p341-434.
-
Strassmann, J. E., (2001), The rarity of multiple mating by females in the social Hymenoptera, Insectes Sociaux, 48, p1-13.
[https://doi.org/10.1007/PL00001737]

- Winston, M.L., (1987), The Biology of the Honey Bee, Harvard University Press, Cambridge.
- Woyke, J., (1973), Instrumental insemination of Apis cerana indica queens , J. Apic. Res, 12(3), p151-158.
- Woyke, J., (1975), Natural and instrumental insemination of Apis cerana indica in India , J. Apic. Res, 14, p153-159.
-
Yoshida, T., J. Saito, and N. Kajigaya, (1994), The mating flight times of native
Apis cerana
japonica Radoszkowski and introduced
Apis mellifera
L. in sympatric conditions
, Apidologie, 25, p353-360.
[https://doi.org/10.1051/apido:19940401]